Abstract
The bioassay-guided fractionation of the aril of M. fragrans (mace spice) yielded five phenolic compounds, one new acyclic bis phenylpropanoid (1) and four previously known phenolic compounds: compounds (1) (S) 1-(3, 4, 5-trimethoxyphenyl)-2-(3-methoxy-5-(prop-1-yl) phenyl)-propan-1-ol, (2) benzenemethanol; α-[1-[2,6-dimethoxy-4-(2-propen-1-yl)phenoxy]ethyl]-3,4-dimethoxy-1-acetate, (3) odoratisol A, phenol, 4-[(2S, 3S)-2,3-dihydro-7-methoxy-3-methyl-5-(1E)-1-propenyl-2-benzofuranyl]-2,6-dimethoxy, (4) 1,3-benzodioxate-5-methanol,α-[1-[2,6-dimethoxy-4-(2-propenyl)phenoxy]ethyl]-acetate, (5) licarin C; benzofuran, 2,3-dihydro-7-methoxy-3-methyl-5-(1E)-1-yl-2-(3,4,5-trimethoxyphenyl). A NMR tube Mosher ester reaction was used in an approach to characterize and determine the assignment of the absolute configuration of the new isolated chiral alcohol (1). The PARP-1 inhibitory activity was evaluated for compound (1) (IC50 = 3.04 μM), compound (2) (IC50 = 0.001 μM), compound (4) (IC50 = 22.07 μM) and compound (5) (IC50 = 3.11 μM). Furthermore, the isolated secondary metabolites were tested for NF-κB and K-Ras inhibitory activities. When tested in the p65 assay, compounds (2) and (4) displayed potent NF-κB inhibition, (IC50 = 1.5 nM and 3.4 nM, respectively).
Keywords: Myristica fragrans, mace, neolignans, acyclic bisphenyl propanoids, PARP-1, NF-κB, K-Ras
1. Introduction
Nutmeg and mace from Myristica fragrans (Houtt) are both Old World spices. M. fragrans is a 9-12 m tall tree with small yellow flowers, native to the Banda Islands in the Maluku Province, Indonesia. This plant is a spice highly consumed by different cultures. The aril of M. fragrans (mace), used in this study, surrounds the seed (nutmeg), and is traditionally used for its culinary and medicinal properties.
Ethnobotanical research has shown that nutmeg and mace are used for stimulant and digestive purposes, and are used in local healing practices to treat certain ailments, such as rheumatism, nervousness, stomach and kidney disorders (Van Gils & Cox, 1994). In addition, M. fragrans is used in traditional Tibetan and Chinese medicine (Duan, Tao, Hao, Gu, & Zhu, 2009). In Ayurvedic medicine, mace is used to treat low fever, asthma, and to alleviate gastro-intestinal complaints (Hattori, et al., 1988). Various biological activities have previously been reported for M. fragrans, such as analgesic, anti-inflammatory (Mueller, Hobiger, & Jungbauer, 2010), antioxidative (Surveswaran, Cai, Corke, & Sun, 2007), antitumor, and antibacterial properties. The uses of M. fragrans, both as a remedy and as a spice, suggest that the active constituents may display potential in prevention and/or treatment of different ailments. Essential oil, fatty acids, glycerides, cyclic, and acyclic bis-phenylpropanoids have previously been identified in M. fragrans (Hattori, et al., 1988). Acyclic bisphenyl propanoids are phenolic secondary metabolites reported only to be found in plants belonging to the Myristicaceae family (Hada, Hattori, Tezuka, Kikuchi, & Namba, 1988).
There is increasing evidence to show that a diet rich in phenolic constituents from food-related plants may have health-beneficial effects (Scalbert, Manach, Morand, Rémésy, & Jiménez, 2005), and may protect against the development of chronic inflammatory diseases (Janega, et al., 2014; Zamora-Ros, et al., 2014). Epidemiological studies suggest that compounds found in food-related plants might protect against various types of cancers, such as lung, colon, prostate, and breast malignancies (Feskanich, et al., 2000; Sun, Yuan, Koh, & Yu, 2006; Tang, Zhou, Wang, Yu, & Ma, 2009; Yan & Spitznagel, 2009). Furthermore, this is supported by studies showing that phytochemical constituents may have potential anticancer activities, e.g.; epigallocatechin-3-gallate found in tea (Chung, Huang, Meng, Dong, & Yang, 1999), genistein from soy (Gong, Li, Nedeljkovic-Kurepa, & Sarkar, 2003), capsaicin found in pepper (Han, Keum, Chun, & Surh, 2002), sulforaphane present in cruciferous vegetables (Dinkova-Kostova, et al., 2002) and curcumin, found in the Indian spice, turmeric (Singh & Aggarwal, 1995).
A study on effects of a phytochemical on regulatory elements involved in transcription and phenotype maintenance, may aid in understanding the mechanism involved in chemoprevention by phytochemicals present in the diet. It has been previously reported that PARP-1 coactivates NF-κB p65 (RelA) during transcription (Hassa, et al., 2005). Inhibitors for these two factors, if found, may be tested for prevention of malignant tumor formation.
The potential health benefits of secondary metabolites found in food-related plants gives scope for identification of a biological target to confer a protective effect against chronic inflammatory conditions. In the present study, isolation of phytochemical constituents from mace (through ethyl acetate partitioning) and an assessment of their effects on PARP-1, NF-κB and K-RAs is reported. The structure-activity relationship of the bioactive constituents is discussed in some detail.
2. Materials and methods
2.1. General
1H, 13C, DEPT, HSQC, and HMBC NMR spectra were measured, using a Bruker Avance 400 MHz spectrometer. The H1-NMR spectra were recorded at 400 MHz, using chloroform-d (CDCl3) and pyridine-d5 solutions (Cambridge Isotope Laboratories, MA, USA). Tetramethylsilane (TMS) was used as an internal standard. Molecular weights were obtained, using a Waters Q-ToF micro mass spectrometer and a Bruker maXis Q-ToF mass spectrometer.
Open column chromatography was performed, using silica gel 60 GF254 (70-230 mesh), Whatman, (GE Healthcare, Piscataway, NJ). Methanol and acetonitrile (Fisher Scientific Fair Lawn, NJ) were used for extraction and isolation. Thin-layer chromatography (TLC) plates F254 (Merck, Darmstadt, Germany) were used to monitor the separation. The TLC plates were developed by using a solution of 10% H2SO4 in ethanol with 1% vanillin and heated to visualize the separation. Preparative and analytical HPLC were carried out, using a Waters 600 controller equipped with a 996 photodiode array detector and a Waters SunFire RP18 column (21.2.× 250 mm, 10 μ m) from Waters (Millford, MA). The isolation was pursued using a trinary solvent system composed of an aqueous phase A (mixture of acetic acid in water (0.025%)) and an organic phase B, composed of methanol and acetonitrile. The optical rotation was obtained in a Perkin Elmer model 345 polarimeter at 20 °C, using a sodium lamp (589 nm). The circular dichronism (CD) measurements were performed using a Jasco Model J-810 spectropolarimeter. The absorbance was measured by using a microplate reader FLUOstar Optima fluorescence plate reader (BMG Labtechnologies GmbH, Inc, Durham, NC, USA). The isolated compounds were characterized and identified by comparing their physical properties and spectroscopic data with literature reports (UV, MS, 1H- and 13C NMR). The carbon assignment for similar compounds has previously been reported (Hattori, et al., 1988).
Compound 2, benzenemethanol, α-[(1, R)-1-[2,6-dimethoxy-4-(2-propen-1-yl)phenoxy]ethyl]-3,4-dimethoxy-1-acetate (2), has previously been reported and described (Duan, Tao, Hao, Gu, & Zhu, 2009).
Compound 3, odoratisol A, phenol, 4-[(2S, 3S)-2, 3-dihydro-7-methoxy-3-methyl-5-(1E)-1-propenyl-2-benzofuranyl]-2,6-dimethoxy (3), has previously been reported and described (Giang, Son, Matsunami, & Otsuka, 2006).
Compound 4, 1,3-benzodioxole-5-methanol,α-[1-[2,6-dimethoxy-4-(2-propenyl)phenoxy]ethyl]-acetate (4) also has previously been isolated, identified, and reported (Forrest, Heacock, & Forrest, 1974; Zacchino & Badano, 1991).
Compound 5, licarin C, benzofuran, 2,3-dihydro-7-methoxy-3-methyl-5-(1E)-1-yl-2-(3, 4, 5-trimethoxyphenyl) (5) has previously been isolated and described (Braz Filho, Mourao, Gottlieb, & Maia, 1976; Wenkert, Gottlieb, Gottlieb, Pereira, & Formiga, 1976).
2.2. Plant material
The dry aril of Myristica fragrans (mace) was purchased at a local Indian grocery market. A voucher specimen was deposited in the research laboratory.
2.3. Extraction, isolation and identification
2.3.1. General
The dry aril of M. fragrans (1.2 kg) was macerated and extracted, using 3 × 2000 ml of methanol to obtain the crude extract. The extract was filtered and dried under vacuum to yield the crude extract. The extract was dissolved in water and sequentially partitioned, using chloroform, ethyl acetate, and butanol (3 × 300 ml). The ethyl acetate layer displayed significant activity in the PARP-1 assay (74% inhibition at 50 mg/ml) and in the NF-κB p65 inhibitory assay and thus was submitted to bioassay-guided isolation. The fractionation was performed over an open column, employing silica gel 60 GF254 (70-230 mesh, Merck) as the stationary phase. The sample was eluted with a gradient composed of a solvent mixture of water: methanol (50→0). The sample was eluted in gradient steps of 100 ml. Each fraction was collected in volumes of 20 ml. During isolation, the separation was monitored, using thin-layer chromatography (TLC). The aluminium plates were pre-coated with silica gel 60 F254. A mixture composed of water and acetonitrile (5:95) was used for separation. The constituents were visualized by spraying with a 10% solution of sulfuric acid and 1% vanillin in ethanol, followed by heating the plates. The fractions were combined according to the results of the TLC analysis. Ten fractions were obtained from this separation (1-10). Analysis of TLC fractions was carried out using reverse-phase HPLC, with a solvent system composed of acetic acid in water (0.025%) (A), and a mixture of methanol and acetonitrile (B), with a gradient of 95:5→0:100, run over 45 minutes. The column was washed by using 100% of organic solvent for 10 minutes, before going back to initial conditions (95:5). The separation was monitored at λ=224 nm.
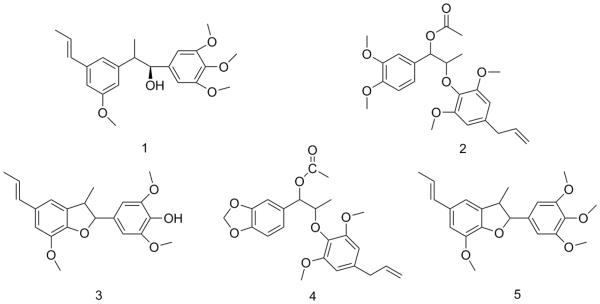
The active fraction was re-chromatographed. Further purification of the active mixture was achieved on a preparative HPLC system with a Waters® SunFire column (10 × 150 mm, 10 μ m), using a trinary solvent system composed of acetic acid in water (0.025%) as eluent mixture A and a mixture of methanol and acetonitrile (1:1) as eluent mixture B. The peaks were chromatographically separated under isocratic conditions (30:70 of solvents A and B) for a duration of 45 minutes. Each run was followed by washing the column with 100% of solvent B, before restoring initial conditions (30:70). The flow-rate during the chromatographic separation was 5 ml/min. Eight peaks were collected at the following time-points; tR= 10 min (2.6 mg), tR = 15 min (6.0 mg), tR = 19 min (22.5 mg) tR 21= min (11.9 mg), tR = 22 min (14.7 mg) and tR = 24 min (22.2 mg), tR = 28 min (4.2 mg) and tR = 40 min (7.8 mg), respectively. Five neolignans were obtained and characterized from this separation (Fig. 1). Compound 1 (22.2 mg) had a retention time of tR = 24 min, compound 2 (22.5 mg) had a tR = 19 min, compound 3 (14.7 mg) had a retention time tR = 22 min, compound 4 (4.2 mg) had a tR = 28 min, and compound 5 (7.8 mg) had a tR = 40 min. The identification was achieved by MS and NMR analysis, and by comparing the obtained spectroscopic data of each compound with previous literature on M. fragrans (Hattori, et al., 1988). The 1H and 13C NMR spectra were determined and the optical rotation was obtained.
Fig 1.
Chemical structures of compounds (1-5) from the ethyl acetate extract of M. fragrans.
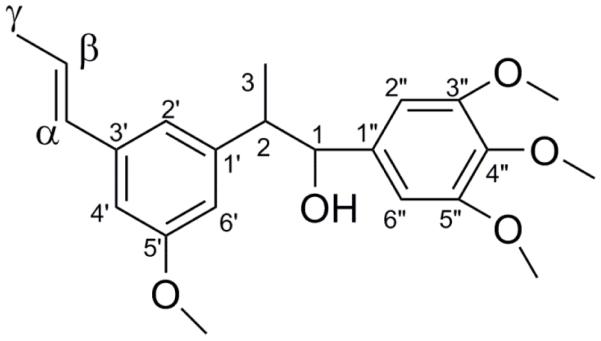
To determine the absolute configuration at C-1 in compound 1 (Fig. 2.), preparation of both the (R) and (S)-MTPA ester derivatives of 1 was pursued following the previously described modified Mosher method (Su, et al., 2002). The amount of 0.5 mg of compound 1 was transferred, using deuterated pyridine-d5 (0.6 ml), to a NMR tube. The chiral substrate was dispensed into the corresponding NMR tube under addition of argon gas. (S)-(+)-α-Methoxy-α-(trifluoromethyl)phenylacetyl chloride (MTPA Cl) (6 μl) was used as a substrate to obtain the (R)-MTPA ester derivative, and (R)-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride was used to obtain the (S)-MTPA ester derivative. Subsequently, the sample mixtures were covered and evenly shaken, the mixtures were allowed to react for 6 h. The chiral substrate MTPA-chloride was coupled to the chiral secondary alcohol to obtain the two configurations. The obtained (R)- and (S)-MTPA Cl ester derivatives were analyzed by 1H-NMR.
Fig 2.
The structure of the novel compound (S) 1-(3, 4, 5-trimethoxy phenyl)-2-(3-methoxy-5-(prop -1-yl) phenyl)-propan-1-ol (1).
2.3.2. (S) 1-(3,4,5-Trimethoxy phenyl)-2-(3-methoxy-5-(prop-1-yl) phenyl)-propan-1-ol (1)
Compound 1 was obtained as a yellow oil; UV (methanol) λmax (log ε) 210 (3.89), 268 nm (4.00): [α] D20 +18 (c 1.0, MeOH). CD (MeOH) λmax (Δε) 245 (−3.67116), 270 (6.65641). The molecular mass was determined (m/z 395.14). The molecular formula was determined to be C22H28O5 on the basis of the positive HRESIMS: m/z 395.1620 [M+Na]+. The formula requires 7° of unsaturation. For 1H NMR and 13C NMR resonances see Table 1.
Table 1.
NMR spectral data of 1H and 13C for (1) (S) 1-(3, 4, 5-trimethoxy phenyl) - 2-(3-methoxy-5-(prop -1-yl) phenyl)-propan-1-ol (in 400 MHz, chloroform-d6)
| Position | Compound 1 (CDCl3) | |
|---|---|---|
| δ C | δH (J in Hz) | |
| 1 | 94.4 | 5.10 (d, J = 9.6) |
| 2 | 46.2 | 3.50 (s) |
| 3 | 18.2 | 1.41 (d, J = 6.7) |
| 1’ | 133.7 | |
| 2’ | 113.8 | 6.77 (s) |
| 3’ | 131.4 | |
| 4’ | 114.6 | 6.82 |
| 5’ | 144.6 | |
| 6’ | 106.1 | 6.46(s) |
| 1” | 132.6 | |
| 2” | 109.3 | 6.98 (s) |
| 3” | 146.3 | |
| 4” | 150.9 | |
| 5” | 146.3 | |
| 6” | 109.3 | 6.98(s) |
| α | 131.4 | 6.40 (dd, J = 1.9, 16) |
| β | 123.6 | 6.10 (dd, J = 6.5, 16) |
| γ | 19.0 | 1.89 (d J = 1.9, 6.5) |
| O-Me | 56.4 | 3.90 (s) |
2.4. PARP-1 inhibitory assay
Poly (adenosine 5’-diphosphate (ADP)-ribose) polymerase 1(PARP-1) is a nuclear chromatin enzyme involved in single strand DNA breaks (SSB). PARP-1 is activated by DNA damage. The small molecules that inhibit the PARP-1 enzyme lead to accumulation of DNA damage, and potentiate anticancer activities by increasing the sensitivity of cancer cells to cytotoxic agents and instigating apoptosis. (Muñoz-Gámez, et al., 2005) The isolated secondary metabolites (1-5) from M. fragrans were tested by using the PARP-1 Chemoluminescent Activity Assay Kit (BPS Bioscience). First, the 96-well plate was coated with histones, by adding 50 μl of prepared histone solution to each well and allowed to incubate at 4°C overnight. The plates were washed three times with ice-cold PBS-Tween (200 μl), before adding blocking buffer to each well. The plates were washed three times with ice-cold PBS-Tween. A reaction mixture, containing activated DNA, PARP-1 buffer, and assay buffer, was added to each well. The plates were washed three times with ice-cold PBS-Tween. The samples tested were diluted in dimethylsulfoxide (10%) and added to the corresponding wells before the addition of PARP-1. The plate was covered and incubated at 20°C for 1 h. The plate was washed three times, before streptavidin-HRP was added to each well, and allowed to incubate at 20°C for 30 min. The chemiluminescent solution was added to each well and luminescence was detected and measured at 410 nm, using a Fluostar Optima plate reader (BMG Labtech Inc).
2.5. NF-κB inhibitory assay
The HeLa human cervical cancer cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and with100 IU/ml of penicillin and streptomycin from Gibco (Rockville, MD, USA). The cells were cultured in 75 ml tissue flasks at a temperature of 37°C in an atmosphere containing 5% CO2. The NF-kB assay was carried out according to previously established protocol (Kim, Lau, Pan, & Carcache De Blanco, 2010). Briefly, a nuclear extract was prepared from the treated and untreated HeLa cells. The analysis of the effects on the NF-κB inflammatory pathway was studied, using a specific ELISA binding assay with a luminescent detection system. The inhibitory effect on transcription factor NF-κB p65 was evaluated in HeLa cells, using the Transcription Assay System (Pierce Biotechnology, Rockford, IL). This allowed the assessment of the binding affinity of the NF-κB subunit p65 to its corresponding biotinylated consensus sequence. The luminescence readings detected in the treated samples were compared to the negative control, treated with tumor necrosis factor-α (TNFα) (Thermo Scientific, Rockford, IL, USA). The inhibitory concentration (IC50) was calculated for the tested compounds and compared to the positive control rocaglamide (IC50 = 0.08 μM) (Enzo Life Sciences, Inc. Farmingdale, NY, USA). Luminescence was detected by using a FLUOstar Optima plate reader (BMG Labtech Inc, Durham, NC). The obtained data were analyzed using Table Curve 2D v4.
2.6. K-Ras inhibitory assay
The assay was performed by following a previously published protocol (Muñoz Acuña, Matthew, Pan, Kinghorn, Swanson & Carcache de Blanco, 2013). The human colorectal HT-29 cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and was cultured in Roswell Park Memorial Institute Media (RPMI-1640) supplemented with 100 IU/ml of penicillin and streptomycin from Gibco (Rockville, MD, USA). The cells were treated and incubated at a temperature of 37°C, in an atmosphere containing 5% CO2, for 3h. This was followed by the removal of the medium and they were then washed three times with phosphate-buffered saline (PBS) and treated with EGF solution (5 ng/ml) for 2 min. Protease inhibitor was added and the cells were then lysed. The aliquots were stored at a temperature of − 80 °C. The protein concentration was determined in each sample, before the K-Ras activity was assessed, by using the Ras GTPase Chemi Elisa kit from Activity Motif (Carlsbad). The primary K-Ras antibody (1:5000) was added to each well and incubated at room temperature for 1h. Subsequently, the HRP-conjugated secondary antibody was used for detection. Chemiluminescence solution was added, and the luminescence was read, using a Fluostar Optima plate reader (BMG Labtech Inc).
2.7. RB Cytotoxicity assay
The anti-proliferative effects of the isolated neolignans from mace were tested on the human colorectal HT-29 cell line, using a previously published protocol (Muñoz Acuña, Nighat, Ahmed, Chang, & Carcache de Blanco, 2013). The cells were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS) and 100 IU/ml of penicillin and streptomycin, both from Gibco (Rockville, MD, USA). The cells were grown in T75 tissue culture flasks as a monolayer at a temperature of 37°C and in 5% CO2. The compounds were diluted in 10 % dimethylsulfoxide and the cells were subsequently treated in a concentration range (0.25 ng/ml-25 μg/ml). The cell suspension was seeded in a 96-well plate (1×105 cells per well) and treated with serial dilutions of the isolated compounds (1-5) and incubated for 72 h at a temperature of 37°C and in an atmosphere of 5% CO2. The cells were fixed, using 100 μl of ice-cold trichloroacetic acid (20%) for 30 min at a temperature of 4°C. The cells were then stained by adding 100 μ l of sulforhodamine (SRB) (0.4%) and kept at a temperature of 20 °C for 30 min, before washing the wells three times with acetic acid (1%) and then letting them air-dry. The stained cells with the bound dye were solubilized by adding 200 μl of Tris base solution (10 mM) to each well. Then, the 96-well plate was placed on a shaker for 5 min. The absorbance reading was performed at a wavelength of 515 nm, using the FLUOstar Optima plate reader (BMG Labtech Inc, Durham, NC). The absorbance values were compared to the untreated control samples and effective concentration (EC50) was calculated, using Table Curve 2Dv4 (System Software Inc., San Jose, CA, USA). Paclitaxel was used as a positive control. The assay was run in triplicate.
2.8. tatistical analysis
Experimental results are presented as means ± SEM and all measurements and analyses were carried out in triplicate. Excel and TableCurve 2D 4v were used for statistical and graphical evaluations in this study.
3. Results and discussion
3.1. Isolation and structure elucidation
Briefly, a new acyclic bis phenylpropanoid was isolated from mace, using a preparative HPLC system with a trinary eluent solvent system composed of a mixture of acetic acid (0.0025%), methanol and acetonitrile. Compound 1 was isolated as yellow oil and identified using NMR spectrometric methods. The HRESIMS data, giving m/z 395.1620 [M+Na]+, established the molecular formula as C22H28O5. The 1H NMR spectrum exhibited two methyl group signals at δH 1.41 ppm (3H, J=6.7 Hz) and δH 1.89 ppm (3H, d J=6.5, 1.9 Hz), respectively. The integration of the signals at δH 3.90 ppm (3H, s) allowed the identification of four methoxy groups. A methine group was detected at δH 3.50 ppm (1H, s), and the spectrum showed a signal of an oxygenated methine at δH 5.10 ppm (1H, d, J= 9.6). The vinylic protons displayed signals at δH 6.10 ppm (1H, dd J= 6.5, 16 Hz) and at δH 6.40 ppm (1H, dd J= 16, 1.9 Hz). Three aromatic signals were found at δH 6.46 ppm (1H, s), δH 6.77 ppm (1H, s) and 6.82 ppm (1H, m) (1H, s). The integration of signals at δH 6.98 ppm (2H, s) allowed the identification of two aromatic protons.
In the 13C spectrum, the carbon signals at δc 132.6 (C-1”), 109.3 (C-2”) (C-6”), 146.3 (C-3”) (C-5”) 150.9 ppm (C-5”) indicated the presence of a 1, 2, 3-trimethoxy-5-methylbenzene moiety. The carbon shifts at δc at 133.7 (C-1’), 113.8 (C-2’), 131.2 (C-3’), 114.6 (C-4’), 144.6 (C-5’), 106.1 (C-6’), and 131.4 (C-α), 123.6 (C-β), and δc 19.0 ppm (C-γ) suggested the presence of a 1-methoxy -3-methyl-5(prop-1-en-yl)benzene moiety in the structure. This was confirmed by the long-range correlations in the HMBC experiment, where the proton at δH 6.46 ppm showed correlation to carbon at δc 113.8 (C-2’) and 133.7 ppm (C-1’). Similarly, this was confirmed by the long-range heterocoupling signals in the HMBC spectrum. The proton at δH 6.82 ppm (H-4’) showed correlation to carbons at δc 133.7 (C-1’), 144.6 (C-5’), and 106.1 (C-6’) ppm. The proton at δH 6.77 ppm showed correlation to carbons at δc 133.7 (C-1’), 47.0 (C-2) and 113.8 (C-2’) ppm. The protons at δH 1.90 ppm (H-γ) showed long range correlations to carbons at δc 123.6 (C-β) and 131.3 ppm (C-α). Carbon signals of a methyl group with carbon shifts δc at 18.2 ppm (C-3) were detected upfield in the carbon spectrum; a methine at δc 47.0 ppm (C-2) and an oxymethine at δc 94.4 ppm (C-1) were also displayed in the spectrum.
The long range correlations (HMBC) were between protons at δH 1.40 ppm (H-3) of the methyl group and carbon at δc 94 ppm (C-1), δc 47.0 ppm (C-2) and with carbon at δc 133.7 ppm (C-1’). The proton at δH 3.90 ppm (O-CH3) showed correlation to carbon δc 146.3 (C-3”) (C-5”) and 150.9 ppm (C-4”). The proton at δH 3.50 ppm (H-2) showed HMBC correlations to carbons at δc 94.4 ppm (C-1), 47.0 ppm (C-2) and 132.6 (C-1”) ppm. The proton at δH 5.10 ppm (H-1) showed correlations to carbons at δc 94.4 (C-1), 47.0 (C-2), 18.2 (C-3), 132.6 (C-1”) and 113.8 (C-2’) ppm. Thus, the complete assignment of the acyclic bis-phenylpropanoid structure of (1) was achieved through the HMBC experiment.
The COSY experiment confirmed the assigned structure and showed 2D correlation between protons at δH 3.50 (H-2) and 5.10 ppm (H-1), and correlations between δH 6.10 ppm (H-β) and δH 6.40 ppm (H-α). Moreover, the NOESY experiment showed correlation between the vicinal protons at 3.50 (H-2), 5.10 (H-1), and 6.98 (H-2”) ppm.
Compound 1 was identified upon comparison with a similarly assigned structure (Wenkert, et al 1976).
The absolute configuration determines the biological and pharmacological activity; thus, it is of great significance to characterize this property on bioactive molecules. The presence of the secondary hydroxyl group on C-1 was used to empirically determine the absolute configuration by employing two chiral substrates, (S)-and (R)-MTPA-chloride, to create the two Mosher ester derivatives with two different stereocentres. The procedure describing the configuration of the stereocentre of a secondary alcohol was previously described (Su, et al., 2002). The stereochemical assignment was based on comparison of the two 1H-NMR spectra. This comparison allowed the assessment of the diamagnetic effect of the α-phenyl group present in the MTPA chloride moiety on the selected proton shifts of H-3. When comparing the two pairs of signals for H-3, the two isomers were distinguishable by the difference in chemical shift δ-values (ΔδS-R). The 1H-NMR spectra were assessed for each one of the enantiomers, 1s and 1r and the difference between the two signals for H-3 of the two chiral residues showed a negative ΔδSR value of −0.002 ppm (Fig. 3) (Ren, et al., 2012). The shielded protons displayed negative ΔδSR values (Su, et al., 2002) and compound (1) was assigned as (S) 1-(3, 4, 5-trimethoxy phenyl)-2-(3-methoxy-5-(prop -1-yl) phenyl)-propan-1-ol.
Fig 3.
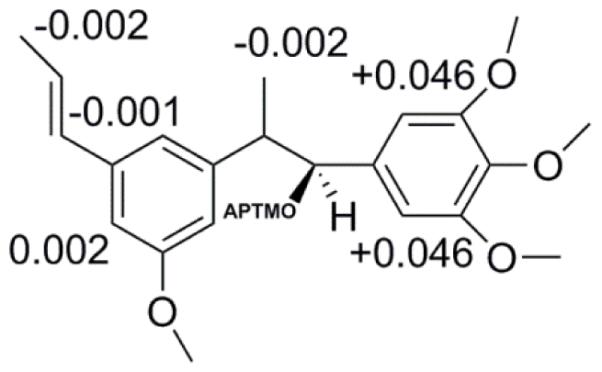
Results obtained from Mosher ester reaction. Illustration of difference in chemical shift δ-values (ΔδS-R) of (1) (S) 1-(3, 4, 5-trimethoxy phenyl)-2-(3-methoxy-5-(prop -1-yl) phenyl)-propan-1-ol.
Compound 2 was isolated as yellow oil. The obtained chemical formula was C24H30O7, calculated for m/z [M+Na]+ 453.11. The 1H, 13C, DEPT, HSQC, and HMBC experiments were used for structural assignment and identification. Based on comparison with literature data, compound (2) was identified as benzenemethanol, α-[(1, R)-1-[2,6-dimethoxy-4-(2-propen-1-yl)phenoxy]ethyl]-3,4-dimethoxy-,1-acetate (Duan, et al., 2009).
Compound 3 was isolated as yellow oil. The m/z 379.12 [M+Na]+ corresponded to a chemical formula of C21H24O5. The proton spectrum was compared to previously published data and led to the identification of phenol, 4-[(2S, 3S)-2, 3-dihydro-7-methoxy-3-methyl-5-(1E)-1-propenyl-2-benzofuranyl]-2,6-dimethoxy (odoratisol A) (Giang, et al., 2006)
Compound 4 was isolated as yellow oil. With a m/z of 437.12 [M+Na]+. The chemical formula corresponded to C23H26O7. The 1H, 13C, DEPT, HSQC and HMBC experiments were used for structural assignment and identification. Based on comparison with literature data, compound (3) was identified as 1,3-benzodioxole-5-methanol,α-[1-[2,6-dimethoxy-4-(2-propenyl)phenoxy]ethyl]-acetate (Forrest, et al., 1974) (Zacchino & Badano, 1991).
Compound 5 was isolated as yellow oil. The elemental formula C22H26O5 was calculated for m/z [M+Na]+ 393.16. The 1H, 13C, DEPT, HSQC and HMBC experiments were used for structural assignment and identification. Based on comparison with literature data, compound (4) was identified as benzofuran, 2, 3-dihydro-7-methoxy-3-methyl-5-(1E)-1-yl-2-(3, 4, 5-trimethoxyphenyl) (Aiba, et al., 1977; Kapooret, et al., 2013; Wenkert, et al., 1976).
3.2. PARP-1 inhibition in vitro
The poly (adenosine 5’-diphosphate (ADP)-ribose) polymerase (PARP-1) is a nuclear chromatin-bound enzyme involved in single strand DNA breaks (SSB). PARP-1 is activated by DNA damage. The small molecules that inhibit the PARP-1 enzyme potentiate anticancer activities by increasing the sensitivity of cancer cells to cytotoxic agents.
The EtOAc layer of M. fragrans displayed 78% inhibition when tested in a PARP-1 enzyme assay. The fractionation of the EtOAc layer yielded a fraction with enriched PARP-1 inhibitory activity (82% at 50 μg/ml). Further, bioassay-guided isolation led to five secondary metabolites from M. fragrans (1-5). Although the four isolated compounds displayed inhibitory activity, compound (2) showed the highest PARP-1 inhibitory activity when tested by the enzyme PARP-1 inhibitory assay. PARP-1 activity was displayed by compound (1) (IC50 = 3.04 μM), compound (2) (IC50 = 0.001 μM), compound (3) (IC50 = 22.1 μM) and compound (4) (IC50 = 3.11 μM).
PARP-1 inhibitory activity has an anti-proliferative effect in malignant cancer cells. It has been shown that PARP-1 inhibition targets, particularly, breast and ovarian cancers with lack of functioning repair proteins BRCA 1 and BRCA 2. PARP-1 inhibitors have been shown to be clinically effective for treatment of hereditary triple negative breast tumors that display loss of function of BRCA 1 and BRCA 2 mutations. Since the active compound originated from a diet-based product (mace), it might be possible to develop this compound as part of a dietary supplement formulation.
3.3. NF-κB p65 inhibitory assay in vitro
The bioassay-guided fractionation of mace led to the isolation of five neolignans (1-5) that were tested for NF-κB activity. The EtOAc layer displayed 39% inhibition in the NF-κB p65 inhibitory assay. Both compound (2) and compound (4) showed potent NF-κB inhibitory activity when tested in HeLa cells (Table 2). Compounds (2) and (4) displayed NF-κB inhibitory concentrations of IC50 = 1.5 nM and IC50 = 3.4 nM, respectively. The inhibitory effect was compared to rocaglamide (IC50 = 75 nM). The transcription factor NF-κB is associated with inflammation and cancer progression, and the results suggested that these neolignans might target inflammatory responses, particularly in malignant cells.
Table 2.
Cytotoxic activity, K-Ras, PARP-1, and NF-κB inhibition of secondary metabolites from mace (1- 5).
| Compound | NF-κB (HeLa)a | K-Ras (HT-29) | PARP-1 | Cytotoxicity (HT- 29)b |
|---|---|---|---|---|
| IC50 (μM) | IC50(μM) | IC50(μM) | IC50(μM) | |
| 1 | Not active | 10.5 | 3.04 | - |
| 2 | 0.0015 | Not active | 0.001 | 3.07 |
| 3 | 62 | Not tested | Not tested | Not tested |
| 4 | 0.0034 | Not active | 22.07 | 0.024 |
| 5 | Not active | Not active | 3.11 | 0.003 |
Data presented as IC50 values with rocaglamide used as the positive control (IC50= 0.075 ± μM).
Data presented as IC50 values with paclitaxel used as the positive control (IC50= 0.5 ± 0.6nM)
The transcription factor NF-κB promotes survival signalling and triggers the resistance of cancer cells to certain tumorigenic agents and chemotherapy (Lerebours, et al.,) . The NF-κB is a heterodimer composed by p65 bound to IκB. The IκB kinase (IKK) is responsible for phosphorylation of IκB. The release from IκB in the cytosol, activates NF-κB before it is translocated to the nucleus (Perkins, 2007). The NF-κB pathway is induced by cytokinin TNF-α (Yin, Chen, & Shu, 2009). This regulatory element exerts its anti-apoptotic effects in malignant cancer cells and it is hypothesized that targeting this regulatory element may sensitize tumor cells to undergo programmed cell death. Thus, NF-κB inhibition triggers apoptosis in cancer cells and induces apoptosis selectively in cancer cells (Darnell, 2002).
The aril of M. fragrans (mace) is a rich source of bio-active neolignans. While compound (1) showed no NF-κB inhibitory activity, suggesting that the hydroxyl substitution in position C-1 interfere with the structure-activity of neolignans (Lin, et al., 1988), the activity of the two esters (2) and (4) suggested that the acetate function may have an essential role in mediating NF-κB inhibition. Also, the presence of four methoxy-substituted groups in both aromatic rings seems to be involved in mediating NF-κB inhibition. Thus, this study and the chemical characterization of neolignans may lead to the optimization of potent NF-κB inhibitors in the near future.
3.4. In vitro K-Ras inhibitory activity
The K-Ras activity was assessed in HT-29 colon cancer cells treated with compounds 1-4. The inhibitory concentration for compound 1 was IC50 = 10.5 μM. The results indicated that the hydroxyl group may be pivotal in mediating K-Ras inhibition; however, structural modifications may lead to optimization of K-RAs inhibitory activity. Since only compound 1 displayed activity of the neolignans in this study, it is suggested that K-Ras inhibition is mediated by a different mechanism of action. The growth factor EGF was used to stimulate the K-Ras pathway. The growth factor EGF is the ligand of the cell membrane-bound receptor EGFR, and induces the intracellular K-Ras protein downstream. The oncogenic K-Ras mutations are commonly found in colon cancers (Nandan, et al., 2008; Telang, Lane, Nelson, Arumugam, & Chesney, 2007). According to previous studies, K-Ras may provide a possible target to prevent the progression of colon cancer (Karapetis, et al., 2008).
3.5. Cytotoxicity in vitro
It has previously been shown that naturally occurring phenyl propanoids are cytotoxic against leukemia (Liu et al., 2006). In this study, we tested the cytotoxicity of the compounds isolated from the aril of M. fragrans in HT-29 human colon cancer cell lines (Table 2). Three of the four compounds showed activity when tested in the SRB-assay. Compound 2 (EC50 = 3.07 μM), compound 4 (EC50 = 0.024 μM) and compound 5 (EC50 = 0.003 μM) showed cytotoxicity in the SRB assay when tested against HT-29 colon cancer cell lines. Compound 3 showed moderate cytotoxicity and compound 4 displayed the highest cytotoxicity in HT-29 cells, when the activity was compared to the positive control paclitaxel (EC50 = 0.0005 μM). This is supported by previous studies that have shown that neolignans exhibit antiproliferative activities against HT-29 colon cancer cells (Kong, Tzeng, & Liu, 2005).
4. Conclusion
The phytochemical investigation of mace afforded five compounds (1-5). The neolignans isolated from mace displayed inhibitory effects on mediators in the inflammatory pathway and, furthermore, showed antiproliferative effects. Bioassay-directed fractionation led to the identification of PARP-1 inhibitory secondary metabolites and NF-κB inhibitors. These findings suggested that neolignans present in food-plants may have health-beneficial effects, possibly in the management of disease states associated with inflammatory conditions. The results of this study warrant future chemopreventive studies, since the secondary metabolites in mace display antineoplastic effects and inhibit regulatory elements involved in DNA maintenance. In summary, the aril of M. fragrans is a sustainable source of neolignans and it comprises a promising source of new phytonutrients with potential great health-beneficial effects.
Highlights.
*Myristica fragrans, a highly consumed spice for its culinary and medicinal properties.
*The present study discovered a new acyclic with K-ras activity that can be chemically optimized.
*This is the first report of neolignans from M. fragrans with PARP-1 and NF-κB inhibitory effects.
Acknowledgements
We would like to thank Dr. Craig McElroy for facilitating the use of NMR instrumentation. The authors are also thankful to Dr. Li Pan for kind guidance and assistance with instrumentation. The authors would like to acknowledge Mr. Mark Apsega and Andrew VanSchoiack for assisting with mass spectrometry (NSF award 1040302) as a purchase service. This work was partially supported by the Diversity Supplement P01 CA125066-S2, funded by the National Cancer Institute, NIH, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Braz Filho R, Mourao JC, Gottlieb OR, Maia JGS. Lanthanide induced shifts as an aid in the structural determination of eusiderins. Tetrahedron Letters. 1976;15:1157–1160. [Google Scholar]
- Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Research. 1999;59:4610–4617. [PubMed] [Google Scholar]
- Darnell JE., Jr. Transcription factors as targets for cancer therapy. Nature Reviews Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proceedings of National Academy of Sciences U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Tao HW, Hao XJ, Gu QQ, Zhu WM. Cytotoxic and antioxidative phenolic compounds from the traditional Chinese medicinal plant, Myristica fragrans. Planta Medica. 2009;75:1241–1245. doi: 10.1055/s-0029-1185506. [DOI] [PubMed] [Google Scholar]
- Feskanich D, Ziegler RG, Michaud DS, Giovannucci EL, Speizer FE, Willett WC, Colditz GA. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. Journal of National Cancer Institute. 2000;92:1812–1823. doi: 10.1093/jnci/92.22.1812. [DOI] [PubMed] [Google Scholar]
- Forrest JE, Heacock RA, Forrest TP. Diarylpropanoids from nutmeg and mace (Myristica fragrans Houtt.) Journal of the Chemical Society, Perkins Transaction 1 Soc Perkin 1. 1974;2:205–209. doi: 10.1039/p19740000205. [DOI] [PubMed] [Google Scholar]
- Giang PM, Son PT, Matsunami K, Otsuka H. New neolignans and lignans from vietnamese medicinal plant Machilus odoratissima Nees. Chemical Pharmaceutical Bulletin. 2006;54:380–383. doi: 10.1248/cpb.54.380. [DOI] [PubMed] [Google Scholar]
- Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702–4709. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- Hada S, Hattori M, Tezuka Y, Kikuchi T, Namba T. Constituents of mace. Part 3. New neolignans and lignans from the aril of Myristica fragrans. Phytochemistry. 1988;27:563–568. [Google Scholar]
- Han SS, Keum YS, Chun KS, Surh YJ. Suppression of phorbol ester-induced NF-kappaB activation by capsaicin in cultured human promyelocytic leukemia cells. Archives of Pharmaceutical Research. 2002;25:475–479. doi: 10.1007/BF02976605. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, Gersbach M, Imhof R, Hottiger MO. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. Journal of Biological Chemistry. 2005;280:40450–40464. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- Hattori M, Yang XW, Shu YZ, Kakiuchi N, Tezuka Y, Kikuchi T, Namba T. Constituents of mace. Part IV. New constituents of the aril of Myristica fragrans. Chemical and Pharmaceutical Bulletin. 1988;36(2):648–653. [Google Scholar]
- Janega P, Klimentová J, Barta A, Kovácsová M, Vranková S, Cebová M, Čierna Z, Matúsková Z, Jakovljevic V, Pechánová O. Red wine extract decreases pro-inflammatory markers, nuclear factor-κB and inducible NOS, in experimental metabolic syndrome. Food Functional. 2014;5:2202–2207. doi: 10.1039/c4fo00097h. [DOI] [PubMed] [Google Scholar]
- Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. The New England Journal. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- Kim JA, Lau EK, Pan L, De Blanco EJ. NF-kappaB inhibitors from Brucea javanica exhibiting intracellular effects on reactive oxygen species. Anticancer Research. 2010;30:3295–3300. [PMC free article] [PubMed] [Google Scholar]
- Kong ZL, Tzeng SC, Liu YC. Cytotoxic neolignans: an SAR study. Bioorganic and Medicinal Chemistry Letters. 2005;15:163–166. doi: 10.1016/j.bmcl.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Lerebours F, Vacher S, Andrieu C, Espie M, Marty M, Lidereau R, Bieche I. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer. 2008;8:41. doi: 10.1186/1471-2407-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E. Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure-activity study. Molecular Pharmacology. 1988;34:200–208. [PubMed] [Google Scholar]
- Liu H, Jensen KG, Tran LM, Chen M, Zhai L, Olsen CE, Søhoel H, Denmeade SR, Isaacs JT, Christensen SB. Cytotoxic phenylpropanoids and an additional thapsigargin analogue isolated from Thapsia garganica. Phytochemistry. 2006;67:2651–2658. doi: 10.1016/j.phytochem.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chemistry. 2010;122:987–996. [Google Scholar]
- Muñoz Acuña U, Matthew S, Pan L, Kinghorn AD, Swanson Steven M, Carcache de Blanco Esperanza J. Apoptosis Induction by 13-Acetoxyrolandrolide through the Mitochondrial Intrinsic Pathway. Phytotherapy Research. 2013;28:1045–1053. doi: 10.1002/ptr.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz Acuña U, Nighat F, Ahmed S, Chang LC, Carcache de Blanco EJ. Apoptotic effect of wortmannolone on cancer cells through potent ROS induction. International Journal of Cancer Research. 2013;47(2):1185–1195. [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Gámez JA, Martín-Oliva D, Aguilar-Quesada R, Cañuelo A, Nuñez MI, Valenzuela MT, Ruiz de Almodóvar JM, De Murcia G, Oliver FJ. PARP inhibition sensitizes p53-deficient breast cancer cells to doxorubicin-induced apoptosis. Biochemical Journal. 2005;386:119–125. doi: 10.1042/BJ20040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J, Babbin BA, Robine S, Yang VW. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008;134:120–130. doi: 10.1053/j.gastro.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nature Reviews Molecular Cell Biology. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Ren Y, Muñoz Acuña UM, Jimenez F, Garcia R, Mejia M, Chai H, Gallucci JC, Farnsworth NR, Soejarto DD, Carcache de Blanco EJ, Kinghorn AD. Cytotoxic and NF-kappaB inhibitory sesquiterpene lactones from Piptocoma rufescens. Tetrahedron. 2012;68:2671–2678. doi: 10.1016/j.tet.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Critical Reviews of Food Science and Nutrition. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] Journal Biological Chemistry. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Su BN, Park EJ, Mbwambo ZH, Santarsiero BD, Mesecar AD, Fong HH, Pezzuto JM, Kinghorn AD. New chemical constituents of Euphorbia quinquecostata and absolute configuration assignment by a convenient Mosher ester procedure carried out in NMR tubes. Journal of Natural Products. 2002;65:1278–1282. doi: 10.1021/np0202475. [DOI] [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis. 2006;27:1301–1309. doi: 10.1093/carcin/bgl024. [DOI] [PubMed] [Google Scholar]
- Surveswaran S, Cai Y-Z, Corke H, Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chemistry. 2007;102:938–953. [Google Scholar]
- Tang NP, Zhou B, Wang B, Yu RB, Ma J. Flavonoids intake and risk of lung cancer: a meta-analysis. Japanese Journal of Clinical Oncologl. 2009;39(6):352–359. doi: 10.1093/jjco/hyp028. [DOI] [PubMed] [Google Scholar]
- Telang S, Lane AN, Nelson KK, Arumugam S, Chesney J. The oncoprotein H-RasV12 increases mitochondrial metabolism. Molecular Cancer. 2007;6:77. doi: 10.1186/1476-4598-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gils C, Cox PA. Ethnobotany of nutmeg in the Spice Islands. Journal of Ethnopharmacology. 1994;42:117–124. doi: 10.1016/0378-8741(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Wenkert E, Gottlieb HE, Gottlieb OR, Pereira M. O. d. S., Formiga MD. Carbon-13 nuclear magnetic spectroscopy of naturally occurring substances. Part 44. Carbon-13 NMR spectroscopy of neolignans. Phytochemistry. 1976;15:1547–1551. [Google Scholar]
- Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. American Journal of Clinical Nutrition. 2009;89:1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- Yin Y, Chen X, Shu Y. Gene expression of the invasive phenotype of TNF-alpha-treated MCF-7 cells. Biomed Pharmacother. 2009;63:421–428. doi: 10.1016/j.biopha.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Zacchino SA, Badano H. Enantioselective synthesis and absolute configuration assignment of erythro-(3,4-methylenedioxy-7-hydroxy-1'-allyl-3',5'-dimethoxy)-8.0.4'-neolignan and its acetate, isolated from nutmeg (Myristica fragrans) Journal of Natural Products. 1991;54:155–160. [Google Scholar]
- Zamora-Ros R, Forouhi NG, Sharp SJ, González CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, Fagherazzi G, Feskens EJ, Franks PW, Grioni S, Katzke V, Key TJ, Khaw KT, Kühn T, Masala G, Mattiello A, Molina-Montes E, Nilsson PM, Overvad K, Perquier F, Redondo ML, Ricceri F, Rolandsson O, Romieu I, Roswall N, Scalbert A, Schulze M, Slimani N, Spijkerman AM, Tjonneland A, Tormo MJ, Touillaud M, Tumino R, van der A DL, van Woudenbergh GJ, Langenberg C, Riboli E, Wareham NJ. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. Journal of Nutrition. 2014;144:335–343. doi: 10.3945/jn.113.184945. [DOI] [PMC free article] [PubMed] [Google Scholar]