Supplemental Digital Content is Available in the Text.
Key Words: HIV testing, test frequency, cost-effectiveness, men who have sex with men, injection drug users
Abstract
Purpose:
Data showing a high incidence of HIV infection among men who have sex with men (MSM) who had annual testing suggest that more frequent HIV testing may be warranted. Testing technology is also a consideration given the availability of sensitive testing modalities and the increased use of less-sensitive rapid, point-of-care antibody tests. We assessed the cost-effectiveness of HIV testing of MSM and injection drug users (IDUs) at 3- and 6-month intervals using fourth-generation and rapid tests.
Methods:
We used a published mathematical model of HIV transmission to evaluate testing intervals for each population using cohorts of 10,000 MSM and IDU. We incorporated HIV transmissions averted due to serostatus awareness and viral suppression. We included costs for HIV testing and treatment initiation, and also treatment costs saved from averted transmissions.
Results:
For MSM, HIV testing was cost saving or cost effective over a 1-year period for both 6-month compared with annual testing and quarterly compared with 6-month testing using either test. Testing IDU every 6 months compared with annually was moderately cost effective over a 1-year period with a fourth-generation test, while testing with rapid, point-of-care tests or quarterly was not cost effective. MSM results remained robust in sensitivity analysis, whereas IDU results were sensitive to changes in HIV incidence and continuum-of-care parameters. Threshold analyses on costs suggested that additional implementation costs could be incurred for more frequent testing for MSM while remaining cost effective.
Conclusions:
HIV testing of MSM as frequently as quarterly is cost effective compared with annual testing, but testing IDU more frequently than annually is generally not cost effective.
INTRODUCTION
More than 1.2 million people were living with HIV in the United States in 2011, among which 14% were undiagnosed.1 HIV testing is the cornerstone of national prevention and care programs. Through diagnosis and antiretroviral therapy (ART), infected persons benefit from reductions in morbidity and mortality, and can reduce onward transmission of HIV through behavior change and viral suppression.2–4
HIV prevalence and incidence in the United States are highest among gay, bisexual, and other men who have sex with men (MSM). In 2010, 74% of persons living with HIV were MSM [67% MSM and 7% MSM/injection drug users (IDUs)] and 13% were IDU.5 The prevalence of undiagnosed infection among MSM and IDU was 16% and 7%, respectively.1 HIV incidence is also rising among MSM; among a large sample of MSM tested in the previous 12 months, more than 7% were newly diagnosed with HIV.6–8 Recent estimates among IDU show a 4% prevalence of newly diagnosed HIV.6 High rates of HIV infection among persons tested in the previous 12 months and high absolute incidence of HIV suggest that testing is not conducted frequently enough.
Centers for Disease Control and Prevention (CDC)'s 2006 Revised Recommendations for HIV testing in health care settings call for testing at least annually for high-risk persons.7 In 2011, CDC suggested that sexually active MSM could benefit from more frequent HIV testing.8 Furthermore, since the 2006 guidelines, HIV testing technologies have become available that detect HIV earlier than previous tests, thus reducing the window period during, which infection is undetectable, and increasing the likelihood that frequent testing will produce earlier diagnoses. Fourth-generation immunoassays detect the virus's p24 antigen, and also the first class of HIV antibodies to appear after infection, allowing detection of HIV infection during the acute, highly infectious stage of disease immediately after HIV acquisition and before HIV antibodies are detectable.9 These fourth-generation combination Ag/Ab tests can therefore increase the benefits of testing at more frequent intervals.
Point-of-care rapid HIV antibody tests are commonly used in the United States because they can be processed outside a laboratory and provide test results in 30 minutes. However, they are typically more expensive and less sensitive than conventional, fourth-generation combination Ag/Ab tests because they have a longer window period of detection, which may lead to false-negative results for persons with early HIV infection.10 Conventional tests typically require a return visit for HIV test results, thus resulting in lower rates of notification of results. The first fourth-generation, Ag/Ab rapid HIV test approved by the Food and Drug Administration has recently been approved for point-of-care use.11,12 In this article, the term “rapid test” refers to antibody-only test, “fourth-generation test” refers to conventional fourth-generation Ag/Ab test and “fourth-generation rapid HIV tests” refers to the fourth-generation rapid, point-of-care Ag/Ab test.
Two US studies that assessed costs and population effects of testing for acute HIV infection concluded that testing MSM and IDU every 6 months with a fourth-generation test could be cost effective.13,14 Significant gaps in our understanding of the cost-effectiveness of increasing HIV testing frequency in high-risk populations remain including the role of test sensitivity and rapid, point-of-care tests. Additionally, the incremental cost-effectiveness of testing every 3 months compared with testing every 6 months, and annual testing has not been evaluated, and no studies have considered additional costs that may be incurred to facilitate the uptake of increasing testing frequency. Our objective was to assess the incremental cost-effectiveness of testing MSM and IDU every 6 months versus annually and quarterly compared with every 6 months with conventional, fourth-generation and point-of-care rapid tests and to investigate circumstances under which frequent testing is cost effective.
METHODS
Analytic Approach
We modified a previously published model of HIV transmission to incorporate HIV detection by testing technology and frequency.15,16 The HIV Detection and Transmission model is a Microsoft Excel-based model that uses Visual Basic Applications to estimate cases of HIV infection detected and one generation of averted secondary transmissions based on the differences in transmission due to awareness of infection, disease stage (acute versus nonacute infection), and viral load suppression from earlier ART initiation. We applied our model to separate, theoretical cohorts of 10,000 MSM and IDU. We estimated the additional HIV infections detected and the resulting HIV transmissions averted for each testing frequency (6-month versus annual testing and 3-month versus 6-month testing) and technology (conventional fourth-generation versus point-of-care rapid testing).
HIV Detection and Transmission
We assumed that all diagnosed infections were new infections, the incidence rate was constant, there was no migration of persons into or out of the model, and that testing occurred on day 90 (quarterly testing) or 180 (testing every 6 months). We estimated the number of new HIV infections expected for each testing frequency using incidence estimates. We then used test window periods to assess the probability infection that was detectable during each testing interval (Table 1). We assumed that infections that occurred during the window period would be detected during the subsequent testing interval. We applied notification rates of 80% for conventional, fourth-generation testing and 99% for rapid point-of-care testing.17 For acute infections, we assumed a 7-day time to receipt of results after a fourth-generation test and immediate results for rapid, point-of-care testing. We then estimated the number of HIV infections detected and duration of awareness of HIV diagnosis for each testing frequency. Averted transmissions were limited to the period of earlier awareness of HIV diagnosis conferred by more frequent testing (eg, 6 months for annual testing). We did not incorporate the clinical benefits or quality-adjusted life years (QALY) of earlier diagnosis to the index case for the 3- to 6-month period of earlier awareness of infection because of more frequent testing. Additional information can be found in the Appendix (see Supplemental Digital Content, http://links.lww.com/QAI/A746).
TABLE 1.
Parameter Values
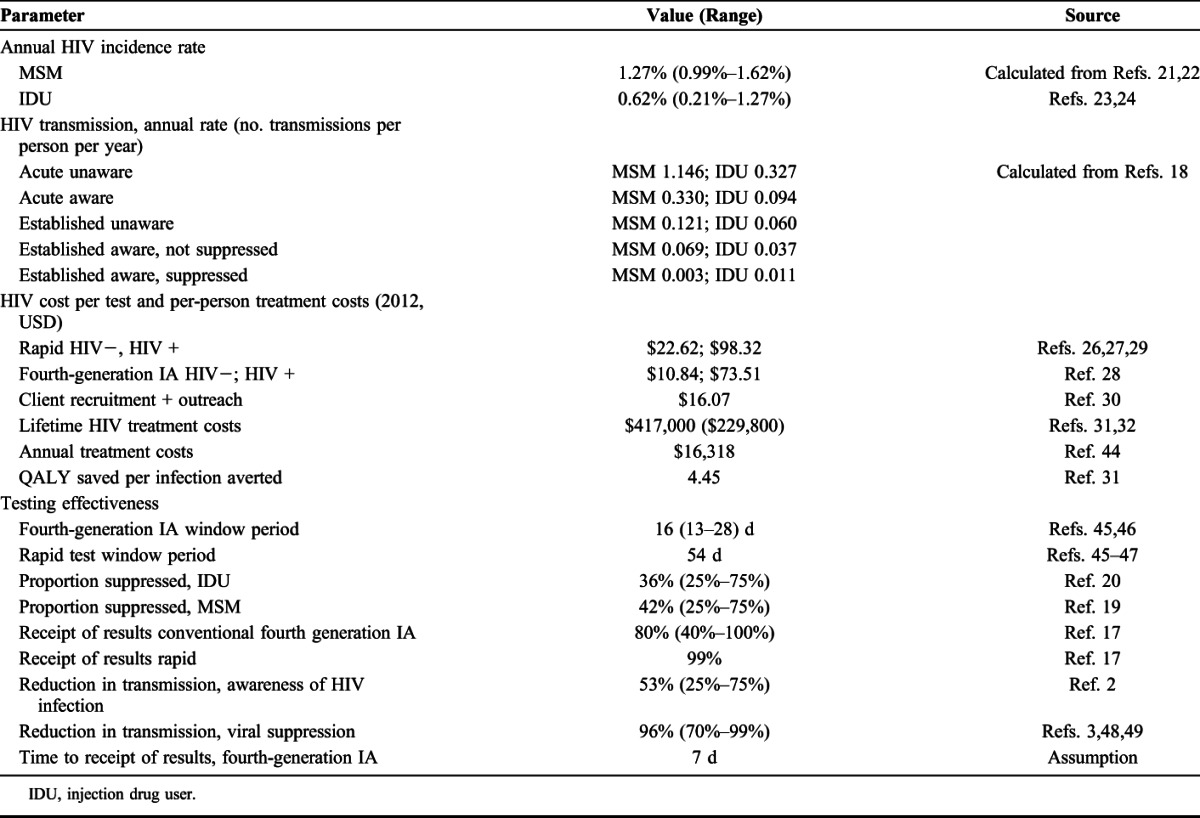
HIV Transmission
We used transmission rates derived from a Bernoulli model to estimate averted transmissions.18 Transmission rates were based on awareness of HIV status, the presence of acute infection, and viral load suppression (acute unaware, acute aware, established unaware, established aware, established aware, and suppressed aware) (Table 1). We assumed that ART is initiated within 3 months of diagnosis that viral suppression confers a 96% reduction in sexual transmission, and behavior changes associated with awareness of HIV positive status confer a 53% reduction in sexual transmission and varied those parameters in sensitivity analysis.2,3 We applied CDC surveillance data on the proportion of prevalent cases with viral suppression, 42.0% MSM and 36.0% IDU, to newly diagnosed persons.19,20
Annual Incidence Rate
We calculated annual HIV incidence rates for MSM and IDU as
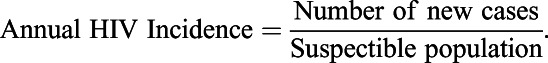 |
For MSM, we calculated an annual HIV incidence rate of 1.27% (range: 0.99%–1.62%) based on 95% confidence limits for 2009 HIV incidence and variation in estimates of the susceptible population.21,22 For IDU, the annual incidence rate estimate was 0.62% (0.02%–1.2%) based on 2009 HIV incidence and recent estimates of the IDU population.23,24
Cost-Effectiveness Analysis
We applied HIV testing and treatment costs and estimated cost per QALY saved for testing every 3 and 6 months compared with annual testing. We used a 1-year time horizon, adjusted all costs and effects to 2012 dollars, and discounted any future costs and effects at a 3% annual rate. We calculated the incremental cost-effectiveness ratio (ICER), which compares cost and outcomes of each HIV testing frequency, relative to the next most effective testing frequency as follows:
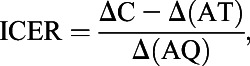 |
where C is the total program cost, A is the number of HIV infections averted, T is the HIV treatment cost saved per infection averted, and Q is the number of QALY saved per infection averted.25
HIV Testing and Treatment Costs
Our HIV testing costs represent testing in clinical settings (Table 1). Costs included laboratory costs, costs for specimen collection and disclosure of results and also costs for client recruitment and outreach that we assume would be required to implement more frequent testing.26–30 For all persons newly diagnosed with HIV, we included treatment costs for the period of earlier detection from diagnosis until the time when annual testing would have occurred. To value averted HIV transmissions, we used lifetime treatment costs of $417,000 discounted to the time of infection31 and evaluated an alternate estimate in sensitivity analysis.32
Scenario Analysis
We modeled a scenario in which a fourth-generation point-of-care, rapid test was used. Based on test performance data, we assumed that such a test would have a window period similar to third-generation HIV tests (22 days), cost 20% more than the current point-of-care rapid tests, and allow 99% of persons to receive their results.33
Sensitivity Analyses
We conducted one-way sensitivity analysis for the range of values for HIV incidence, test window periods for fourth-generation tests, receipt of HIV test results for fourth-generation tests, lifetime HIV treatment costs, and the reduction in transmission because of awareness of positive HIV status and viral load suppression (Table 1).
We conducted 2-way sensitivity analyses pairing highest and lowest values for reduction in HIV transmission due to viral suppression (70%–99%) and the probability of viral suppression among diagnosed persons (25%–75%) (Table 1). We also conducted threshold analyses to determine how much program costs could increase under each testing scenario and still remain, or become, cost effective at the $100,000 per QALY saved threshold.
RESULTS
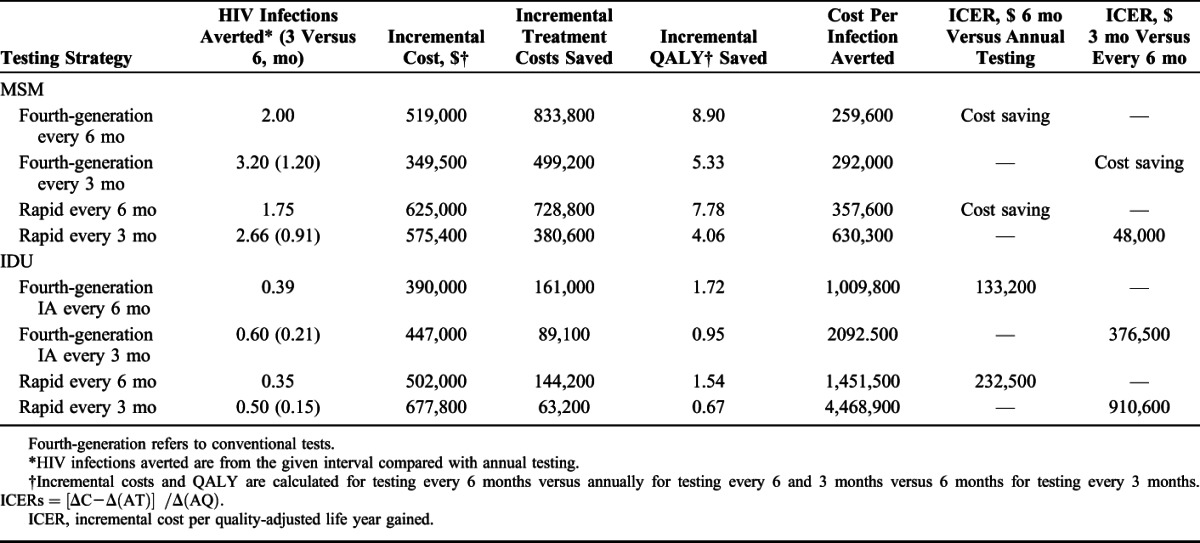
For our cohort of MSM, increasing testing frequency to every 6 months compared with annual testing averted 2.00 and 1.75 HIV transmissions with a conventional fourth-generation or rapid test, respectively, over a 1-year period and was cost saving (Table 2). Compared with a strategy of testing every 6 months, testing quarterly averted an additional 1.20 transmissions and was cost saving with a fourth-generation test and averted an additional 0.91 HIV transmissions with an ICER of $48,000 per QALY saved for rapid testing. For IDU, increasing testing to every 6 months compared with annually averted 0.39 HIV transmissions over 1 year with an ICER of $133,200 per QALY for fourth-generation tests and 0.35 HIV transmissions averted with an ICER of $232,500 saved for rapid testing. For IDU, a quarterly testing strategy compared with testing every 6 months yielded 0.21 and 0.15 HIV transmissions averted, respectively, for fourth-generation testing and rapid testing with ICERs greater $375,000 and $900,000 per QALY.
TABLE 2.
Cost-Effectiveness of HIV Testing Frequencies for a Cohort of 10,000 MSM or IDUs With Fourth-Generation or Point-of-Care Rapid Tests
Scenario Analysis
For MSM, the use of a fourth-generation, point-of-care rapid test would be less favorable than conventional, laboratory-based fourth-generation testing although ICERs remained cost saving for semiannual testing and $34,700 per QALY saved for quarterly testing. For IDU, ICERs for a fourth-generation, point-of-care rapid test would be slightly more favorable than conventional rapid testing with an ICER of $204,600 per QALY saved for testing every 6 months and greater than $700,000 for quarterly testing (data not shown).
Sensitivity Analyses
For MSM, our results were robust and remained cost saving in all cases of semiannual testing and most cases of quarterly testing to variations in the value of the fourth-generation test window period, notification of HIV test results, viral suppression, and the transmission benefit of awareness of positive HIV status (data not shown). When we applied lifetime treatment costs of $229,800, ICERs for fourth-generation testing were less than $15,000 per QALY for semiannual and quarterly testing. For quarterly rapid testing of MSM, results were sensitive to HIV incidence, the proportion of positives with viral suppression and lower lifetime treatment costs with ICERs near $100,000 per QALY at the lower bound and cost saving at the upper bound (0.99%–1.62% HIV incidence and 25%–75% suppression).
The cost-effectiveness of more frequent testing was sensitive to a number of parameter variations for IDU with semiannual testing (Fig. 1). The range of values of HIV incidence among IDU (0.21%–1.27%) yielded ICERs less than $100,000 per QALY saved at the highest incidence, and for the lowest incidence, $400,000 and $700,000 for fourth-generation and rapid testing, respectively. The most favorable values for receipt of results (100%) and the proportion suppressed (75%) resulted in ICERs less than $100,000 per QALY saved for fourth-generation testing. Our results were not sensitive to changes in the window period for fourth-generation HIV tests or changes in the reduction in HIV transmission (25%–75%) related to behavior change for persons aware of their HIV infection. For quarterly testing, only the upper bound of IDU incidence produced an ICER less than $100,000 per QALY saved (data not shown).
FIGURE 1.
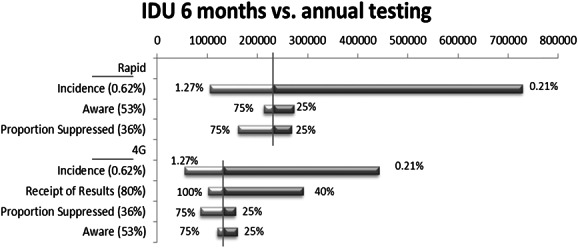
Tornado Diagrams of one-way sensitivity analyses of the cost-effectiveness of semiannual rapid and fourth-generation HIV testing for IDU. The horizontal bar shows the range in cost-effectiveness ($ per QALY saved), given the variation in model parameters and the parameter values explored in sensitivity analyses. The vertical line shows the base case cost-effectiveness. The legend shows base case parameter values. 4G, fourth-generation HIV test.
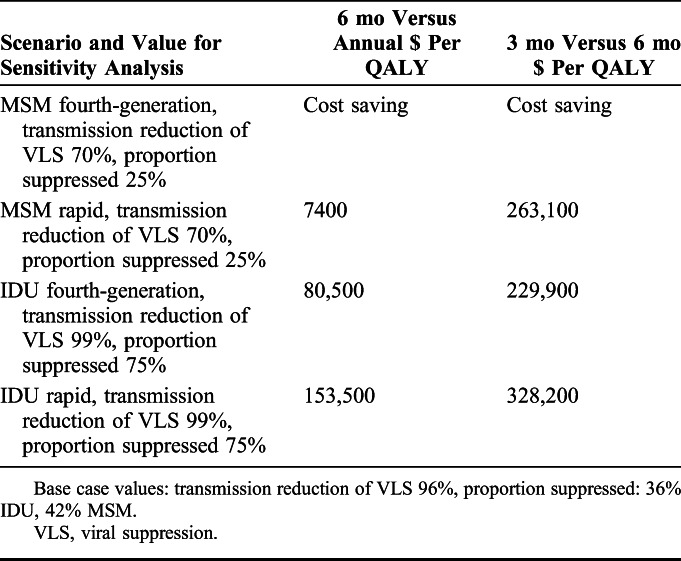
In 2-way sensitivity analyses of viral suppression parameters, we combined the least optimistic values for MSM and the most optimistic values for IDU (reduction in HIV transmission due to suppression 70% or 99%, proportion suppressed 25% or 75%) given our base case findings. Our results remained robust for MSM for fourth-generation testing at both 6-month and quarterly testing intervals; however, for quarterly rapid testing of MSM, the least optimistic viral suppression parameters resulted in ICERs greater than $200,000 per QALY saved (Table 3). For IDU, the ICERs for semiannual testing became more attractive and less than $100,000 per QALY for fourth-generation testing with optimistic viral suppression parameters, whereas the ICERs remained above $200,000 per QALY for quarterly testing (Table 3).
TABLE 3.
Two-Way Sensitivity Analysis on Reduction in Transmission due to Viral Suppression and Proportion Suppressed
Threshold Analyses
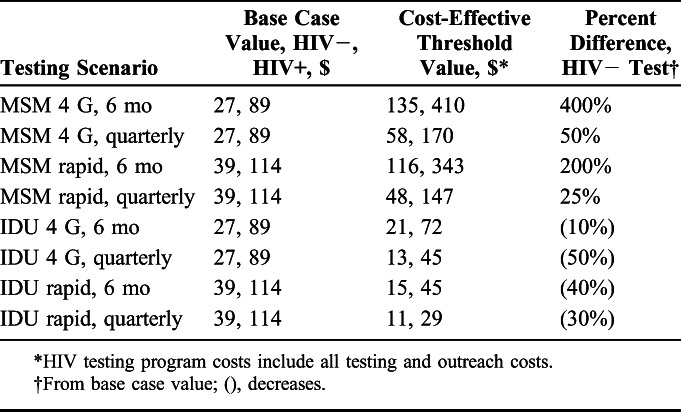
In threshold analyses of MSM testing frequencies, testing costs could increase substantially from base case values and still remain cost effective at a $100,000 per QALY threshold (Table 4). For testing at 6-month intervals, costs could increase from $27 to $135 for a fourth-generation and from $39 to $116 for a rapid test and still remain cost effective. Quarterly testing would remain cost effective with costs in the $50–$60 range for fourth-generation and rapid testing. For IDU, costs for fourth-generation tests would have to drop 10% to $21% and 50% to $13 to be cost effective for 6-month and quarterly testing, respectively. Testing costs would have to drop by over one-third for rapid testing to be cost effective for IDU.
TABLE 4.
Threshold Analyses of HIV Testing Program Costs for Testing MSM and IDU
DISCUSSION
Testing MSM at 3-month and 6-month intervals was cost effective even with the less-sensitive rapid test, whereas for IDU, testing at the lowest testing frequency evaluated (every 6 months) combined with a more sensitive (fourth-generation) test was moderately cost effective.
Testing MSM as frequently as every 3 months was very cost effective or cost saving under almost all scenarios evaluated. Although we did not consider targeting to high-risk MSM such as young MSM or African American MSM, our sensitivity analyses suggest that such an approach would result in cost savings.22 Cost savings are rarely achieved for health interventions. Thus, more frequent testing for MSM compares favorably to widely used interventions such as annual mammography at $61,000 per QALY saved and diabetes prevention at $31,500 per QALY saved.34,35
Semiannual testing of IDUs could be cost effective using a laboratory-based, fourth-generation test, whereas testing with a point-of-care, rapid test was not cost effective. Quarterly testing of IDUs was also not cost effective. Integrating testing with services such as drug abuse treatment may be a good option if more frequent testing is to be undertaken.36 Lower HIV incidence among IDU compared with MSM was a major driver, the difference in our cost-effectiveness results. Declining rates in annual testing among IDU are also a consideration; recent data from the National HIV Behavioral Surveillance System show a decline (from 66% to 49%) in testing in the past 12 months among IDU.6,37 Thus, there is a need to balance the benefits of investing in increased annual testing of IDU with investing in testing IDU more frequently than annually.
Because additional costs will likely be required to have clients accept testing at more frequent intervals, we included in our base case costs ($16) for client recruitment and outreach, which have not been considered in previous studies.30 In addition, our threshold analysis showed that for MSM, $77–$108 per person could be added to the cost of semiannual testing, and $9–$31 per person could be added to the cost of quarterly testing while remaining cost effective at the $100,000 per QALY saved threshold. These additional costs could be applied to efforts to increase outreach such as mobile van testing at $21 per person ($2012), to cover costs associated with increased testing volumes or enhance linkage services.30 In contrast, for IDU, the only plausible scenario below the $100,000 per QALY saved threshold occurred if per-person costs of testing with a fourth-generation test decreased by $6 and $14 for semiannual and quarterly testing, respectively. This leaves no room for spending on mobile vans or other methods to increase testing uptake and capacity, which reduces the feasibility of frequent testing in this hard-to-reach population.
Testing method is an important consideration for increasing testing frequency. In a survey of MSM in Australia, respondents indicated a willingness to test frequently if testing were rapid, nonclinic-based, and used saliva or finger stick.38 In the United States, the rapid antibody test currently surpasses conventional tests as a proportion of the 2.5 million tests annually funded by CDC due to its easier use outside the clinical setting.17 The ability to provide rapid, point-of-care testing to people at higher risk than testing in a clinical setting may also improve cost-effectiveness ratios. The trade-off with rapid antibody testing, nearly 100% notification of results but lower test sensitivity, resulted in fewer averted transmissions compared with a fourth-generation test at the same testing frequencies. However, higher rapid testing costs resulted in higher ICERs. Our scenario analysis indicated that a fourth-generation, point-of-care rapid test was more cost effective than rapid antibody tests, although not as attractive as a fourth-generation laboratory-based test due to slightly lower sensitivity and higher costs. Nonetheless, there are wide variations in HIV test costs, and the entrance of a fourth-generation, point-of-care rapid test into the marketplace may result in changes in relative prices of HIV testing technologies, ultimately making cost-effectiveness difficult to predict.28
Our costs were calculated based on the HIV testing algorithm that has been in place since 1989. However, the CDC has updated laboratory testing guidelines for the diagnosis of HIV; the new testing algorithm uses more sensitive screening (fourth-generation) and confirmatory tests to facilitate detection of acute and early infection.39 Costs for the new algorithm are generally estimated to be lower than the 1989 algorithm.28 Additionally, frequent testing increases the proportion of persons diagnosed with acute and early infection; thus using the new algorithm is likely to improve cost-effectiveness.
Our study is subject to several limitations. We did not consider benefits to the index client because of earlier diagnosis and treatment initiation. Two randomized clinical trials have recently provided clear evidence that early ART improves clinical outcomes compared with delayed ART.40,41 However, the clinical benefits of the treatment for the short 3- to 6-month period of earlier detection afforded by greater testing frequency are unknown at this time. We also did not incorporate the effect of partner notification or serosorting, both of which could increase the cost-effectiveness of testing by reducing the likelihood of HIV transmission. Additionally, we did not consider home tests, which could promote uptake of more frequent testing or, because of lower test sensitivity, could increase the rate of false-negative test results and delay linkage to care.42 We also limited our analysis to a first generation of transmissions, which could underestimate cost-effectiveness, particularly in high-risk populations. Our use of a 96% transmission reduction for persons with a suppressed viral load, which was obtained from a randomized controlled trail conducted in stable heterosexual partnerships who used condoms, may overestimate the true population-level benefit in MSM and IDU with various levels of adherence.3 However, this is offset by limiting this benefit to the period of early detection due to more frequent testing. Although we incorporated the benefits of acute phase detection, we did not account for early infection, the period up to 90 days after infection during which viremia is also heightened, which could underestimate cost-effectiveness. We made the simplifying assumption that all diagnosed infections are incident infections, which may underestimate cost-effectiveness because we do not consider the benefits (transmissions averted) of identifying previously diagnosed persons who may be relinked to care. We also assumed that timing of testing did not vary over the 3- or 6-month testing interval. If there were variation, it could impact the likelihood that testing occurred during the window period and the duration of serostatus awareness. Finally, the lifetime treatment costs we used to value averted transmissions represent costs for persons receiving optimal HIV care and thus may overestimate the costs and the benefit of testing for those who do not receive that level of care. Nevertheless, the “net effect” of the potential sources of bias and even sensitivity analyses do not suggest the findings lean in one direction or the other.
Our findings are generally consistent with other studies that have applied different modeling approaches—dynamic compartmental modeling and mathematical optimization modeling.13,14,43 Long reported an ICER of $18,000 per QALY saved for semiannual, fourth-generation testing of MSM, which is in line with our cost savings results. Key differences in the Long study compared with ours include higher test and counseling costs and lower estimated incidence among MSM. Our findings for quarterly testing of MSM also seem to be in line with a cost-benefit analysis that found the optimal frequency for high-risk persons is quarterly testing.43 Compared with Cipriano et al, our findings were less favorable, although still moderately cost effective ($133,000 versus $30,000 per QALY saved) for semiannual, fourth-generation testing of IDU. A key difference is that Cipriano et al used a drug treatment center setting and assumed 100% of infected IDUs received their HIV test results, whereas we assumed 80% of results were received. Other factors that can account for differences in these studies include differences in model structure and type and also time horizon (20 years in the dynamic compartmental models versus 1 year in our study).
We explicitly compared increased testing frequency with currently recommended annual testing. This comparison is important to policy makers and program managers who often need to consider near-term differences in testing outcomes. We also considered the most common testing method, rapid antibody testing, which was not as cost effective as laboratory-based testing. And although the full costs of increasing testing frequency among these high-risk groups remains unknown, we addressed a question important to many policy makers, “How much can be spent on implementation and still be cost effective?”
In summary, we found that compared with annual testing, it was cost effective to test MSM as frequently as every 3–6 months even with increased investment in testing recruitment and implementation. More frequent than annual testing of IDU was generally not cost effective for most IDU.
Footnotes
Author Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2012. HIV Surveillance Supplemental Report. Atlanta, Georgia: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 2.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–1450. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26:893–896. [DOI] [PubMed] [Google Scholar]
- 5.CDC. HIV Surveillance Report, 2011. [Google Scholar]
- 6.Broz D, Wejnert C, Pham HT. HIV infection and risk, prevention, and testing behaviors among injecting drug users—national HIV behavioral surveillance system, 20 U.S. Cities, 2009. Morb Mortal Wkly Rep. 2014;63:1–51. [PubMed] [Google Scholar]
- 7.CDC. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17; quiz CE11–14. [PubMed] [Google Scholar]
- 8.CDC. HIV testing among men who have sex with men—21 cities, United States, 2008. Morb Mortal Wkly Rep. 2011;60:694–699. [PubMed] [Google Scholar]
- 9.Branson BM, Mermin J. Establishing the diagnosis of HIV infection: new tests and a new algorithm for the United States. J Clin Virol. 2011;52(suppl 1):S3–S4. [DOI] [PubMed] [Google Scholar]
- 10.Branson BM. The future of HIV testing. J Acquir Immune Defic Syndr. 2010;55(suppl 2):S102–S105. [DOI] [PubMed] [Google Scholar]
- 11.FDA. August 9, 2013 Approval Letter—Alere Determine HIV-1/2 Ag/Ab Combo. Rockville, MD: U.S. Food and Drug Administration; 2013. [Google Scholar]
- 12.PR Newswire. U.S. Food and Drug Administration Grants CLIA Waiver for Alere Determine™ HIV-1/2 Ag/Ab Combo Test. Waltham, MA: PR Newswire; 2014. [Google Scholar]
- 13.Long EF. HIV screening via fourth-generation immunoassay or nucleic acid amplification test in the United States: a cost-effectiveness analysis. PLoS One. 2011;6(11):e27625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cipriano LE, Zaric GS, Holodniy M, et al. Cost effectiveness of screening strategies for early identification of HIV and HCV infection in injection drug users. PLoS One. 2012;7:e45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchinson AB, Patel P, Sansom SL, et al. Cost-effectiveness of pooled nucleic acid amplification testing for acute HIV infection after third-generation HIV antibody screening and rapid testing in the United States: a comparison of three public health settings. PLoS Med. 2010;7:e1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchinson AB, Farnham PG, Duffy N, et al. Return on public health Investment: CDC's expanded HIV testing Initiative. J Acquir Immune Defic Syndr. 2011;59:281–286. [DOI] [PubMed] [Google Scholar]
- 17.Huang A, Hutchinson A, Hollis ND, et al. Notification following new positive HIV test results. Int J STDS AIDS. 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Yaylali E, Farnham P, Schneider KD, et al. From theory to practice: implementation of a resource allocation model in health departments. J Public Health Manag Pract. 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC. Men living with diagnosed HIV who have sex with men: progress along the continuum of HIV care—United States. Morb Mortal Wkly Rep. 2014;63:829–833. [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2011. HIV Surveillance Supplemental Report. Atlanta, Georgia: Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 21.Purcell DW, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J. 2012;6:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006-2009. PLoS One. 2011;6:e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lansky A, Finlayson T, Johnson C, et al. Estimating the number of persons who inject drugs in the united states by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One. 2014;9:e97596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tempalski B, Lieb S, Cleland CM, et al. HIV prevalence rates among injection drug users in 96 large US metropolitan areas, 1992-2002. J Urban Health. 2009;86:132–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinkerton SDHD. A method for evaluating the economic efficiency of HIV behavioral risk reduction interventions. AIDS Behav. 1998;2:189–201. [Google Scholar]
- 26.Pinkerton SD, Bogart LM, Howerton D, et al. Cost of rapid HIV testing at 45 U.S. hospitals. AIDS Patient Care STDS. 2010;24:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchinson AB, Farnham PG, Lyss SB, et al. Emergency department HIV screening with rapid tests: a cost comparison of alternative models. AIDS Educ Prev. 2011;23(3 suppl):58–69. [DOI] [PubMed] [Google Scholar]
- 28.Hutchinson AB, Ethridge SF, Wesolowski LG, et al. Costs and outcomes of laboratory diagnostic algorithms for the detection of HIV. J Clin Virol. 2013;58(suppl 1):e2–e7. [DOI] [PubMed] [Google Scholar]
- 29.Farnham PG, Hutchinson AB, Sansom SL, et al. Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep. 2008;123(suppl 3):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha RK, Clark HA, Sansom SL, et al. Cost-effectiveness of finding new HIV diagnoses using rapid HIV testing in community-based organizations. Public Health Rep. 2008;123(suppl 3):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013;64:183–189. [DOI] [PubMed] [Google Scholar]
- 32.Schackman BR, Fleishman JA, Su AE, et al. The lifetime medical cost savings from preventing HIV in the United States. Med Care. 2015;53:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masciotra S, McDougal JS, Feldman J, et al. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(suppl 1):S17–S22. [DOI] [PubMed] [Google Scholar]
- 34.Stout NK, Rosenberg MA, Trentham-Dietz A, et al. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98:774–782. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Zhang P, Barker LE, et al. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010;33:1872–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CDC. Integrated prevention services for HIV infection, viral hepatitis, sexually Transmitted diseases, and Tuberculosis for persons who Use drugs Illicitly: summary Guidance from CDC and the U.S. Department of health and human services MMWR Morb Mortal Wkly Rep. 2012;61:1–40. [PubMed] [Google Scholar]
- 37.CDC. HIV infection and HIV-associated behaviors among injecting drug users—20 cities, United States, 2009. MMWR Morb Mortal Wkly Rep. 2012;61:133–138. [PubMed] [Google Scholar]
- 38.Gray RT, Prestage GP, Down I, et al. Increased HIV testing will modestly reduce HIV incidence among gay men in NSW and would be acceptable if HIV testing becomes convenient. PLoS One. 2013;8:e55449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention and Asssociation of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. 2014. Available at http://stacks.cdc.gov/view/cdc/23447. Accessed September 24, 2014. Published June 27, 2014. [Google Scholar]
- 40.National Institutes of Health. Starting Antiretroviral Treatment Early Improves Outcomes for HIV-infected Individuals. Available at: http://www.nih.gov/news/health/may2015/niaid-27.htm. Published May 27, 2015. Accessed [July 14, 2015]. [Google Scholar]
- 41.Danel C, Gabillard D, Le Carrou J. Early ART and IPT in HIV-infected African adults with high CD4 count (Temprano Trial). CROI. 2015. [Google Scholar]
- 42.Katz DA, Cassels SL, Stekler JD. Response to the modeling analysis by Katz et al. on the impact of replacing clinic-based HIV tests with home testing among men who have sex with men in Seattle: authors' reply. Sex Transm Dis. 2014;41:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas A, Armbruster B. The cost-effectiveness of expanded HIV screening in the United States. AIDS. 2013;27:795–801. [DOI] [PubMed] [Google Scholar]
- 44.Gebo KA, Fleishman JA, Conviser R, et al. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24:2705–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. [DOI] [PubMed] [Google Scholar]
- 46.Louie B, Liska SL, Klausner JD, et al. Reactivity of an array of HIV antibody assays with specimens from HIV acutely infected individuals. Available at: http://www.hivtestingconferencearchive.org/hivtesting2007/abstracts/abstract13.pdf. 2007.
- 47.Owen SM, Yang C, Spira T, et al. Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. J Clin Microbiol. 2008;46:1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormick AW, Walensky RP, Lipsitch M, et al. The effect of antiretroviral therapy on secondary transmission of HIV among men who have sex with men. Clin Infect Dis. 2007;44:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson DP, Law MG, Grulich AE, et al. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372:314–320. [DOI] [PubMed] [Google Scholar]


