Abstract
Background:
HIV-associated Kaposi sarcoma (KS) is one of the most common malignancies in sub-Saharan Africa. The diagnosis is often based on clinical suspicion, without histopathologic confirmation. When biopsies are performed, the accuracy of interpretation by local pathologists is poorly understood. We assessed the accuracy of clinical suspicion and pathologic diagnosis of KS in 2 East African countries.
Methods:
At 2 large HIV care sites in Uganda and Kenya, we evaluated consecutive biopsies performed from October 2008 to January 2013 on HIV-infected adults with clinically suspected KS. Biopsies were interpreted by both local African pathologists and a group of US-based dermatopathologists from a high volume medical center. For the purpose of this analysis, the US-based dermatopathologist interpretation was used as the gold standard. Positive predictive value was used to characterize accuracy of local African clinical suspicion of KS, and concordance, sensitivity, and specificity were used to characterize accuracy of local pathologic diagnosis.
Results:
Among 1106 biopsies, the positive predictive value of clinical suspicion of KS was 77% (95% confidence interval: 74% to 79%). When KS was not histopathologically diagnosed, clinically banal conditions were found in 35%, medically significant disorders which required different therapy in 59% and life-threatening diseases in 6%. Concordance between African pathologists and US-based dermatopathologists was 69% (95% confidence interval: 66% to 72%). Sensitivity and specificity of African pathologic diagnoses were 68% and 89%, respectively.
Conclusions:
Among East African HIV-infected patients, we found suboptimal positive predictive value of clinical suspicion of KS and specific, but not sensitive, histopathologic interpretation. The findings call for abandonment of isolated clinical diagnosis of KS in the region and augmentation of local dermatopathologic services.
Key Words: Kaposi sarcoma, cancer, diagnosis, sub-Saharan Africa, pathology, dermatopathology
INTRODUCTION
Kaposi sarcoma (KS) is an angioproliferative cancer caused by infection with Kaposi sarcoma–associated herpesvirus (KSHV), which primarily targets the skin, lymphatics, and aerodigestive tract in immunocompromised hosts.1 In sub-Saharan Africa, high endemic KSHV seroprevalence and the HIV epidemic have resulted in KS being among the most commonly reported malignancies.2 For example, in Uganda, KS is the second most frequently reported cancer in men and fifth most common in women.2 Not only is KS common in sub-Saharan Africa, it is also often lethal; 1-year mortality after KS diagnosis is 19%–24%.3–5 Not surprisingly, patients diagnosed with KS frequently have an indication for chemotherapy, which itself may cause adverse effects, thus highlighting the importance that the KS diagnosis is correct. Although population data on chemotherapy use for KS in Africa are limited, 1 study from South Africa found chemotherapy given to 29% of patients.6
In resource-rich settings (eg, the U.S. and Europe), clinically suspected KS is routinely confirmed with histopathology. With lesions typically present on the skin, tissue for microscopic confirmation is easily obtained with a simple skin punch biopsy. In sub-Saharan Africa, despite the clinical relevance of KS, its diagnosis is often made on clinical grounds (ie, macroscopic visual inspection) without histologic confirmation (Naftali Buzakhala, MBChB, MMed, Miriam Laker-Oketta MBChB MSc, and Mwebesa Bwana MBChB, MMed; personal communication July 2008). In perhaps the most comprehensive published population-level ascertainment of KS diagnoses (in Malawi), only 17% of all KS diagnoses were biopsy-confirmed.7 Of potential concern is that this high-frequency of “clinical” diagnosis occurs despite the accuracy of this approach being poorly understood. When biopsies are performed, they often occur in the context of pathologists without expertise in dermatopathology8 and who lack diagnostic adjuncts, such as histochemical stains.9,10 It is thus unclear how accurate African pathologists are in diagnosing KS.
To address the limitations in knowledge regarding accuracy of clinical and pathologic diagnosis of KS in sub-Saharan Africa, we introduced skin punch biopsy services for KS to a group of HIV clinics in what is traditionally known as the hotbed of KS in the world—East Africa. Using US-based dermatopathologists' diagnoses as gold standard reference for the purposes of this analysis, we evaluated the accuracy of clinically suspected diagnoses of KS in East Africa and investigated the accuracy of local African pathologic interpretation.
METHODS
Study Design
At 2 HIV care sites in East Africa, we evaluated the histopathologic outcomes of a consecutive sample of HIV-infected adult patients who had skin biopsies performed because of clinical suspicion for KS. The biopsies were independently interpreted by both local African pathologists and a group of US-based dedicated dermatopathologists from a high volume center. Both African and US-based pathology interpretations were performed using routine clinical practice processes. The US-based readings were used to estimate the positive predictive value of clinical suspicion for KS by African clinicians and to estimate the accuracy of the African pathologists.
Participants and Samples
In 2008, in response to the observation that virtually all cases of KS were being diagnosed on clinical grounds alone (ie, macroscopic visual inspection), we provided training in the performance of skin punch biopsy and requisite equipment to 2 HIV care sites that were participating in the East Africa International Epidemiologic Databases to Evaluate AIDS Consortium.11,12 The first site, in Mbarara, Uganda, was the Immune Suppression Syndrome Clinic at the Mbarara Regional Referral Hospital. The Immune Suppression Syndrome Clinic is a rural-based, university-affiliated HIV clinic, which has cared for over 22,000 HIV-infected adults since its inception in 1998. The second site was the Academic Model Providing Access to Healthcare (AMPATH) network in western Kenya, which was established in 2001, is also university-affiliated, and has enrolled over 160,000 HIV-infected patients at over 60 different clinics.
At each site, a training session was provided for all clinicians on the epidemiology, diagnosis, and management of KS. The training included discussion of the clinical mimickers of KS, the importance of establishing a microscopic diagnosis, and a demonstration of the skin punch biopsy procedure. After this, 4-mm skin punch biopsies were made available as part of routine program services, free-of-charge through same day referral, and performed by a small group of practitioners (“skin biopsy teams”) at each site.13 Hemostasis was achieved in most cases with manual pressure and Gelfoam (Pfizer, New York, NY), and thus patients did not have to return to have sutures removed. The objective of all steps of the process was to reduce obstacles to acquiring a skin biopsy. All primary care clinicians at the 2 sites were informed that the skin biopsy service was intended for patients for whom the clinician suspected KS. Because the service was not backed by trained local dermatopathologists, the clinicians were also told that the service was not intended to obtain pathologic diagnoses for patients for whom clinicians were strongly entertaining diagnoses other than KS. In general, the biopsy practitioners were instructed to perform a biopsy in all cases in which they believed KS was a possible diagnosis and a biopsy could be safely obtained. In practice, this meant that they were asked to provide a technical service for all requesting clinicians by performing a biopsy unless, in their view, KS was unlikely and a biopsy would only place the patient at needless risk. We evaluated the histopathologic outcomes in a consecutive series of patients referred for a skin biopsy at each site from October 2008 to January 2013. Institutional review boards at each site and at the University of California, San Francisco (UCSF) and Indiana University gave approval.
Measurements
Clinical Suspicion of KS
Patients were initially assessed by local primary care providers during the course of routine clinical care and referred for biopsy when there was clinical suspicion of KS. The level of medical training among providers was variable, reflecting the heterogeneity in the local provider workforce, and ranged from clinical officers (equivalent to the US nurse practitioners) to physicians. Although the exact number of providers referring patients for biopsy was not recorded, it is estimated, after accounting for staff turnover, to be over 50 across the 2 health care systems.
African Pathology Interpretation
At AMPATH, formalin-fixed biopsy samples were submitted to the Department of Pathology at Moi University, where they were interpreted by 1 of 5 local pathologists as part of routine clinical care. Each of the pathologists has general duties in surgical and forensic pathology, and none has specialized training in dermatopathology. Pathologic evaluation was restricted to observation of microscopic features on tissue slices through routine hematoxylin and eosin (H&E) staining. Beginning in the second quarter of 2012, the laboratory began to test the use of immunohistochemical staining for the latency-associated nuclear antigen-1 (LANA-1) of KSHV. Subsequently, use of the LANA-1 stain was adopted variably by the different pathologists. Because of inconsistent staffing at the Ugandan site, there was not pathologic interpretation there, and, instead, formalin-fixed samples were sent to the Dermatopathology Service at UCSF.
US-Based Dermatopathology Interpretation
The Dermatopathology Service at UCSF is a tertiary referral center consisting of 9 board-certified dermatopathologists with extensive expertise in HIV-related dermatopathology; its annual volume of skin specimens is among the highest in the Western U.S. Many faculty members have been practicing since the onset of the HIV/AIDS epidemic and were the first to describe some of the clinical mimickers of KS.14–16 As noted, Ugandan formalin-fixed samples were directly shipped to the UCSF Dermatopathology Service. From AMPATH, tissue blocks and slides were shipped to UCSF after local African pathologist reading. The UCSF dermatopathologists evaluated the African-derived specimens in line with US specimens, after routine practice protocols. They could, at their discretion, recut and restain (H&E) specimens or stain with monoclonal antibodies for the LANA-1 protein of KSHV (Vector Laboratories, Burlingame, CA) or other immunohistochemical stains. UCSF dermatopathologists were masked to the interpretations of the Kenyan pathologists, and the results from the UCSF dermatopathologists were provided back to Kenya approximately 2 months after receipt. The only communication between the UCSF and Kenyan pathologists was in the form of the returned biopsy interpretations; there was no real-time or case-specific discussion.
Statistical Analysis
Data were analyzed through contingency tables with resultant proportions and 95% confidence intervals (CIs) (SAS version 9.4; SAS Institute, Cary, NC). The accuracy of the African clinicians' visual suspicion of KS was evaluated by estimating its positive predictive value. Using the US-based dermatopathologic interpretation as the gold standard for the purposes of this analysis, the positive predictive value was defined as the percentage of all biopsies that were interpreted as having KS present by the US dermatopathologists of the total that were given a definitive KS present or KS absent interpretation by the US dermatopathologists; ie, all indeterminate US-based interpretations were excluded from this analysis. We compared the African pathology interpretation and the US-based interpretation in terms of overall concordance and sensitivity and specificity. For determination of sensitivity and specificity, we used the US-based interpretation as the gold standard given their pathologists' specialized training and experience in dermatopathology, ability to recut specimens and use specialized immunohistochemical stains, and ready access to other faculty consultation. The objective was to compare the performance of the African pathologists and the US-based pathologists, both as implemented during routine clinical care and not in the artificial setting of research.
RESULTS
A total of 1152 unique HIV-infected adult patients at 2 sites in Uganda and Kenya had skin biopsies performed because of clinical suspicion of KS and had pathologic interpretation by US-based dermatopathologists (Table 1). Of these 1152, 897 of the biopsies also had interpretation by African-based pathologists. A total of 46 (4.0% overall; 4.6% in Kenya and 1.6% in Uganda) biopsies were deemed indeterminate by the US-based dermatopathologists. Reasons included problems with tissue block or slide quality [eg, crush and desiccation artifact, errors in sectioning, fixation, or embedding that could not be resolved by resectioning of the tissue block (33%)], insufficient tissue quantity (eg, no dermis present) to definitively rule out KS (46%), and scenarios in which the US-based dermatopathologist was suspicious of KS but the KSHV-specific anti-LANA-1 immunostain was negative and an alternate specific diagnosis could not be rendered (22%).
TABLE 1.
US-Based Dermatopathologic Interpretations of Skin Biopsies Performed on HIV-Infected Adult Patients Clinically Suspected to Have KS in East Africa

Positive Predictive Value of Clinical Suspicion of KS in Uganda and Kenya
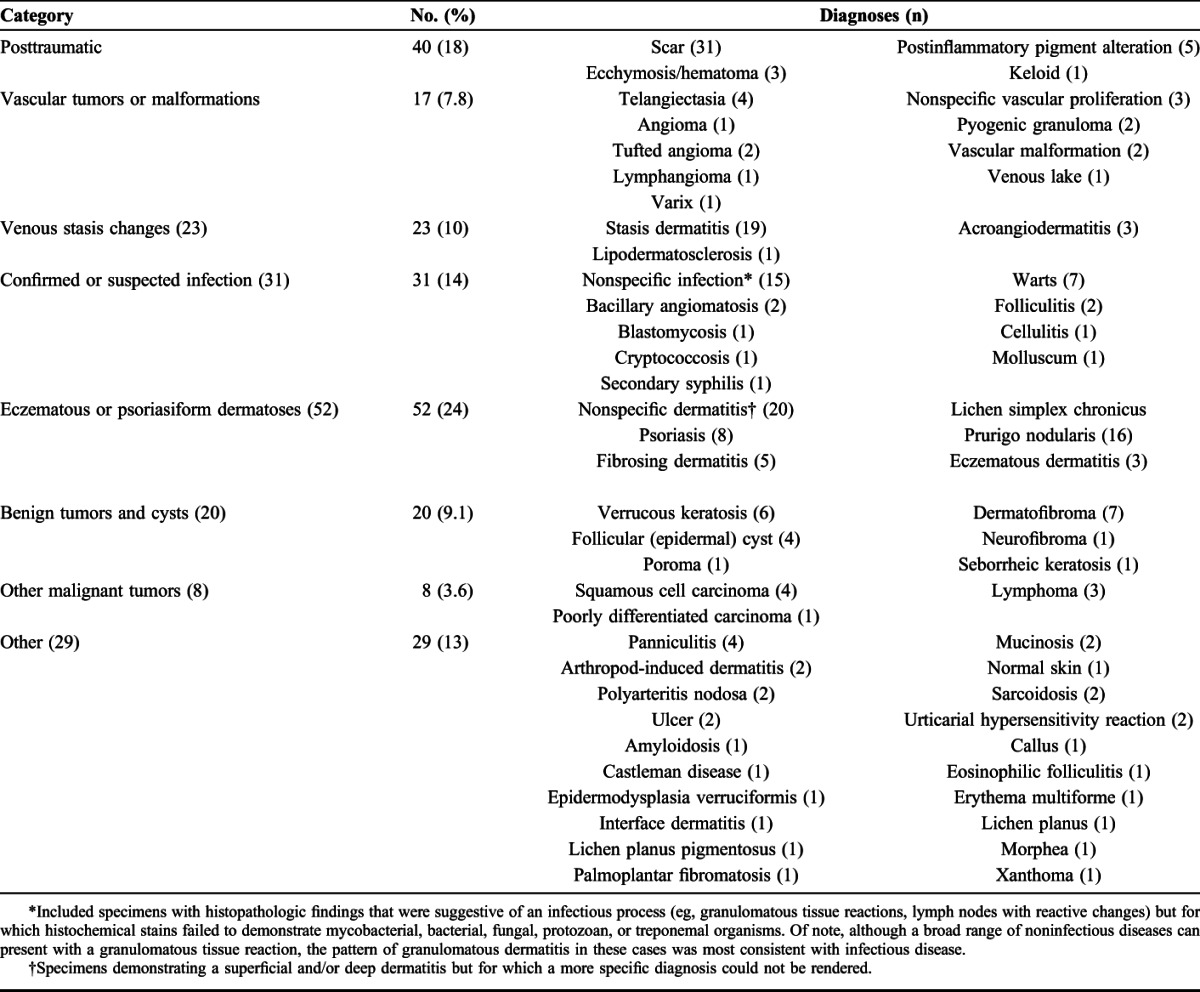
Among the 1106 biopsies for which a definitive diagnosis could be made by the US-based dermatopathologists, 77% (95% CI: 74% to 79%) were interpreted as KS, which corresponds to the positive predictive value of clinical suspicion for KS. There was no significant difference between countries (76% in Kenya and 80% in Uganda; P = 0.22) or over time (P = 0.60). When KS was not found, a wide variety of other diagnoses were made by the US-based dermatopathologists (Table 2). Overall, 35% (N = 77) of alternate diagnoses were clinically banal conditions (eg, wart and dermatofibroma), and 6% (N = 13) were acute or life-threatening conditions (eg, bacillary angiomatosis, lymphoma, squamous cell carcinoma, and cryptococcosis). The remaining 59% (N = 130) were medically significant (eg, psoriasis and sarcoidosis), meriting management that is different than for KS.
TABLE 2.
Pathologic Diagnoses Made by US-Based Dermatopathologists When KS Was Not Present (N = 220)
Accuracy of African Pathologic Diagnosis
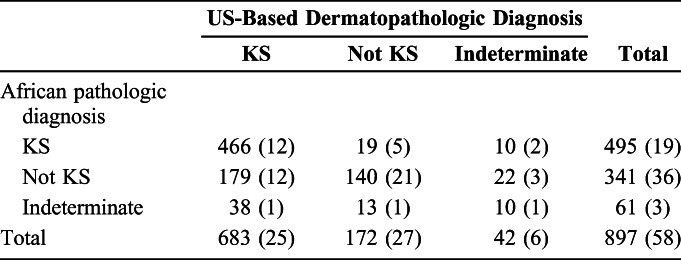
Overall concordance between the interpretations of the East African-based pathologists and US-based dermatopathologists was 69% (95% CI: 66% to 72%) (Table 3). Among the 281 interpretations which were discordant, in 217 (77%) the US interpretation was KS, whereas the African read was not KS or indeterminate. Anti-LANA-1 immunostaining was performed by the US-based dermatopathologists on 13 (6.0%) of these 217 specimens and all were positive. Among 32 (11%) discordant results, the US read was not KS, whereas the African read was KS or indeterminate. LANA-1 staining was performed by the US-based dermatopathologists on 6 (19%) of these 32 specimens, all of which were negative. For the remaining 32 (11%) discordant results, the US read was indeterminate, whereas the African read was KS or not KS. LANA-1 staining was performed by the US-based dermatopathologists on 5 (16%) of these specimens and was nonreactive for 2 (40%) and indeterminate for the remaining 3 (60%). Using US-based dermatopathologist interpretation as the gold standard, the sensitivity of the African pathologic diagnosis for KS was 68% (95% CI: 65% to 72%) and the specificity was 89% (95% CI: 83% to 93%). There was no significant difference in the sensitivity (P = 0.61) or specificity (P = 0.058) of African pathologic diagnoses over time.
TABLE 3.
Comparison Between African and US-Based Pathologic Interpretations of Skin Biopsies Performed on HIV-Infected Adult Patients Clinically Suspected to Have KS in East Africa. Number With US-Based Anti-LANA-1 Staining Performed is Shown in Parentheses
DISCUSSION
Despite the clinical relevance of KS in sub-Saharan Africa, little is known about the accuracy of current diagnostic practices for this cancer in the region. Using consecutive skin biopsies performed for clinical suspicion of KS in large clinic networks spanning 2 countries in East Africa, we examined the accuracy of clinical suspicion and local pathologic diagnosis of KS using US-based dermatopathology as the gold standard reference. We found only moderate (77%) positive predictive value of clinical suspicion of KS and modest (69%) concordance between African and US-based pathologists.
Our findings are consistent with previous studies of KS and other malignancies in Africa. In a large study (n = 321 cases of clinically suspected KS) from the more resource-replete South Africa, the positive predictive value of clinical diagnosis of KS (67%) was similar to our estimate.17 Methodological comparability to our study is unclear, however, because the South African work used locally performed LANA-1 staining (on all specimens) instead of external expert histodermatopathologic interpretation as the gold standard. LANA-1 staining, depending upon the ambient infrastructure and experience of the operators, may or may not perform as well as expert histodermatopathologic interpretation. In a smaller study (n = 23) of biopsies originating from 7 different African countries in which, similar to our work, US-based dermatopathology was used as the gold standard, the positive predictive value of clinical diagnosis for KS (57%) was lower.18 In another class of malignancies—lymphomas—which, similar to KS, also often have externally overt manifestations, the accuracy of clinical diagnosis has been equally as poor as our findings. In 2 different studies of childhood non-Hodgkin lymphoma in Uganda, the positive predictive value of a clinical diagnosis was 75%.19,20 Estimates of the accuracy of local African pathologic diagnoses of other malignancies have been more variable. Depending on the exact cancer in question, the reported sensitivity of local African pathology compared with an external gold standard has ranged from 36% to 98% and specificity from 28% to 94%.20,21
Our study has several limitations. In the realm of clinical diagnosis, it is conceivable that clinicians were correct about their suspicion of KS but, because of sampling error, biopsied the wrong lesion. We do not know how frequently this may have occurred, but it is more plausible when the final pathologic interpretation was a “normal” condition (eg, scar) than a disease condition. In addition, 4% of samples were deemed indeterminate by the US-based dermatopathologists, often because of poor quality or insufficient size of specimens. If all, or a large fraction, of these specimens were indeed KS, the true-positive predictive value of clinical suspicion of KS would have been higher. Regarding the pathologic comparison, to calculate sensitivity and specificity of the African pathologic interpretation, we considered the US-based dermatopathologic interpretations as the gold standard, but this is a distinction that could be contended. Our argument for this claim is based on the extensive dedicated training in dermatopathology that the US-based practitioners have undertaken, their greater resources (which translates to better equipment and reagents, such as LANA-1 staining), and the broad consultation they have available (with access to many other faculty) for difficult cases. Yet, it is best to think of the US-based dermatopathologic interpretations that we had available as one operational definition of a gold standard but not necessarily the true and only gold standard. Establishing a true gold standard in what is inherently a subjective measurement would be very difficult. It would require multiple interpretations by several very experienced readers armed with all of the best technology and then some policy as to how to resolve anything short of consensus. We believed this was beyond the scope of what we could accomplish over a reasonable timeline. Finally, although our work spanned 2 large HIV care systems, our findings are inherently operator-dependent and hence may not generalize throughout the region.
In terms of clinical practice, the finding of a 77% positive predictive value of clinical diagnosis of KS suggests that reliance on visual impression alone should be abandoned. From a dermatologic perspective, this is not surprising given the many morphologic variants of KS and the numerous conditions that mimic these variants. In other words, there is very little morphologically that is highly specific for KS. When KS was not present, alternate histopathologic diagnoses ranged from minor ailments, such as warts, to diseases with high acuity, such as lymphoma. The implications of these missed diagnoses are self-evident. For minor ailments, an incorrect diagnosis of KS may lead to the administration of unnecessary chemotherapy; for serious conditions, an incorrect KS diagnosis may lead to life-threatening failure to provide correct therapy. Of note, we did not estimate the negative predictive value of clinical suspicion, that is, how often a lesion that is not believed to be KS on clinical grounds (and hence not biopsied) is truly not KS. However, we speculate that this, too, may be suboptimal because the nonspecific nature of KS makes it difficult to be sure that a variety of hyperpigmented lesions of indeterminate duration are not KS. These points clearly explain why biopsy is the gold standard for KS diagnosis in resource-rich settings.
The modest concordance in the histopathologic diagnosis of KS between African and US-based pathologists is likewise not surprising, given the complexity inherent in KS diagnosis. The African interpretations were more specific than sensitive, meaning they were more likely to “miss” KS than to incorrectly label a specimen as KS. This may reflect the lack of familiarity with the more subtle or atypical histopathologic presentations of KS. For example, characteristic attributes of KS may not be fully developed or may not be recognized in early lesions (eg, patch stage or early plaque KS), when the density of cellularity is low or when obscuring inflammation is present. Additionally, unfamiliarity with atypical histomorphologic variants of KS, including lymphangiomatous, pseudogranulomatous, lobular, or “tufted” configurations, among others, may result in a false-negative interpretation.22 Conversely, less frequent false-positive interpretations may occur in the setting of other dermal spindle-cell or vascular processes, such as dermatofibroma or bacillary angiomatosis, which are in both the clinical and histopathologic differential diagnosis of KS. In cases when the diagnosis of KS is not obvious on routine H&E staining, the use of immunohistochemistry, primarily LANA-1 staining, constitutes an important diagnostic adjunct not available to most African pathologists. Interestingly, the UCSF pathologists used LANA-1 staining as a diagnostic aid in just 8.5% of discordant cases, suggesting they were usually able to render a diagnosis on H&E staining alone. These data, along with the imperfect sensitivity of LANA-1 staining for the diagnosis of KS,23,24 imply that immunohistochemical staining ought not be considered a substitute for expertise and training in dermatopathology but, rather, one piece of an integrated approach to augmenting pathology services.
Solutions to improving the diagnosis of KS will require additional resources, but these need not be insurmountable. In terms of performance of biopsy, we have recently shown how task shifting of tissue procurement from surgeons/physicians to low- or mid-level providers is feasible.13 Alternatively, teledermatology approaches could be considered to, at a minimum, decide on visual grounds which patients do not require biopsy.18 Regarding pathology, given the paucity of trained pathologists (estimated to be, at best, one-tenth the capacity of resource-rich nations10), an overall increased investment would be optimal. However this investment is a long-term goal, a more attainable short-term goal is the training and placement of a few well-trained dermatopathologists in select regional pathology centers of excellence across sub-Saharan Africa in combination with an efficient system to transport specimens to these centers.9,25 Indeed, regional air travel is sophisticated in sub-Saharan African and could, with political will, be the backbone of a fast and inexpensive system of transport. A cold chain is not required to transport specimens, and dermatopathology results are rarely needed emergently, making this idea feasible. In terms of what to specifically focus on when training African pathologists in the nuances of KS-related dermatopathology, the deficiencies we found in sensitivity (as opposed to specificity) point toward the need to use approaches that emphasize the detailed detection of the pathologic features that indicate the presence of KS. Expanded availability of LANA-1 staining might help in this regard, but performance and interpretation of immunohistochemical staining is not trivial; it requires training, experience, and resources to produce and consistently maintain valid results. Also, reliance on LANA-1 staining only pertains to the diagnosis of KS and does not help to enhance the diagnosis of other skin conditions. Instead of placing LANA-1 stains in all pathology laboratories in sub-Saharan Africa, we would rather first see the creation of regional centers of excellence in pathology, which could manage the entire spectrum of dermatopathologic conditions. Finally, newer technologies, such as telepathology, may also play a role.21,26
In summary, our data suggest that the accuracy of clinical and histopathologic diagnosis of KS in East Africa is suboptimal. The findings call for immediate action in several realms: clinical-only diagnosis of KS must give way to biopsy and dermatopathologic interpretation must improve. The findings are also important harbingers for other cancers. As the overall proportion of the disease burden attributable to cancer in sub-Saharan Africa is projected to rise by as much as 85% by 2030,27 investment in a better equipped workforce to perform biopsies and a better pathology infrastructure to interpret these specimens will only become more critical.
ACKNOWLEDGMENTS
The authors thank Michael Kanyesigye, Christine Ngabirano, Elyne Rotich, and all of the clinical and histopathology laboratory staff at the ISS-Uganda and AMPATH-Kenya facilities.
Footnotes
Supported by National Institutes of Health (NIH) grants U01 AI069911, R01 CA119903, D43 CA 153717, U54 CA190153, and the G. D. Hsuing, PhD Student Research Fellowship at Yale University School of Medicine.
Presented in part at the 13th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies, November 7–8, 2011, Bethesda, MD.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No.11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr. Accessed April 30, 2015. [Google Scholar]
- 3.Asiimwe SB, Laker-Oketta M, Bennett JP, et al. Impact of Kaposi's sarcoma on survival in HIV-infected African adults on antiretroviral therapy. Paper presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston, MA.
- 4.Maskew M, Fox MP, van Cutsem G, et al. Treatment response and mortality among patients starting antiretroviral therapy with and without Kaposi sarcoma: a cohort study. PLoS One. 2013;8:e64392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makombe SD, Harries AD, Yu JK, et al. Outcomes of patients with Kaposi's sarcoma who start antiretroviral therapy under routine programme conditions in Malawi. Trop Doct. 2008;38:5–7. [DOI] [PubMed] [Google Scholar]
- 6.Chu KM, Mahlangeni G, Swannet S, et al. AIDS-associated Kaposi's sarcoma is linked to advanced disease and high mortality in a primary care HIV programme in South Africa. J Int AIDS Soc. 2010;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banda LT, Parkin DM, Dzamalala CP, et al. Cancer incidence in Blantyre, Malawi 1994-1998. Trop Med Int Health. 2001;6:296–304. [DOI] [PubMed] [Google Scholar]
- 8.Tsang MW, Kovarik CL. Global access to dermatopathology services: physician survey of availability and needs in sub-Saharan Africa. J Am Acad Dermatol. 2010;63:346–348. [DOI] [PubMed] [Google Scholar]
- 9.Kaschula RO. The practice of pathology in Africa. Arch Pathol Lab Med. 2013;137:752–755. [DOI] [PubMed] [Google Scholar]
- 10.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14:e152–e157. [DOI] [PubMed] [Google Scholar]
- 11.International Epidemiologic Databases to Evaluate AIDS-East Africa [Internet]. Available at: https://www.iedea-ea.org. Accessed April 30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Ekouevi DK, Williams C, et al. Cohort profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laker-Oketta M, Wenger M, Semeere A, et al. Task shifting and skin punch for the histologic diagnosis of Kaposi's sarcoma in sub-Saharan Africa: a public health solution to a public health problem. Oncology. 2015;89:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger TG, Tappero JW, Kaymen A, et al. Bacillary (epithelioid) angiomatosis and concurrent Kaposi's sarcoma in acquired immunodeficiency syndrome. Arch Dermatol. 1989;125:1543–1547. [PubMed] [Google Scholar]
- 15.LeBoit P, Egbert B, Stoler M, et al. Epithelioid haemangioma-like vascular proliferation in AIDS: manifestation of cat scratch disease bacillus infection? Lancet. 1988;331:960–963. [DOI] [PubMed] [Google Scholar]
- 16.LeBoit P. Proliferations of spindled cells. Am J Dermatopathol. 2001;23:158–159. [DOI] [PubMed] [Google Scholar]
- 17.Van Bogaert LJ. Clinicopathological proficiency in the diagnosis of Kaposi's sarcoma. ISRN AIDS. 2012;2012:e565463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsang MW, Kovarik CL. The role of dermatopathology in conjunction with teledermatology in resource-limited settings: lessons from the African teledermatology project. Int J Dermatol. 2011;50:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogwang MD, Zhao W, Ayers LW, et al. Accuracy of burkitt lymphoma diagnosis in constrained pathology settings: importance to epidemiology. Arch Pathol Lab Med. 2011;135:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orem J, Sandin S, Weibull CE, et al. Agreement between diagnoses of childhood lymphoma assigned in Uganda and by an international reference laboratory. Clin Epidemiol. 2012;4:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wamala D, Katamba A, Dworak O. Feasibility and diagnostic accuracy of internet-based dynamic telepathology between Uganda and Germany. J Telemed Telecare. 2011;17:222–225. [DOI] [PubMed] [Google Scholar]
- 22.Ramdial PK. Dermatopathological challenges in the human immunodeficiency virus and acquired immunodeficiency syndrome era. Histopathology. 2010;56:39–56. [DOI] [PubMed] [Google Scholar]
- 23.Hong A, Davies S, Lee CS. Immunohistochemical detection of human herpesvirus 8 (HHV8) latent nuclear antigen-1 in Kaposi's sarcoma. Pathology. 2003;35:448–450. [DOI] [PubMed] [Google Scholar]
- 24.Hammock L, Reinsauer A, Wang W, et al. Latency-associated nuclear antigen expression and human herpesvirus-8 polymerase chain reaction in the evaluation of Kaposi sarcoma and other vascular tumors in HIV-positive patients. Mod Pathol. 2005;18:463–468. [DOI] [PubMed] [Google Scholar]
- 25.Vento S. Cancer control in Africa: which priorities? Lancet Oncol. 2013;14:277–279. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein RS, Graham AR, Lian F, et al. Reconciliation of diverse telepathology system designs. Historic issues and implications for emerging markets and new applications. APMIS. 2012;120:256–275. [DOI] [PubMed] [Google Scholar]
- 27.Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13:790–801. [DOI] [PubMed] [Google Scholar]


