Abstract
Introduction:
The evidence has begun to mount for diminishing the frequency of CD4 count testing. To determine whether these observations were applicable to an urban US population, we used New York City (NYC) surveillance data to explore CD4 testing among stable patients in NYC, 2007–2013.
Methods:
We constructed a population-based retrospective open cohort analysis of NYC HIV surveillance data. HIV+ patients aged ≥13 years with stable viral suppression (≥1 viral load the previous year; all <400 copies per milliliter) and immune status (≥1 CD4 the previous year; all ≥200 cells per cubic millimeter) entered the cohort the following year beginning January 1, 2007. Each subsequent year, eligible patients not previously included entered the cohort on January 1. Outcomes were annual frequency of CD4 monitoring and probability of maintaining CD4 ≥200 cells per cubic millimeter. A multivariable Cox model identified factors associated with maintaining CD4 ≥200 cells per cubic millimeter.
Results:
During 1.9 years of observation (median), 62,039 patients entered the cohort. The mean annual number of CD4 measurements among stable patients was 2.8 and varied little by year or characteristic. Two years after entering, 93.4% and 97.8% of those with initial CD4 350–499 and CD4 ≥500 cells per cubic millimeter, respectively, maintained CD4 ≥200 cells per cubic millimeter. Compared to those with initial CD4 ≥500 cells per cubic millimeter, those with CD4 200–349 cells per cubic millimeter and CD4 350–499 cells per cubic millimeter were more likely to have a CD4 <200 cells per cubic millimeter, controlling for sex, race, age, HIV risk group, and diagnosis year.
Conclusions:
In a population-based US cohort with well-controlled HIV, the probability of maintaining CD4 ≥200 cells per cubic millimeter for ≥2 years was >90% among those with initial CD4 ≥350 cells per cubic millimeter, suggesting that limited CD4 monitoring in these patients is appropriate.
Key Words: HIV, monitoring, CD4 count, cohort, New York City
INTRODUCTION
Although there have been great advances in the treatment of persons living with HIV since the beginning of the epidemic, there have been fewer innovations in the routine laboratory monitoring of HIV-infected patients. One of the first laboratory tests that emerged for such patients, the CD4 cell count, is still used by clinicians to determine a patient's level of immunosuppression at diagnosis and to determine the need for either initiation or discontinuation of prophylaxis for certain opportunistic infections (OIs). However, once a patient achieves stable virologic suppression [one of the goals of antiretroviral therapy (ART)], and is no longer near a threshold for OI prophylaxis, the benefit of CD4 cell count testing has been called into question.1
This lack of clarity about the utility of ongoing CD4 cell count testing in certain situations was, until very recently, reflected in disparate recommendations about the frequency of such monitoring in clinical guidelines and protocols, both national and local.2–4 In addition, certain systems for performance measurement, such as HIVQUAL (a national continuous quality control project), required CD4 monitoring every 6 months.5 This confusion has left clinicians in a situation where they might have felt compelled to order CD4 tests at least biannually to match the guidelines and/or quality management protocols, despite the fact that such testing would have often been of limited clinical utility, and, therefore, might represent an overutilization of health care resources.6 Expectations by patients for such testing with each blood draw, based on years of having understood this to be the single most important indicator of their HIV-related health, may also have fueled such testing.
However, over the past 2 years, the evidence has begun to mount for both (1) using virologic monitoring as the preferred monitoring approach and (2) diminishing the frequency of CD4 cell count testing. In terms of the former, a recent systematic review7 included 2 studies (1 a randomized clinical trial; 1 an observational study) that found a longer duration of viremia and longer time to switching to a second-line regimen with clinical and immunologic monitoring alone versus clinical and immunologic monitoring plus virologic monitoring8,9; a mortality advantage in patients with virologic monitoring was found in the observational study as well.8 Ultimately, both studies provided the basis for 2013 World Health Organization guidelines that strongly recommended the use of virologic monitoring to both diagnose and confirm ART failure in all settings, including low-resource ones10; it was specified that clinical and immunologic monitoring should only diagnose treatment failure in the absence of the availability of virologic monitoring.
In terms of the rationale for the diminished frequency of CD4 cell count testing, at least 5 recent analyses suggest that less frequent testing among certain HIV-infected patients might be appropriate. Using electronic record data from 1 Veteran's Administration HIV clinic paired with selective chart review, Gale et al11 determined that less frequent CD4 count testing might be appropriate, based on the fact that, among other findings, no clinically stable patient in their analysis with a CD4 count ≥300 cells per cubic millimeter had a CD4 dip (defined as a CD4 count <200 cells per cubic millimeter) after 2 years of continuous viral suppression. Similarly, using data from the ARTEMIS clinical trial, Girard et al12 found that CD4 testing was beneficial in the first 48 weeks of ART, but those patients who achieved virologic suppression and had CD4 counts equal or above 200 cells per cubic millimeter had little clinical benefit beyond 48 weeks. In addition, 1 recent retrospective chart review from Australia demonstrated that none of the 162 decisions to modify or discontinue ART over a 30-month period were based on a CD4 count.13 However, all of these studies include a small sample that may not be representative of the entire population for various reasons. A more recent study also found uncertain utility of CD4 monitoring among a large program-based cohort of South African patients in a very poor township,14 but these patients may not be representative of patients outside of the resource-limited setting. Even more recently, in an observational cohort in the Asia–Pacific region, there was no significant difference in time to a confirmed CD4 <200 cells per cubic millimeter between biannual and annual CD4 measurement cohorts.15 This mounting evidence was reflected in 2014 US guidelines proposing that CD4 count monitoring for stable virologically suppressed patients with CD4 counts consistently between 300 and 500 be conducted annually; the guidelines considered CD4 count monitoring for stable virologically suppressed patients with CD4 counts consistently >500 cells per cubic millimeter to be optional.2
To determine whether these observations were applicable to a large urban US population receiving care under usual conditions, outside the structure of a research cohort or clinical trial, we used New York City (NYC) surveillance data to explore current CD4 testing patterns among virologically and immunologically stable HIV-infected patients in NYC, from 2007 through 2013. We also sought to determine whether some patients could undergo less frequent CD4 testing without detriment to their clinical care.
METHODS
Study Design and Data Source
We conducted a population-based retrospective open cohort study using the NYC HIV registry data. AIDS diagnoses have been reportable in New York State since 1981. The New York State law expanded AIDS case reporting on June 1, 2000 to include diagnoses of non-AIDS HIV infection as well as laboratory tests of CD4 counts less than 500 cells per cubic millimeter and detectable viral loads (VLs), and on June 1, 2005 to include all CD4 and VL values. The NYC HIV registry contains all NYC HIV cases and is continuously updated with newly confirmed diagnoses and laboratory results and vital status on new and existing case records. As of June 30, 2014, NYC HIV registry contained a cumulative total of over 230,000 cases and more than 8 million laboratory tests. The analysis used surveillance data and was a public health practice activity, not human subject research. Thus, it was exempt from institutional review board approval.
HIV-infected patients aged ≥13 years were considered “stable” if they met both of the following 2 conditions for an entire calendar year and included for follow-up beginning on January 1 of the next year: (1) stable viral suppression, defined as having at least 1 VL test, with all measurements <400 copies per milliliter and (2) stable immune status, defined as having at least 1 CD4 test, with all measurements ≥200 cells per cubic millimeter. As a full year of VL and CD4 data are needed to determine the eligibility of each patient, and as the year 2006 was the first full year of comprehensive HIV-related laboratory reporting in New York State, January 1, 2007 was the earliest date a patient could enter the cohort and be included in the analysis. Each subsequent year, eligible patients not previously included entered the cohort on January 1. Patients were followed through December 31, 2013, and censored at first VL ≥400 copies per milliliter or the very last CD4/VL. Patients who exited the cohort because of a failure (CD4 count below 200 cells per cubic millimeter) or being censored (VL ≥400 copies per milliliter) were allowed to reenter the cohort after they became eligible again with a stable immune status (all CD4 counts ≥200 cells per cubic millimeter) and a stable viral suppression status (all VL <400 copies per milliliter) for an entire calendar year.
Measurements
The primary outcome measure was the frequency of CD4 monitoring in a year between 2007 and 2013; the secondary outcome measure was the probability of the CD4 dropping <200 cells per cubic millimeter. The initial CD4 count on January 1 (when the patient entered the cohort) was calculated based on the values of the last CD4 count in the previous year and the first CD4 count on or after January 1 of the cohort entry year, assuming a linear change by time between the 2 tests.
Statistical Analysis
For the primary outcome measure analysis, we first described the frequency of CD4 monitoring by year, using mean (SD), median [interquartile range (IQR)], and frequency categories. We then limited our analysis to the 2013 population to examine the frequency of CD4 monitoring in subgroups, by sex, race/ethnicity, age, transmission risk, and year of diagnosis. Data from 2013 were used because they were the most recent year for which complete data were available due to the slight lag in laboratory reporting.
For the secondary outcome measure analysis, the Kaplan–Meier product limit method was used to estimate the cumulative proportion of patients maintaining CD4 count ≥200 cells per cubic millimeter after they entered the cohort, stratified by sex (male/female), race/ethnicity, age, transmission risk, year of diagnosis, and initial CD4 count, with log-rank tests to assess differences between groups. Patients were censored at the date of their first VL ≥400 copies per milliliter, after they entered or reentered the cohort, or last CD4/VL test, if patients' CD4 count never dropped to below 200 cells per cubic millimeter by the end of the analysis period, December 31, 2013. A multivariable Cox proportional hazard regression model with 6 independent variables (sex, race/ethnicity, age, transmission risk, year of diagnosis, and initial CD4 count) was used to identify factors associated with maintaining CD4 count ≥200 cells per cubic millimeter and to calculate the respective adjusted hazard ratios.
All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
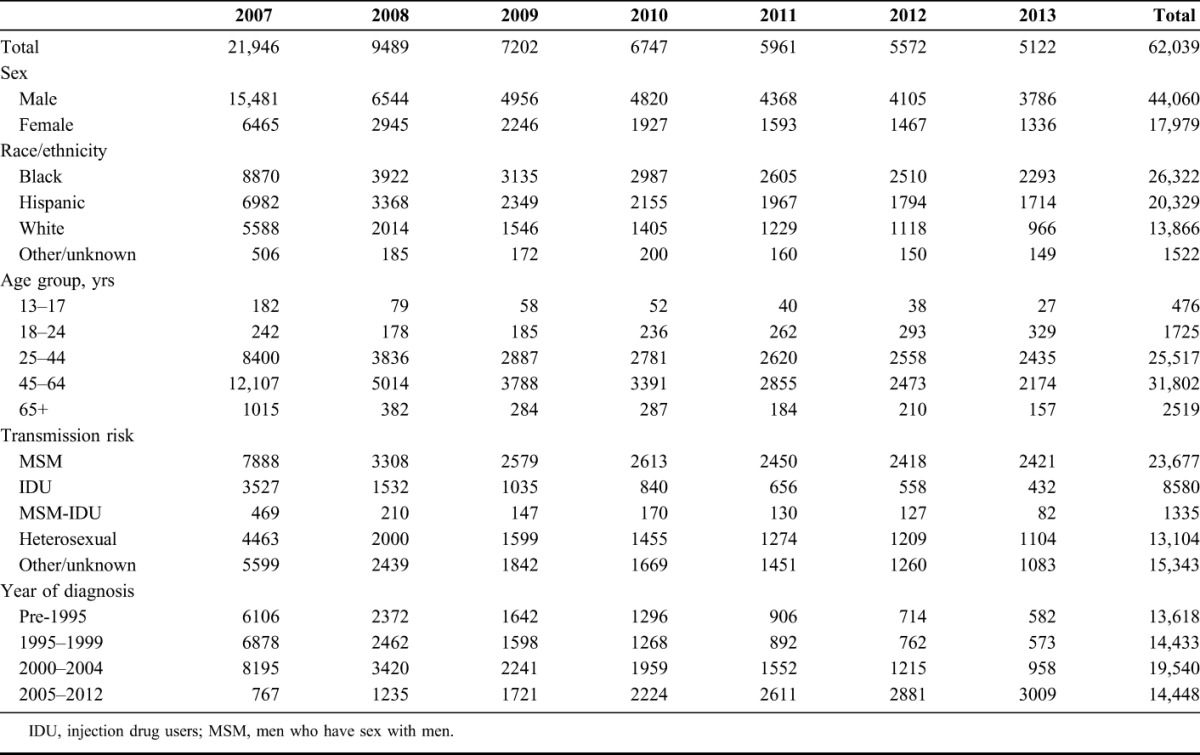
A total of 62,039 unique patients were included in the open cohort and were added by year as follows: 21,946 (2007), 9489 (2008), 7202 (2009), 6747 (2010), 5961 (2011), 5572 (2012), and 5122 (2013) (Table 1). The number of patients who reentered the cohort was 13,242, assigned by year: 1765 (2009), 2680 (2010), 2900 (2011), 2893 (2012), and 3004 (2013) (data not shown in Table 1).
TABLE 1.
Characteristics of Stable Patients With HIV (N = 62,039) Enrolled in a Retrospective Open Cohort in NYC, by Year of Enrollment, 2007–2013
Frequency of CD4 Testing Among Stable Patients With HIV
Over the interval, the mean annual number of CD4 measurements among stable patients was between 2.7 and 2.9 and showed no significant variation by year (2007–2013) (Table 2). In 2013, 23.5% of stable patients with HIV had 4 or more CD4 measurements, and the annual number of CD4 measurements among stable patients was found to vary little by sex, race/ethnicity, transmission risk, or year of diagnosis, but the most frequent testing seemed to occur among patients at the extremes of age: 33.3% of persons 13–17 years, and 29.6% of persons 65 and older had ≥4 tests per year, compared with 25.0% of persons 18–24 years, 20.7% of persons 25–44 years, and 24.9% of persons 45–64 years (Table 3).
TABLE 2.
Frequency of CD4 Testing Among Stable Patients With HIV in NYC, 2007–2013
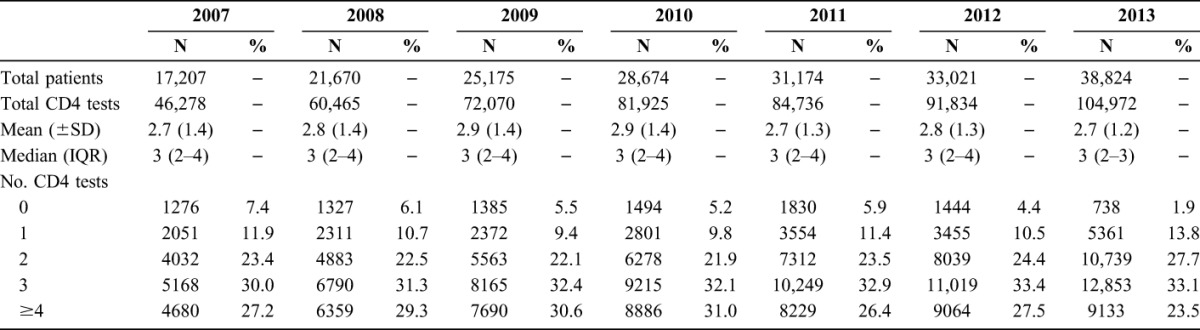
TABLE 3.
Frequency of CD4 Testing Among Stable Patients With HIV (N = 38,824) in NYC, 2013
Probability of CD4 Dropping to <200 Cells Per Cubic Millimeter
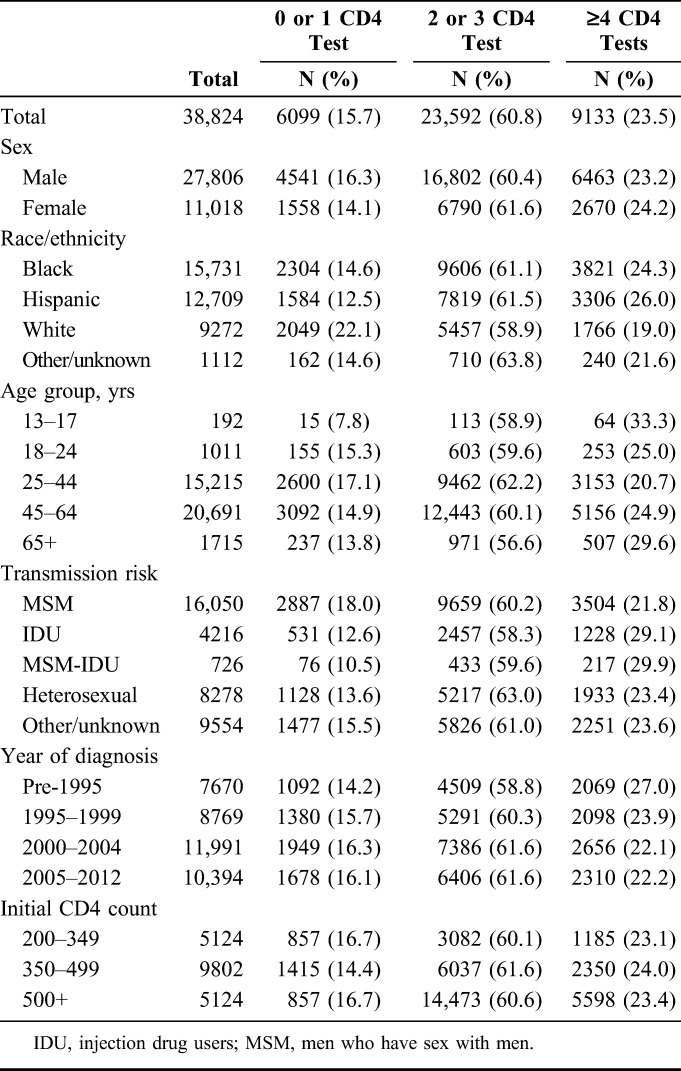
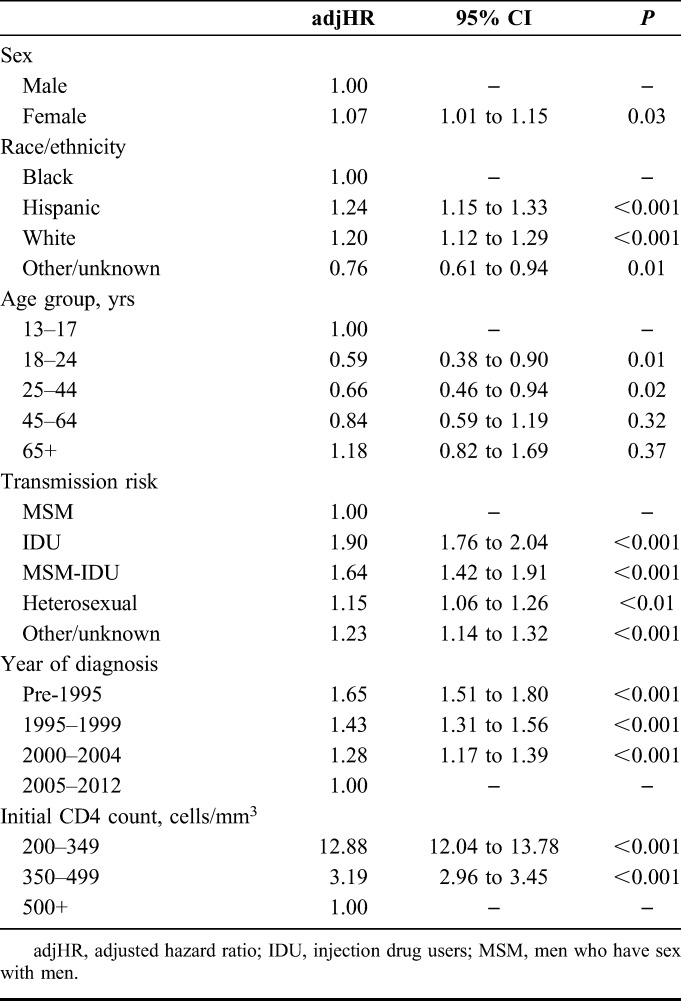
There were a total of 201,546 patient-years of observation in total, with a per-patient mean of 2.7 and a median of 1.9 (IQR, 0.8–4.3) patient-years. Two years after entering the cohort, 71.6%, 93.4%, and 98.1% of those with initial CD4 cell counts of 200–349 (N = 11,025), 350–499 (N = 16,347), and ≥500 cells per cubic millimeter (N = 34,667), respectively, maintained CD4 ≥200 cells per cubic millimeter (Fig. 1). It took 1.5 years for 25% of patients with an initial CD4 of 200–349 to experience a CD4 count <200 cells per cubic millimeter. And even after a median of 5 years of observation, only 4.6% of those with an initial CD4 cell count of ≥500 cells per cubic millimeter experienced a CD4 count <200 cells per cubic millimeter. In 2013, compared to those with initial CD4 ≥500 cells per cubic millimeter, those with CD4 200–349 cells per cubic millimeter and CD4 350–499 cells per cubic millimeter were significantly more likely to have a CD4 dip <200 cells per cubic millimeter, controlling for sex, race/ethnicity, age, transmission risk, and diagnosis year (Table 4).
FIGURE 1.
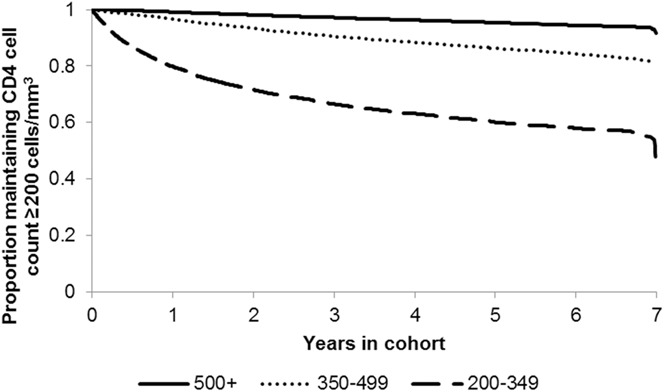
Proportion of stable patients with HIV maintaining CD4 ≥200 cells per cubic millimeter in NYC, by initial CD4 cell count.
TABLE 4.
Factors Associated With CD4 Count Dropping to Below 200 Cells Per Cubic Millimeter Among Stable Patients With HIV (N = 38,824) in NYC, 2013
DISCUSSION
In this large population-based, urban US cohort with well-controlled HIV, initial CD4 at cohort entry was a strong independent predictor of maintaining CD4 ≥200 cells per cubic millimeter. Specifically, the probability of maintaining CD4 ≥200 cells per cubic millimeter for at least 2 years was 96.7% among those with an initial CD4 ≥350 cells per cubic millimeter. These findings support the recent shift in national and local guidelines, which now recommend annual monitoring for patients with consistent viral suppression. The findings also support the growing consensus about CD4 testing in resource-limited settings that was reflected in the proceedings of a September, 2013 WHO expert consultation on the future role of CD4 testing for ART monitoring.14 In addition, these findings provide further support to the decision to decouple CD4 testing from VL testing in stable, virologically suppressed patients and to focus exclusively on the maintenance of virologic suppression as the sole indicator of successful ongoing therapy. Paired with the somewhat diminished role of CD4 cell count staging in determining the need for ART at initial diagnosis,2–4 these findings might signal the waning importance of CD4 testing more broadly, although it is worth noting that the CD4 cell count, particularly when conducted at the point of care, has a key operational role in linkage to HIV care.16
Over the 2007–2013 interval, in the absence of any clinical recommendations to the contrary, clinicians maintained a practice of obtaining almost 3 CD4 measurements per year. In fact, 85% of virologically and immunologically stable patients had testing at least twice yearly. Compared with the frequency of VL testing in 2013 [mean (SD), 2.8 (1.2); median (IQR), 3 (2–4)], the frequency of CD4 measurements implies that many NYC clinicians continued to include CD4 testing on the panel of tests every time that a patient had blood drawn for other testing during this interval despite previous evidence of virologic suppression. Whether practice patterns will change going forward will depend on a number of factors, including provider awareness and understanding of the new recommendations and the ability of clinical practice sites to adjust operationally. A related factor will be the willingness of patients (and providers) to forgo this additional “check” of their immune status. Physician factors can likely be overcome with operational changes, which might include modifying standard laboratory order templates used in HIV primary care settings (to drop CD4 cell count testing); patient factors can likely be managed through thoughtful conversations by providers and other forms of patient education. Ultimately, removal of the CD4 cell count from expected monitoring may diminish patient and provider anxiety that previously arose from natural fluctuations in CD4 cell count values.13
The finding that CD4 testing varied little by year of diagnosis was initially surprising. Anecdotally, patients diagnosed earlier in the epidemic were characterized as being more wedded to following (and perhaps even requesting) this indicator of their health and immune status, even in the face of virologic suppression. But ultimately, those diagnosed before 1995 were only slightly more likely than persons diagnosed in more recent years to have had ≥4 CD4 tests in 2013 (27.0% vs. 22.2% for all other year of diagnosis combined).
In contrast with at least 1 previous analysis,11 we allowed patients to reenter the cohort after they became eligible again (with a stable immune status and a stable viral suppression status for an entire calendar year); we felt that this decision would allow our study population to more accurately reflect the true population of clinically stable patients. Importantly, however, when we repeated our analysis using only one data point for each patient, we found essentially the same results: 2 years after entering the cohort, 72.2%, 93.4%, and 98.3% (vs. 71.6%, 93.4%, and 98.1%) of those with initial CD4 cell counts of 200–349, 350–499, and ≥500 cells per cubic millimeter, respectively, maintained CD4 ≥200 cells per cubic millimeter. Also, in contrast with at least 1 other previous analysis,15 we used a single CD4 drop to <200 as an endpoint. However, in an additional analysis (not shown) using a “confirmed” CD4 drop <200 (ie, 2 CD4 cell counts <200), the findings are even more robust: 2 years after entering the cohort, 94.9%, 99.2%, and 99.8% of those with initial CD4 cell counts of 200–349, 350–499, and ≥500 cells per cubic millimeter, respectively, maintained CD4 ≥200 cells per cubic millimeter.
This analysis has several important limitations. First, the use of surveillance data for the analytic cohort is limited by the fact that only CD4 measurements obtained in the jurisdiction could be considered. This could lead to an underestimate of both the CD4 testing frequency and the probability of the CD4 dipping to <200 cells per cubic millimeter. However, as the number of patients receiving CD4 monitoring in more than one jurisdiction is likely to be small, and as there is no reason to suspect more frequent dips in CD4 counts generated by the same cohort during testing events outside of NYC, this “incomplete” CD4 reporting may have minimal impact on our analysis. Furthermore, other covariates, such as factors that might drive CD4 trajectories or measurement frequency (eg, concomitant chemotherapy, any other immunosuppressive medication, ART use) are not available without chart review. However, our finding that the probability of the CD4 dipping to <200 cells per cubic millimeter was still quite small even with the inclusion of such patients only underscores the minimal likelihood that stable patients without any of these comorbidities will experience a dip to CD4 <200 cells per cubic millimeter. Another major limitation is that the definition of stable viral suppression (VL <400 copies per milliliter) exceeds that used currently for NYC HIV surveillance analyses (VL ≤200 copies per milliliter) and also exceeds that used for years by clinicians. It is a vestige of an early era of less precision in laboratory monitoring. However, if a more strict definition (VL ≤200 copies per milliliter) had been used, we would have expected to observe an even higher probability that stable patients were able to maintain their CD4 count ≥200 cells per cubic millimeter, thereby proving even stronger evidence that stable patients need less frequent CD4 monitoring.
Limitations aside, the implications of these findings are quite clear. As 2 sets of national guidelines already recommend, limited CD4 monitoring is appropriate for patients with CD4 ≥350 cells per cubic millimeter who are stably virologically suppressed. Additional testing in this context is unlikely to require clinical action (eg, initiation of prophylaxis for OIs). And continued CD4 monitoring for virologically suppressed patients whose CD4 counts have been consistently >500 cells per cubic millimeter for at least 2 years may be considered optional. The fact that our analysis uses population-based data derived from a large surveillance data set (rather than data from a clinical trial or program-based cohort) further underscores the relevance and importance of the findings.
As others have reported, these findings also have important fiscal implications.6,13,15 At the New York State Medicaid rate of US $64.93 for a CD4 test (2013), for stable patients with CD4 ≥350 cells per cubic millimeter, NYC in 2013 would have saved ∼US $1.5 million if CD4 monitoring had been limited to twice yearly, and ∼US $3.7 million if CD4 monitoring were limited to once yearly. Cost savings will be even greater in coming years as the number of persons living with HIV in NYC and the US increases, and new guidelines recommending ART for all HIV-infected patients regardless of CD4 count are implemented.2,17,18 Curbing these unnecessary expenses can help reduce the overall cost of HIV care at a time when there is uncertainty in funding. Moreover, forgoing laboratory testing of questionable necessity provides important albeit less tangible benefits such as prevention of provider and patient anxieties surrounding test results of uncertain significance. Future analyses are planned to study the impact of various new sets of clinical guidelines released in 2014 that also advise less frequent monitoring of stable patients and to ensure that these initial findings are robust as patients are followed over a greater number of years of virologic suppression.
ACKNOWLEDGMENTS
The authors acknowledge Franklin Laufer, PhD, for providing the Medicaid rate for a CD4 test and Amina Khawja for assisting with article preparation.
Footnotes
Supported in part by the New York City Department of Health and Mental Hygiene and by a cooperative agreement from the Centers for Disease Control and Prevention for HIV prevention.
Presented in part at the 21st Conference on Retroviruses and Opportunistic Infections, March 3–6, 2014, Boston, MA.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sax PE. Editorial commentary: can we break the habit of routine CD4 monitoring in HIV care? Clin Infect Dis. 2013;56:1344–1346. [DOI] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdoles- centGL.pdf. Accessed November 25, 2015. [Google Scholar]
- 3.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–425. [DOI] [PubMed] [Google Scholar]
- 4.New York State Department of Health AIDS Institute. Diagnostic, Monitoring, and Resistance Laboratory Tests for HIV; Available at http://www.hivguidelines.org/wp-content/uploads/2014/01/diagnostic-monitoring-and-resistance-laboratory-tests-for-hiv.pdf. Accessed November 25, 2015. [Google Scholar]
- 5.Health Resources and Services Administration. HIV Core Clinical Performance Measures; 2014. Available at: http://hab.hrsa.gov/deliverhivaidscare/archivedallages.pdf. Accessed October 5, 2014. [Google Scholar]
- 6.Hyle EP, Sax PE, Walensky RP. Potential savings by reduced CD4 monitoring in stable patients with HIV receiving antiretroviral therapy. JAMA Intern Med. 2013;173:1746–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker JD, Bien CH, Easterbrook PJ, et al. Optimal strategies for monitoring response to antiretroviral therapy in HIV-infected adults, adolescents, children and pregnant women: a systematic review. AIDS. 2014;28(suppl 2):S151–S160. [PubMed] [Google Scholar]
- 8.Keiser O, Chi BH, Gsponer T, et al. Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS. 2011;25:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mermin J, Ekwaru JP, Were W, et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial. BMJ. 2011;343:d6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach [2010 Revision]. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 11.Gale HB, Gitterman SR, Hoffman HJ, et al. Is frequent CD4+ T-lymphocyte count monitoring necessary for persons with counts >=300 cells/muL and HIV-1 suppression? Clin Infect Dis. 2013;56:1340–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girard PM, Nelson M, Mohammed P, et al. Can we stop CD4+ testing in patients with HIV-1 RNA suppression on antiretroviral treatment? AIDS. 2013;27:2759–2763. [DOI] [PubMed] [Google Scholar]
- 13.Chow E, Read T, Chen M, et al. Routine CD4 cell count monitoring seldom contributes to clinical decision-making on antiretroviral therapy in virologically suppressed HIV-infected patients. HIV Med. 2014;16:196–200. [DOI] [PubMed] [Google Scholar]
- 14.Ford N, Stinson K, Davies MA, et al. Is it safe to drop CD4+ monitoring among virologically suppressed patients: a cohort evaluation from Khayelitsha, South Africa. AIDS. 2014;28:2003–2005. [DOI] [PubMed] [Google Scholar]
- 15.Ahn JY, Boettiger D, Law M, et al. Effects of CD4 monitoring frequency on clinical endpoints in clinically stable HIV-infected patients with viral suppression. J Acquir Immune Defic Syndr. 2015;69:e85–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynberg E, Cooke G, Shroufi A, et al. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc. 2014;17:18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torian LV, Xia Q, Wiewel EW. Retention in care and viral suppression among persons living with HIV/AIDS in New York City, 2006-2010. Am J Public Health. 2014;104:e24–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. HIV Surveillance Report, 2011. Atlanta, GA: CDC; 2013. [Google Scholar]


