Supplemental Digital Content is Available in the Text.
Key Words: HIV, hepatitis B, hepatitis C, people who inject drugs, behaviors, image and performance enhancing drugs
Abstract
Background:
Infection risks among people who inject drugs (PWID) are widely recognized, but few studies have focused on image and performance enhancing drugs (IPEDs). Globally, concern about IPED injection has increased and, in the United Kingdom, IPED injectors have become the largest group using Needle and Syringe Programmes. Blood-borne virus prevalence trends among IPED injectors are explored.
Method:
Data from 2 surveys of IPED injectors (2010–2011; 2012–2013) and the national bio-behavioral surveillance system for PWID (1992–1997; 1998–2003; 2004–2009) were merged. Psychoactive drug injectors and women were excluded. Logistic regression analyses explored temporal changes.
Results:
Between 1992 and 2009, median age increased from 25 to 29 years (N = 1296), years injecting from 2 to 4. There were 53 men who had sex with men (MSM). Overall, 0.93% had HIV, 4.4% ever had hepatitis B (HBV), and 3.9% hepatitis C (HCV, from 1998, N = 1083). In multivariable analyses, HIV increased in 2004–2009 [adjusted odds ratio (AOR) = 10 (95% confidence interval (CI): 0.94 to 106) vs. 1992–2003], and remained elevated (AOR = 4.12, 95% CI: 0.31 to 54, 2012–2013); HBV also increased in 2004–2009 (AOR = 3.98, 95% CI: 1.59 to 9.97). HCV prevalence increase was only borderline significant (AOR = 2.47, 95% CI: 0.90 to 6.77, 2010–2011). HIV and HBV were associated with MSM and HCV with sharing needles/syringes. Uptake of diagnostic testing for HIV and HCV, and HBV vaccination increased (to 43%, 32% and 44% respectively). Condom use was consistently poor; needle/syringe sharing occurred.
Conclusion:
Blood-borne virus prevalences among IPED injectors have increased and for HIV, is now similar to that among psychoactive drug injectors. Targeted interventions to reduce risks are indicated.
INTRODUCTION
It is widely recognized that people who inject drugs (PWID) are vulnerable to blood-borne viral (BBV) infections. However, whilst BBVs have been extensively studied among individuals who inject psychoactive drugs (such as opiates and stimulants),1–3 few studies have focused on those who inject image and performance enhancing drugs (IPEDs).4–7 A wide range of illicit drugs can be injected with the aim of altering body image and/or performance. These drugs range from human growth hormone,8 a range of peptide hormones,9,10 the most commonly injected IPED anabolic androgenic steroids,3,11 to tanning drugs, such as “Melanotan II.”12
Globally, there is increasing concern about the extent and public health consequences of IPED use,4,10,13 and recently a number of studies have raised concerns about infections, including HIV, among those who inject IPEDs.6,7 In particular, there has been concern that HIV and hepatitis B virus (HBV) infections might have increased over time among this group in the United Kingdom (UK).7 This has occurred during a time when the number of people who inject IPEDs in contact with needle and syringe programmes (NSPs) has grown in the UK14 and Australia.15
Infection risks for BBVs among IPED injectors are, for a number of reasons, likely to be different to those among people injecting psychoactive drugs. Firstly, users' behaviors, and therefore risks of infection, can be impacted by the effects of psychoactive drugs, with the use of these leading to disinhibition and compulsive usage,16 although there is increasing evidence to support some levels of dependence amongst IPED users and for these drugs having hedonic effects.17–19 Secondly, there are differences in injecting practice, as IPEDs are injected only subcutaneously or intramuscularly and usually require much less preparation than psychoactive drugs.3,7,14 Finally, IPEDs are typically injected less frequently than psychoactive drugs and their use can be cyclical.4,7,14 These differences have been thought to place those who inject IPEDs at a much lower risk of injection-related infections than those who inject psychoactive drugs.4,14 However, studies suggest that those using IPEDs may have greater sexual risks, as they commonly report risky sexual behaviors4,7,20,21 and low levels of condom use.7,20,22
In response to the increasing concerns about IPED use and the possible increase in the prevalence of BBVs among this population, data from a number of related sero-behavioral surveys were used to examine temporal changes in the UK. The aim of this study was to describe changes between 1992 and 2012 in (1) injecting risk; (2) sexual behaviors; and (3) BBV prevalence among people injecting IPEDs.
METHODS
Data from cross-sectional sero-behavioral studies were extracted and analyzed.
Surveys
In England and Wales, PWID have been recruited into a voluntary unlinked-anonymous monitoring (UAM) survey since 1990; methodological details of which have been published previously.23,24 Briefly, agencies providing services to PWID (eg NSPs and addiction services) at sentinel locations invite clients who have ever injected drugs to participate. Sentinel sites are selected so as to reflect both the geographic distribution and the range of services offered to PWID. Those who consent to participate provide a biological sample and self-complete a brief questionnaire. This survey has multi-site ethics approval.
From 1990 to 2009, this survey had a single questionnaire focused on psychoactive drug use and collected oral fluid samples. During 2010, the biological sample was changed to a dried blood spot (DBS) and the questionnaire was reviewed. At the same time a UAM subsurvey focused on IPED use was implemented, in response to the increased concerns about risk in this group.14 The initial IPED subsurvey during 2010–2011 collected oral fluid samples and used a modified questionnaire focused on IPED use.7 After this initial subsurvey, a routine biennial IPED subsurvey was established using a similar questionnaire, but collecting DBS samples. The first wave of this routine survey was undertaken during 2012–2013.25
The samples collected in the surveys were tested for antibodies to HIV (anti-HIV), hepatitis B core antigen (anti-HBc), and hepatitis C virus (anti-HCV), using previously published methods by the same laboratory.7,26,27 Common data items were extracted from the main UAM Survey and the 2 subsurveys for male participants who reported injecting only IPEDs during the preceding year. The main UAM Survey had collected limited data on the drugs used before 1992 and, because of the small number of IPED users recruited into this survey annually, three 6-year time periods (1992–1997, 1998–2003, 2004–2009) were used. When combining data from 6 survey years into a single time period only an individual's first participation during that period was included (self-reported previous participations were used to exclude repeats). Data analyzed were thus for 5 time periods; 3 from the main UAM Survey (1992–1997, 1998–2003, 2004–2009) plus the 2 IPED subsurveys (2010–2011, 2012–2013).
Risk factors from the questionnaire data were analyzed in categorical form for the most part, either based on yes/no responses or predefined categories in the questionnaire, or groups in the case of continuous variables. The latter were chosen to provide roughly equal size groups with a sufficient number in each. Factors of interest included the 5 time periods, region [Southern and Eastern England; the Midlands (England); Northern England; Wales], age (<25, 25–34, 35+ years), injecting duration (<2, 2–5, >5 years), UK born, ever received a used needle/syringe, ever used a NSP, HBV vaccination uptake, number of sexual partners (preceding year; none, 1, 2–9, 10+), condom use (always, sometimes, never), sexuality [men who have sex with men (MSM)], and ever tested for HCV or HIV.
Statistical Methods
Changes in risk factors over time were assessed through χ2 tests with survey period for categorical variables; and for some continuous variables, the association with time was measured through Pearson's correlation coefficient. Risk factors for BBV infection (HCV, HBV, and HIV) were analyzed through logistic regression. Tests for HCV and HBV had reduced sensitivity before the introduction of DBS; we used a routine based on the expectation-maximization algorithm to account for imperfect sensitivity and specificity28 implemented through the Stata command logitem. Although we examined the relationship between past diagnostic testing for HIV/HCV and HBV vaccination with infection status, these variables were not included in multivariable analysis, as there is not a causal relationship between previous testing and infection status, nor for HBV vaccination and HCV/HIV status; ie, these variables may show an association due to higher uptake in high-risk individuals, but the test/vaccine does not in itself change the risk of infection.
As there was a substantial proportion of missing data for some variables, a multiple imputation approach was employed in the primary analysis. Missing data can lead to a loss of power, but more significantly, informatively missing data may lead to biased results in complete case analyses, where only observations with no missing data are analyzed.29 We used the approach of chained equations, which produces multiple datasets of predicted values based on the relationships between variables, and combines the results to account for uncertainty in predictions according to Rubin's rules30 (see Appendix A, Supplemental Digital Content, http://links.lww.com/QAI/A747).
Due to the small number of observed infections, it was desirable to find a parsimonious model to describe the data. As the number of potential covariates was not large, a complete search of all possible models was undertaken for each infection, and the Akaike31 information criteria used for comparisons. Time-period was included in all models, as trends over time are of principal interest.
RESULTS
Descriptive Statistics
In total, 1296 participations were included in the analyses, with 611 (47%) from 2010 onwards and the fewest during 2004–2009 (129, 10%). Figure 1 and Table 1 show distributions of participant characteristics over time. The age and injecting duration of those sampled increased (P < 0.001 for both), with a median injecting duration of 2 years (IQR 0–4) in 1992–1997 and 4 years (IQR 1–10) in 2012–2013; and a median age of 25 years (IQR 22–29) in 1992–1997 and 29 years (IQR 23–35) in 2012–2013. Condom use showed no significant change over time (P = 0.355); overall only 20.5% reporting always using a condom, with 39.5% sometimes doing so, and 40% never. Significant differences in reported number of sexual partners during the preceding year was observed over time (P = 0.012) although this did not show any overall trend. Overall, 6.8% reported no partners, 42% 1, 42% 2–9 and 9.9% 10 or more.
FIGURE 1.
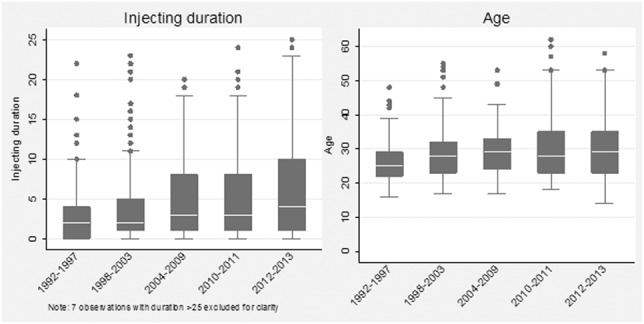
Distribution of reported injecting duration and age (at time of survey) over time, men injecting IPEDs, England and Wales: 1992–2013. Box and whisker plots with medians, 25th and 75th percentiles and whisker defined as 1.5 times the interquartile range.
TABLE 1.
Characteristics of Participations by Men Injecting IPEDs Over Time, England and Wales: 1992–2013
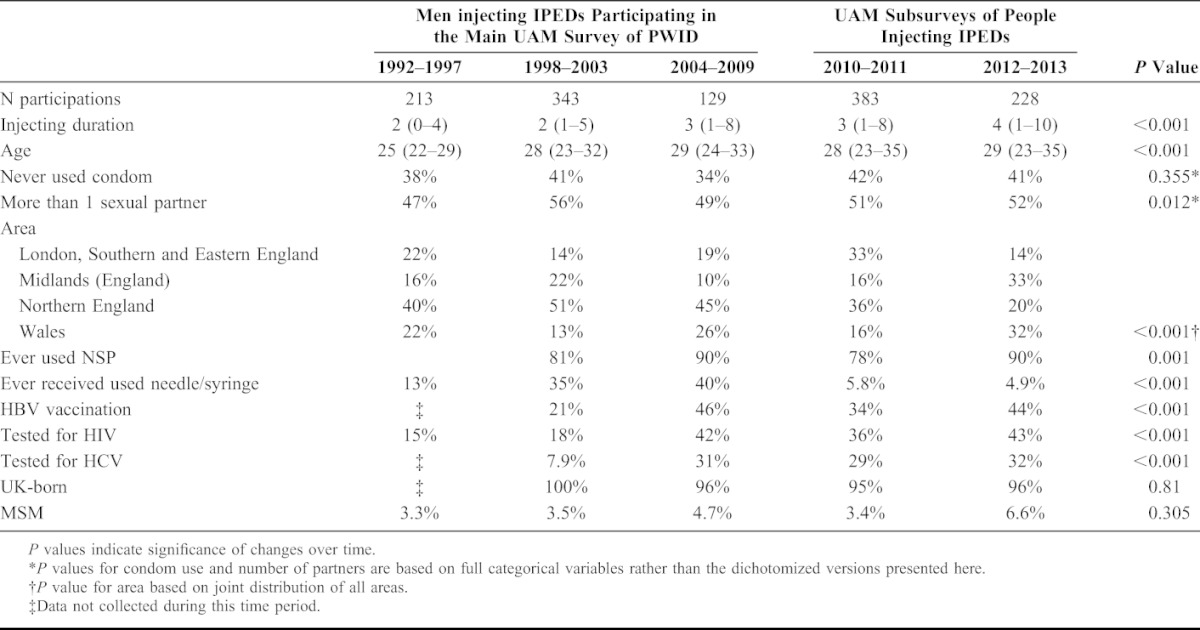
Other behavioral and demographic variables showed some changes over time; ever used NSP varied between 78% and 90%. Reporting ever receiving used needles/syringes increased from 13% in 1992–1997 to 34.5% in 1998–2003 and 40% in 2004–2009 before dropping to less than 6% from 2010; this drop might be related to the move to the IPED focused questionnaire in 2010 even though questions were similar. Vaccination for HBV and testing for HCV and HIV increased between 1998 and 2003 and 2004 and 2009, and stayed roughly stable thereafter. The proportion born in the UK and MSM have both remained stable over time, with 712/746 (95%) UK-born overall (data collected from 2004 only) and 53 (4.1%) MSM.
In total 42/1083 (3.9%) participations tested positive for anti-HCV; 57/1296 (4.4%) for anti-HBc; and 12/1296 (0.93%) for anti-HIV. Figure 2 shows prevalence of these 3 infections over time. Prevalence rose sharply for all 3 infections in the 2004–2009 period, but fell again in 2010–2011 and 2012–2013.
FIGURE 2.
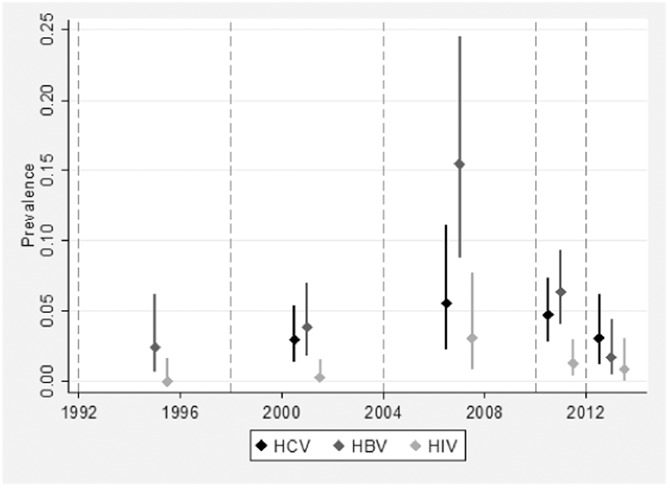
Observed prevalence and 95% confidence intervals of anti-HCV, anti-HBc, and anti-HIV prevalence over time, accounting for imperfect test sensitivity and specificity, men injecting IPEDs, England and Wales: 1992–2013. No data are available for anti-HCV before 1998 when testing was first introduced into the survey.
Patterns of Missing Data
Injecting duration had the largest amount of missing data (mainly due to age first injected being missing); most of the other variables contained relatively small amounts (<5%) of missing data and a number of data items (eg UK born) were not collected in earlier survey years and therefore were missing for these (see Appendix A, Supplemental Digital Content, http://links.lww.com/QAI/A747). Crucially, those missing injecting duration were more likely to have positive test results for HCV and HIV (P = 0.077 and 0.153 respectively); and this difference increased over time, with those missing injecting duration having prevalences 2–3 times as high in 2012–2013 for all 3 infections. This indicates a risk of bias in complete case analysis of trends.
Model Selection
The model selection phase, based on the imputed data, did not yield a clear choice in model structure in terms of a single best-scoring model, although some variables could be excluded with relative certainty (where variables did not appear in any of the best-scoring models). For HCV and HBV, number of partners and condom use did not feature in any of the best-scoring models, and being born in the UK featured infrequently; these variables were therefore omitted and all other variables were retained. This resulted in some “redundant” variables, but allowed a more direct comparison between the results for HCV and HBV. For HIV, results were even more uncertain, with MSM appearing in all best scoring models but little certainty as to which other variables should be included; the only firm conclusion was that very few variables could be included without over-fitting to the data. We selected MSM, age, ever received used needles/syringes and condom use for the final model. Detailed tables of the model selection results and Akaike information criteria scores are shown in Appendix B (see Supplemental Digital Content, http://links.lww.com/QAI/A747).
Logistic Regression Results
Table 2 shows results from multivariable models for HCV, HBV and HIV, based on data from the multiple imputation (MI) analysis. In most cases, the univariable complete case, univariable MI and MI multivariable analyses give fairly similar results; we therefore focus on the multivariable MI results, but highlight differences between the analyses where they occur. Full details of the different models are given in Appendix C (see Supplemental Digital Content, http://links.lww.com/QAI/A747).
TABLE 2.
Multivariable Logistic Regression Results for Anti-HCV, Anti-HBc, and Anti-HIV, Based on Multiple Imputation Model Data, Men Injecting IPEDs, England and Wales: 1992–2013
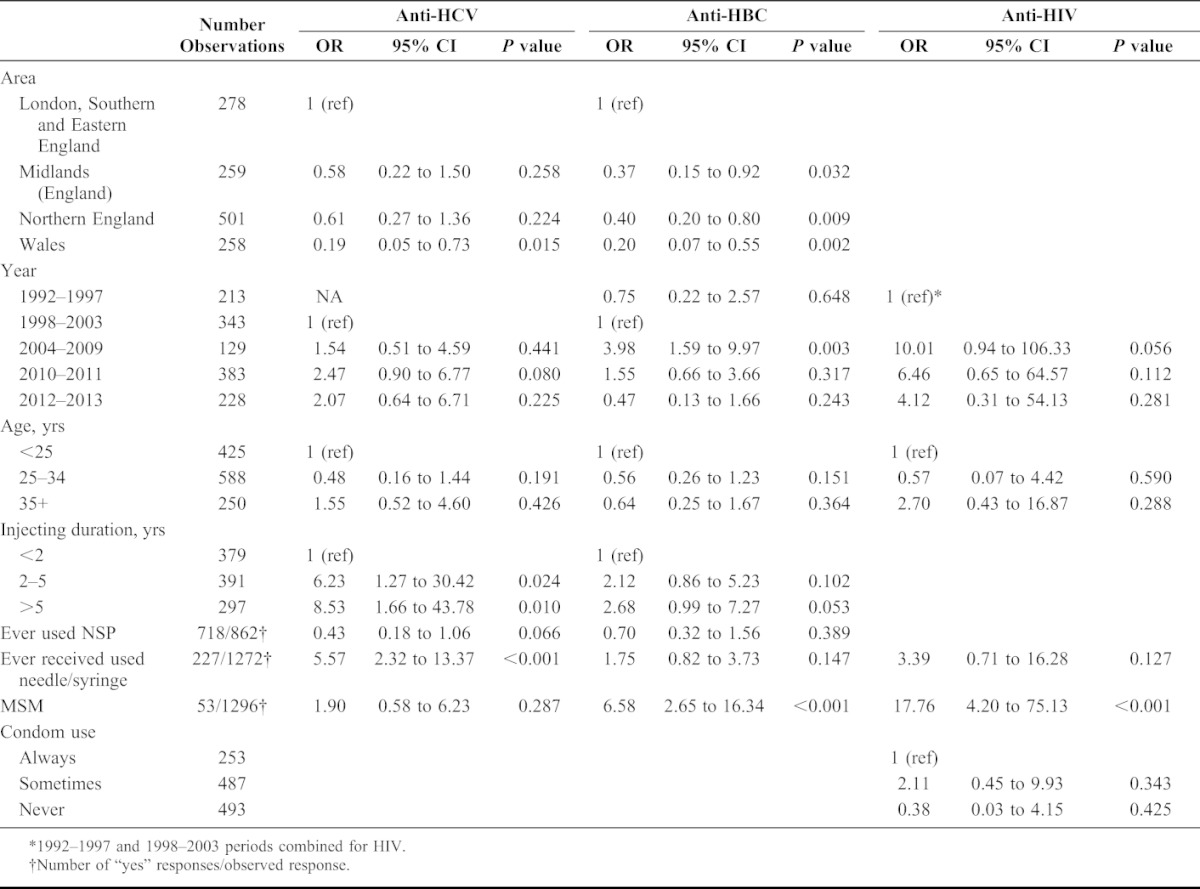
Differences between regions followed a similar pattern for all 3 infections, with Southern and Eastern England having the highest prevalence and Wales the lowest. HCV prevalence increased over time, although was only borderline significant in 2010–2011 with an odds ratio (OR) of 2.47 (95% CI: 0.90 to 6.77) vs. 1999–2003. The pattern is rather different to the unadjusted ORs, which had an increase in 2004–2009 then decreasing subsequently; the difference in the multivariable results is largely due to adjusting for ever receiving used needles/syringes; numbers reporting ever receiving needles/syringes dropped significantly in the last 2 periods, but prevalence of HCV did not fall correspondingly.
There are some similarities in the temporal pattern for HBV, but prevalence increased more markedly in 2004–2009 with an OR of 3.98 (95% CI: 1.59 to 9.97), then decreasing over the next 2 periods. The pattern for HIV was similar, but with an extremely high, albeit uncertain increase in 2004–2009 with an OR of 10.01 (95% CI: 0.94 to 106.3) and falling in the subsequent 2 periods. The ORs were attenuated in the multivariable analyses compared with univariable results, but all results exhibit substantial uncertainty.
For each of the 3 infections, prevalence was similar for those aged 25–34 vs. <25, but significantly higher in the 35+ age group in univariable analysis; however, there was no association after adjusting for injecting duration, which is highly correlated with age. Injecting duration was significantly associated with HCV status, with ORs of 6.23 (95% CI: 1.27 to 30.42) and 8.53 (95% CI: 1.66 to 43.78) for 2–5 years and 5+ vs. <2 years respectively. Interestingly, despite the high risk of bias identified due to missing data, results for the univariable complete case analysis were very similar. The effect of injecting duration on HBV followed a similar pattern, but weaker; and results for HIV were similar to HBV, but highly uncertain. The different patterns in injecting risk for the 3 infections were further borne out by results for ever receiving used needles/syringes, which had a strong effect for HCV with an OR of 5.57 (95% CI: 2.32 to 13.37) and positive, but nonsignificant effect for HBV and HIV. Ever using a NSP was found to be protective for HCV (P = 0.066) but nonsignificant for HBV.
Sexuality was found to be an important risk factor for HBV and HIV, with MSMs having a far higher prevalence, with ORs of 6.58 (95% CI: 2.65 to 16.34) for HBV and 17.76 (95% CI: 4.20 to 75.13) for HIV, and nonsignificant for HCV. Apart from survey period, MSM was the only variable to remain significant in the multivariable analysis of HIV. Reported number of partners and condom use had little association with any infections, even in univariable analyses. We attempted to examine whether the effects of other variables differed according to sexuality, ie through interactions with MSM, but data were too sparse to do so.
Previous diagnostic testing for HCV and HIV showed significant positive associations with all 3 infections; as mentioned previously this must be interpreted as a difference in underlying risk in those seeking tests and not a causal factor; ie testing does not cause an increased risk of infection. HBV vaccination showed little association with HCV and HBV status, but HIV prevalence was somewhat higher in univariable analyses. Finally, being UK born showed no significant associations, although numbers of non-UK born are low, and the effect could not be estimated for HIV, as there were no infections in this group.
DISCUSSION
Our findings confirm that BBV infections are a problem among people who inject IPEDs, though in part this reflects an overlap with the MSM population. Even so, the prevalence of HIV is similar to that among those injecting psychoactive drugs in the UK.32 Though the prevalences of HBV and HCV are lower than among those who inject psychoactive drugs,33,34 they are most probably higher than the levels in the general population.33–35 Worryingly, even though the prevalence of these 3 infections might have peaked in the second half of the 2000's, they are currently still higher than during the 1990's.
This study has a number of limitations. Firstly, the representativeness of those recruited is impossible to measure, as the marginalization, comparative rarity and illicit nature of injecting drug use all impeded the construction of a sampling frame. Although the survey used an established methodology for recruiting PWID23,24; its robustness for people who inject IPEDs is not known and cannot be assessed as information on the nature and size of this group is limited.4,14 Secondly, the findings here rely on self-reports; although the reliability of these has not been assessed among IPED users, they have been found to be reliable for people injecting psychoactive drugs.36,37 The reliability of the sexuality data is a particular concern as this was based on the response to a single question about male sexual partners and therefore the extent of sex with men may be underreported. Responses were occasionally incomplete leading to missing data, particularly in relation to age at first injection. This problem was partly overcome by the use of MI methods, although it is unclear to what degree bias due to systematic missingness may persist; there is no guarantee that the observed relationships between variables in the complete data hold for those with missing data. In general, MI and complete case analyses were in close agreement; although this does not validate the MI approach, it is reassuring that radical differences are not observed. Thirdly, though the infection data were based on the testing of biological samples, analysis was limited by the small numbers, particularly for HIV. Finally, though the data are drawn from the same programme of sero-behavioral surveys there is some methodological variation over time; in particular the first three time points drew on a survey of PWID focused on psychoactive drug use, whilst the 2 more recent time periods relate to purposive surveys of IPED injectors. There may be differences between the risk profiles of those captured in these 2 survey variants, and considering the higher level of sharing, those captured in the general PWID survey might have been a higher risk group. Considering these limitations, caution is needed when attempting to generalize our findings.
The 3 infections examined here share a common route of transmission through injecting, but HBV and HIV are also readily transmitted sexually. The sexual transmission of HCV is rare,38 though this may be more common among some groups of MSM, particularly when infected with HIV. The results here, although uncertain, reflect this risk pattern: the associations of injecting duration and receiving used needles/syringes with HCV infection are stronger than those for HBV and HIV, with the latter presumably being diluted by the sexual transmission route, which might be assumed relatively high in this population.7,14 This also appears to be confirmed by the very strong association of HBV and HIV infection with MSM, which was not observed for HCV. If transmission routes for the 3 infections were identical, one would expect the temporal trends to be similar; indeed, there are some differences, with a large “spike” in prevalence anti-HBc in 2004–2009 followed by a tailing off, which is similar to the pattern of HIV, compared with a relatively flat profile over time for HCV. The differences in transmission routes are also borne out by the correlation between infections; HIV has no evidence of an association with HCV (although data are sparse) with an OR of 2.09 (0.27–16.40) but a strong association with HBV: OR = 14.27 (4.68–46.72). HCV and HBV have an OR of 7.91 (3.75–16.68); in general, all 3 infections are likely to be correlated due to the shared route of injecting, but for HIV and HBV this is expected to be stronger due to these also being sexually transmitted. These theories would be better confirmed by modeling all 3 infections jointly to separate out the effect of sexual transmission, but due to the paucity of data this was not possible to test formally.
The extent of these infections and the indication of an increase in prevalence over time for HIV and HCV are a concern. Particularly, as our data indicate that the uptake of diagnostic testing for HIV and HCV among men injecting IPEDs (42.2% and 32.7%, respectively, in 2012–2013) is much lower than among those injecting psychoactive drugs,33,39 and HBV vaccine uptake (44.5% in 2012–2013) is poor. Considering the injecting and sexual risks in this population, there is a substantial potential for extensive unrecognized spread of BBVs among those injecting IPEDs. Though the relative roles of injecting and sexual risks in the transmission of HIV and HBV need further examination, these findings indicate that those providing services to PWID need be alert to the infection risks among those who inject IPEDs. In particular, they need to be aware that injecting practices associated with IPED use differ from those for psychoactive drugs4,7,14 and of the potentially greater sexual risks.4,7,14 Services working with PWID need to ensure that those injecting IPEDs have access to appropriate injecting equipment, targeted harm reduction advice, testing for BBVs, hepatitis B vaccinations, sexual health services, and condoms.
This study reports on the largest sample so far of people who use IPEDs analyzed in relation to BBV infections and is the first to examine risk over time, however, the results need to be interpreted with caution. Though further work is needed, the findings indicate that both sexual and injection risks lead to BBV transmission among those injecting IPEDs. The extent of IPED use, the characteristics of the users and the drug use profiles of IPED injectors attending NSPs in the UK have all changed over time.14,40 However, the role of these factors in the changing BBV prevalence requires further investigation. These results also demonstrate the need for targeted interventions to address sexual health and drug use risks among those injecting IPEDs.
ACKNOWLEDGMENTS
The authors are grateful to all of the people who took part in the surveys and to the various services across the England and Wales who assisted with their recruitment. They thank the support staff who worked on the surveys and those who undertook the laboratory work.
Footnotes
Supported by Public Health England, and its predecessor bodies, and it is covered by Crown Copyright and made available through the Open Government license. The views expressed here are those of the authors and do not necessarily reflect the views of Public Health England.
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Aceijas C, Stimson GV, Hickman M, et al. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS. 2004;18:2295–2303. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McVeigh J, Evans-Brown M, Bellis MA. Human enhancement drugs and the pursuit of perfection. Adicciones. 2012;24:185–190. [PubMed] [Google Scholar]
- 4.Evans-Brown M, McVeigh J, Perkins C, et al. Human Enhancement Drugs: The Emerging Challenges to Public Health. Liverpool, United Kingdom: North West Public Health Observatory; 2012. ISBN: 978-1-908929-01-3. [Google Scholar]
- 5.Crampin AC, Lamagni TL, Hope VD, et al. The risk of infection with HIV and hepatitis B in individuals who inject steroids in England and Wales. Epidemiol Infect. 1998;121:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day CA, Topp L, Iversen J, et al. Blood-borne virus prevalence and risk among steroid injectors: results from the Australian Needle and Syringe Program Survey. Drug Alcohol Rev. 2008;27:559–561. [DOI] [PubMed] [Google Scholar]
- 7.Hope VD, McVeigh J, Marongiu A, et al. Prevalence of, and risk factors for, HIV, hepatitis B and C infections among men who inject image and performance enhancing drugs: a cross-sectional study. BMJ Open. 2013;3:e003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans-Brown M, McVeigh J. Injecting human growth hormone as a performance-enhancing drug-perspectives from the United Kingdom. J Subst Use. 2009;14:267–288. [Google Scholar]
- 9.Kimergård A, McVeigh J, Knutsson S, et al. Online marketing of synthetic peptide hormones: poor manufacturing, user safety, and challenges to public health. Drug Test Anal. 2014;6:396–398. [DOI] [PubMed] [Google Scholar]
- 10.Stensballe A, McVeigh J, Breindahl T, et al. Synthetic growth hormone releasers detected in seized drugs: new trends in the use of drugs for performance enhancement. Addiction. 2015;110:368–369. [DOI] [PubMed] [Google Scholar]
- 11.Sagoe D, Molde H, Andreassen CS, et al. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014;24:383–398. [DOI] [PubMed] [Google Scholar]
- 12.Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. [DOI] [PubMed] [Google Scholar]
- 13.Brennan BP, Kanayama G, Pope HG., Jr Performance-enhancing drugs on the web: a growing public-health issue. Am J Addict. 2013;22:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Advisory Council on the Misuse of Drugs. Consideration of the Anabolic Steroids. London, United Kingdom: Home Office; 2010. [Google Scholar]
- 15.Iversen J, Topp L, Wand H, et al. Are people who inject performance and image-enhancing drugs an increasing population of Needle and Syringe Program attendees? Drug Alcohol Rev. 2013;32:205–207. [DOI] [PubMed] [Google Scholar]
- 16.Simmonds L, Coomber R. Injecting drug users: a stigmatised and stigmatising population. Int J Drug Policy. 2009;20:121–130. [DOI] [PubMed] [Google Scholar]
- 17.Kanayama G, Brower KJ, Wood RI, et al. Treatment of anabolic-androgenic steroid dependence: emerging evidence and its implications. Drug Alcohol Depend. 2010;109:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pope HG, Jr, Wood RI, Rogol A, et al. Adverse health consequences of performance-enhancing drugs: an endocrine society scientific statement. Endocr Rev. 2014;35:341–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrandt T, Lai JK, Langenbucher JW, et al. The diagnostic dilemma of pathological appearance and performance enhancing drug use. Drug Alcohol Depend. 2011;114:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korkia P, Stimson G. Anabolic Steroid Use in Great Britain: An Exploratory Investigation. Final Report to the Department of Health for England, Scotland and Wales. London, United Kingdom: The Centre for Research on Drugs and Health Behaviour; 1993. [Google Scholar]
- 21.Midgley S, Heather N, Best D, et al. Risk behaviours for HIV and hepatitis infection among anabolic-androgenic steroid users. AIDS Care. 2000;12:163–170. [DOI] [PubMed] [Google Scholar]
- 22.Bolding G, Sherr L, Maguire M, et al. HIV risk behaviours among gay men who use anabolic steroids. Addiction. 1999;94:1829–1835. [DOI] [PubMed] [Google Scholar]
- 23.Noone A, Durante AJ, Brady AR, et al. HIV infection in injecting drug users attending centres in England and Wales, 1990–1991. AIDS. 1993;7:1501–1507. [DOI] [PubMed] [Google Scholar]
- 24.Hope VD, Judd A, Hickman M, et al. HIV prevalence among injecting drug users in England and Wales 1990 to 2003: evidence for increased transmission in recent years. AIDS. 2005;19:1207–1214. [DOI] [PubMed] [Google Scholar]
- 25.Public Health England, Centre for Infectious Disease Surveillance & Control and Microbiology Services. Unlinked Anonymous Monitoring Survey of People Who Inject Drugs in Contact with Specialist Services: Data Tables. People Who Inject Image and Performance Enhancing Drugs. London, United Kingdom: Public Health England; 2014. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/326898/UAM_Survey_data_tables_2014_IPED.pdf. Accessed July 16, 2015. [Google Scholar]
- 26.Connell JA, Parry JV, Mortimer PP, et al. Novel assay for the detection of immunoglobulin G antihuman immunodeficiency virus in untreated saliva and urine. J Med Virol. 1993;41:159–164. [DOI] [PubMed] [Google Scholar]
- 27.Judd A, Parry J, Hickman M, et al. Evaluation of a modified commercial assay in detecting antibody to hepatitis C virus in oral fluids and dried blood spots. J Med Virol. 2003;71:49–55. [DOI] [PubMed] [Google Scholar]
- 28.Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146:195–203. [DOI] [PubMed] [Google Scholar]
- 29.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 31.Akaike H. A new look at the statistical model identification. IEEE T Automat Contr. 1997;19:716–724. [Google Scholar]
- 32.Hope VD, Harris RJ, De Angelis D, et al. Two decades of successes and failures in controlling the transmission of HIV through injecting drug use in England and Wales, 1990 to 2011. Euro Surveill. 2014;19:pii: 20762. [DOI] [PubMed] [Google Scholar]
- 33.Hepatitis C in the UK: 2014 Report. London, United Kingdom: Public Health England; 2014. Available at: https://www.gov.uk/government/publications/hepatitis-c-in-the-uk. Accessed July 16, 2015. [Google Scholar]
- 34.Hope VD, Eramova I, Capurro D, et al. Prevalence and estimation of hepatitis B and C infections in the WHO European region: a review of data focusing on the countries outside the European Union and the European Free Trade Association. Epidemiol Infect. 2014;142:270–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Department of Health. Getting Ahead of the Curve: A Strategy for Combating Infectious Diseases (Including Other Aspects of Health Protection). A Report by the Chief Medical Officer. London, United Kingdom: Department of Health; 2002. [Google Scholar]
- 36.Latkin CA, Vlahov D, Anthony JC. Socially desirable responding and self-reported HIV infection risk behaviors among intravenous drug users. Addiction. 1993;88:517–526. [DOI] [PubMed] [Google Scholar]
- 37.De Irala J, Bigelow C, McCusker J, et al. Reliability of self-reported human immunodeficiency virus risk behaviors in a residential drug treatment population. Am J Epidemiol. 1996;143:725–732.8651235 [Google Scholar]
- 38.Balogun M, Ramsay ME, Parry JV, et al. A national survey of genitourinary medicine clinic attenders provides little evidence of sexual transmission of hepatitis C virus infection. Sex Transm Infect. 2003;79:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unlinked Anonymous HIV and Viral Hepatitis Monitoring Among PWID: 2014 Report. Health Protection Report; 2014. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/341008/hpr2614_hivUAM.pdf. Accessed July 16, 2015. [Google Scholar]
- 40.McVeigh J, Beynon C, Bellis MA. New challenges for agency based syringe exchange schemes: analysis of 11 years of data (1991 to 2001) in Merseyside and Cheshire, UK. Int J Drug Policy. 2003;14 399–405. [Google Scholar]


