Abstract
Angiogenesis is a requirement for tumor growth and metastasis. The angiogenic process depends on vascular endothelial cell migration and invasion, and is regulated by various cell adhesion receptors. Integrins are such a family of receptors that facilitate the cellular adhesion to and migration on extracellular matrix proteins in the intercellular spaces and basement membranes. Among 24 members of the integrin family, αvβ3 is studied most extensively for its role in tumor angiogenesis and metastasis. The αvβ3 is expressed at relatively low levels on epithelial cells and mature endothelial cells, but it is highly expressed on the activated endothelial cells of tumor neovasculature and some tumor cells. This restricted expression makes αvβ3 an excellent target to develop antiangiogenic drugs and diagnostic molecular imaging probes. Since αvβ3 is a receptor for extracellular matrix proteins with one or more RGD tripeptide sequence, many radiolabeled cyclic RGD peptides have been evaluated as “αvβ3–targeted” radiotracers for tumor imaging over the last decade. This article will use the dimeric and tetrameric cyclic RGD peptides developed in our laboratories as examples to illustrate basic principles for development of αvβ3–targeted radiotracers. It will focus on different approaches to maximize the radiotracer tumor uptake and tumor/background ratios. This article will also discuss some important assays for pre-clinical evaluations of integrin–targeted radiotracers. In general, multimerization of cyclic RGD peptides increases their integrin binding affinity and the tumor uptake and retention times of their radiotracers. Regardless of their multiplicity, the capability of cyclic RGD peptides to bind other integrins (namely αvβ5, α5β1, α6β4, α4β1 and αvβ6) is expected to enhance the radiotracer tumor uptake due to the increased integrin population. The result from preclinical and clinical studies clearly show that radiolabeled cyclic RGD peptides (such as 99mTc-3P-RGD2, 18F-Alfatide-I and 18F-Alfatide-II) are useful as the molecular imaging probes for early cancer detection and noninvasive monitoring of the tumor response to antiangiogenic therapy.
Keywords: Integrins, αvβ3, PET and SPECT radiotracers, tumor imaging
Graphical Abstract
Introduction
Cancer is the second leading cause of death worldwide.1 Most cancer patients will survive if it can be detected at the early stage. The sooner cancer is diagnosed and treated, the better chance a cancer patient will have for a full recovery. Thus, early detection is of great clinical importance for implementation of a therapeutic regimen before primary tumors become widely spread. In fact, early detection is the best option to reduce deaths from cancer substantially.
There are several imaging modalities available for diagnosis of cancer, including X-ray computed tomography (CT), ultrasound (US), nuclear magnetic resonance imaging (MRI), positron emission tomography (PET) and single photon emission computed tomography (SPECT). While CT, US and MRI techniques are better suited for anatomic analysis of solid tumors, it is difficult to use them for evaluation of biochemical changes in tumor tissues due to the fact that they require a high concentration of contrast agent in order to achieve sufficient contrast. The specificity and sensitivity of CT and US for high-incidence tumors (e.g. breast, colorectal, lung and prostate) are relatively low.2 In contrast, PET or SPECT offers significant advantages with respect to specificity and sensitivity (~10−10 M for SPECT and 10−10 - 10−12 M for PET).2–4 Both modalities are able to provide the detailed information related to biochemical changes in tumor tissues at the cellular and molecular levels.
Nuclear imaging with PET or SPECT requires administration of radiopharmaceuticals (also called radiotracers), which are drugs containing a radionuclide for routine diagnosis of diseases. According to their biodistribution characteristics, diagnostic radiotracers can be divided into two classes: those whose biodistribution is determined almost exclusively by their chemical and physical properties; and those whose ultimate distribution properties are determined by their receptor binding affinity and receptor population in the diseased tissue. The latter ones are often called target-specific radiotracers. A number of target-specific radiotracers have been developed to target the receptors overexpressed on tumor cells and/or tumor vasculature.2–10 In many cases, small peptides are used as targeting biomolecules (BM) for receptor binding in order to achieve high tumor specificity and selectivity.
Many radiolabeled cyclic RGD peptides have been evaluated as SPECT and PET radiotracers for tumor imaging.11–50 A number of review articles have appeared to cover their nuclear medicine applications.51–65 Instead of being an exhaustive review of current literature on radiolabeled cyclic RGD peptides, this article will use the dimeric and tetrameric cyclic RGD peptides developed in our laboratories as examples to illustrate the basic principles for development of αvβ3-targeted radiotracers. It will focus on different approaches to maximize radiotracer tumor uptake and tumor/background (T/B) ratios. It will also discuss some important biological assays for evaluations of αvβ3-targeted radiotracers, and their potential as molecular imaging probes for noninvasive monitoring of tumor metastasis and early detection of tumor response to treatment (chemotherapy, antiangiogenic therapy, radiation therapy or combination thereof). The same basic principles illustrated in this review article may also apply to the radiotracers based on other receptors. Whenever possible, it will use the references published over the last 10 years. The author would apologize to those whose work has not been cited in this article.
Radiotracer Design
Requirements for New Radiotracers
For a new radiotracer to be successful, it must show clinical indications for several of high-incidence tumor types (namely breast, lung and prostate cancers). Its localization of in tumors has to be sufficient so that clinically useful diagnostic images can be obtained within hours after intravenous administration of the radiotracer. Since most of high-incidence tumor types occur in the torso (e.g. lung, colorectal and breast cancers), renal excretion without significant kidney retention is necessary in order to maximize the T/B ratios. The main objective of tumor imaging is to achieve one or more of the following goals: (1) to detect the presence of tumor at early stage, (2) to distinguish between benign and malignant tumors, (3) to follow the tumor growth and tumor response to a specific therapy (chemotherapy, antiangiogenic therapy, radiation therapy or combination thereof), (4) to predict the success or failure of a specific therapeutic regimen, and (5) to assess the prognosis of a particular tumor in a specific cancer patient.
Basics of Receptor Binding
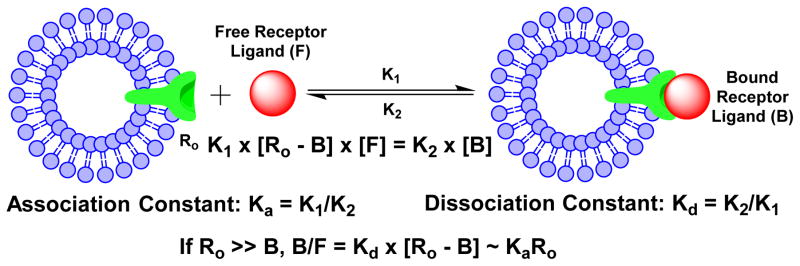
Receptor–ligand binding is defined by non-covalent interactions between a receptor and its ligand. These include hydrogen-bonding, lipophilic interactions, and static interactions between negative and positive charges. Using steady-state approximation, the association (Ka) and dissociation (Kd) constants could be calculated according to the equations illustrated in Chart I. Frequently the dissociation constant Kd is preferred as the receptor binding affinity of a specific receptor ligand. K1 and K2 are association and dissociation rates, respectively. For receptor-based radiotracers, Ro is the total receptor population (bound and unbound), [B] is the concentration of receptor-bound radiotracer, and [F] is the unbound free radiotracer in blood circulation. If the total receptor population is much higher than the number of radiotracer molecules (radiolabeled receptor ligand), the target/background ratio will be directly proportional to Ka and Ro (Chart I). Both binding affinity of the targeting biomolecule and receptor population are important to the tumor uptake and T/B ratios of a receptor-based radiotracer. Ligand binding is characterized in terms of the concentration of ligand at which half of the receptor binding sites are occupied, known as the IC50, which is related to but different from the association and dissociation constants. If two ligands were present at the same time, more of the higher-affinity ligand would be bound to the available receptor binding sites. Binding affinity is most commonly determined using a radiolabeled ligand, known as hot ligand. Competitive binding experiments involve binding-site competition between a hot ligand and a cold ligand (untagged ligand). Non-labelled methods such as surface plasmon resonance and dual polarization interferometry can also quantify the affinity from concentration based assays but also from the kinetics of association and dissociation.66 Microscale thermophoresis (MST) is a method that allows the determination of binding affinity without any limitation to the ligand’s molecular weight.67
Chart 1.
Schematic Presentation of Receptor Binding.
Integrin-Targeted Radiotracer
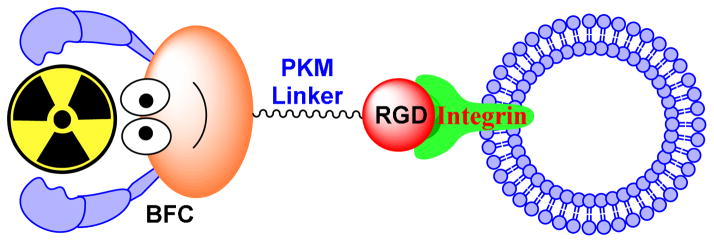
Figure 1 shows the schematic illustration of an integrin-targeted radiotracer. A cyclic RGD peptide serves as the targeting biomolecule to carry an isotope to integrins expressed on tumor cells and activated endothelial cells of tumor neovasculature. BFC is a bifunctional coupling agent to attach the isotope to cyclic RGD peptide.68 PKM is the pharmacokinetic modifying linker which is often used to improve the excretion kinetics of radiotracers.68–70
Figure 1.
Schematic presentation of integrin-targeted radiotracers. The cyclic RGD peptide (monomeric, dimeric or multimeric) serves as the targeting biomolecule to carry an isotope to the integrins (particularly αvβ3 and αvβ5). The bifunctional coupling agent (BFC) is used to attach the isotope to the targeting biomolecule. The PKM linker is often utilized to modify its pharmacokinetics.
Radionuclide
The choice of radionuclide depends on the imaging modality (SPECT vs. PET). More than 80% of radiotracers for SPECT in nuclear medicine are 99mTc compounds due to the optimal nuclear properties of 99mTc and its easy availability at low cost.68–70 The 6 h half-life is long enough to allow radiopharmacists to carry out radiosynthesis and for physicians to collect clinically useful images. At the same time, it is short enough to permit administration of 20 – 30 mCi of 99mTc without imposing a significant radiation dose to the cancer patient. The most clinically relevant PET isotopes are 18F, 64Cu and 68Ga. 18F is a cyclotron-produced isotope. Despite its short half-life (t1/2 = 110 min), the commercial availability of preparative modules makes 18F radiotracers more accessible to clinicians. 64Cu is another PET isotope to develop target-specific radiotracers. It has a half-life of 12.7 h and a β+ emission (abundance: 18%; and Emax = 0.655 MeV). Despite poor nuclear properties, its long half-life makes it feasible to prepare, transport and deliver 64Cu radiotracers for clinical applications.71 The breakthroughs in the production of 64Cu with high specific activity have made it more available to research institutions without the on-site cyclotron facilities.7,71 64Cu is a viable alternative to 18F for research programs that wish to incorporate high resolution and sensitivity of PET, but cannot afford to maintain the expensive isotope production infrastructure.71 68Ga is generator-produced PET isotope with a half-life of 68 min. The 68Ge-68Ga generator can be used for more than a year, allowing PET studies without the on-site cyclotron. If the radiotracer is properly designed, 68Ga can be as useful for PET as 99mTc for SPECT.72 The 68Ga-labeled somatostatin analogs have been studied for PET imaging of somatostatin-positive tumors in pre-clinical animal models and cancer patients.72–75 Gallium chemistry and related nuclear medicine applications have been reviewed recently.72
Bifunctional Coupling Agent (BFC)
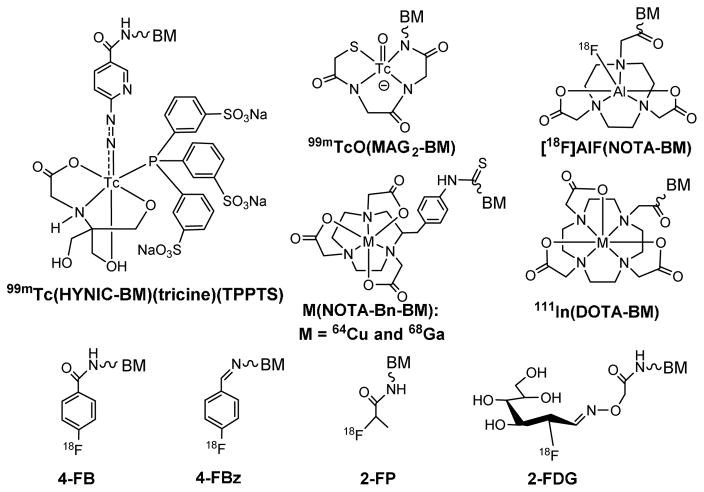
The choice of BFC depends on radionuclide. Among various BFCs (Figure 2), HYNIC is of great interest due to its high 99mTc-labeling efficiency, the high solution stability of its 99mTc complexes, and the use of co-ligands to modify biodistribution properties of 99mTc radiotracers.46,47,68–70 HYNIC and related radiochemistry have been reviewed.70 In contrast, DOTA and NOTA derivatives (Figure 2) have been widely used for 68Ga and 64Cu-labeling of biomolecules due to the high hydrophilicity and high stability of their 68Ga/64Cu chelates.7,71,72 Because of the short half-life of 68Ga (t1/2 = 68 min), fast and efficient radiolabeling is important for 68Ga radiotracers. Organic prosthetic groups (Figure 2: 4-FB, 4-FBz, 2-FP and 2-FDG) are needed for 18F-labeling.76–89 Because of aromatic rings, 4-FB, 4-FBz and 2-FP groups often have relatively high lipophilicity, which might lead to more hepatobiliary excretion. The results from recent studies indicate that Al(NOTA) (Figure 2) is highly efficient for routine radiosynthesis of 18F-labeled small biomolecule radiotracers using the kit formulation.90–97 The Al(NOTA) chelate offers significant advantages over organic prosthetic groups (e.g. 4-FB, 4-FBz and 2-FP) with respect to the 18F-labeling efficiency and hydrophilicity, which is important for rapid renal excretion of 18F radiotracers and minimization of their accumulation in normal organs, such as liver and lungs.
Figure 2.
Examples of BFCs useful for radiolabeling of small biomolecules, such as cyclic RGD peptides. HYNIC and MAG2 are useful for 99mTc-labeling. DOTA, NOTA and their derivatives are better suited for chelation of 64Cu, 68Ga and 111In. For 18F-labeling, 4-FB, 4-FBz, 2-FP and 2-FDG are often used as prosthetic groups. The Al(NOTA) chelate is highly efficient for routine radiosynthesis of 18F radiotracers using a kit formulation.
Integrins as Molecular Targets for Tumor Imaging
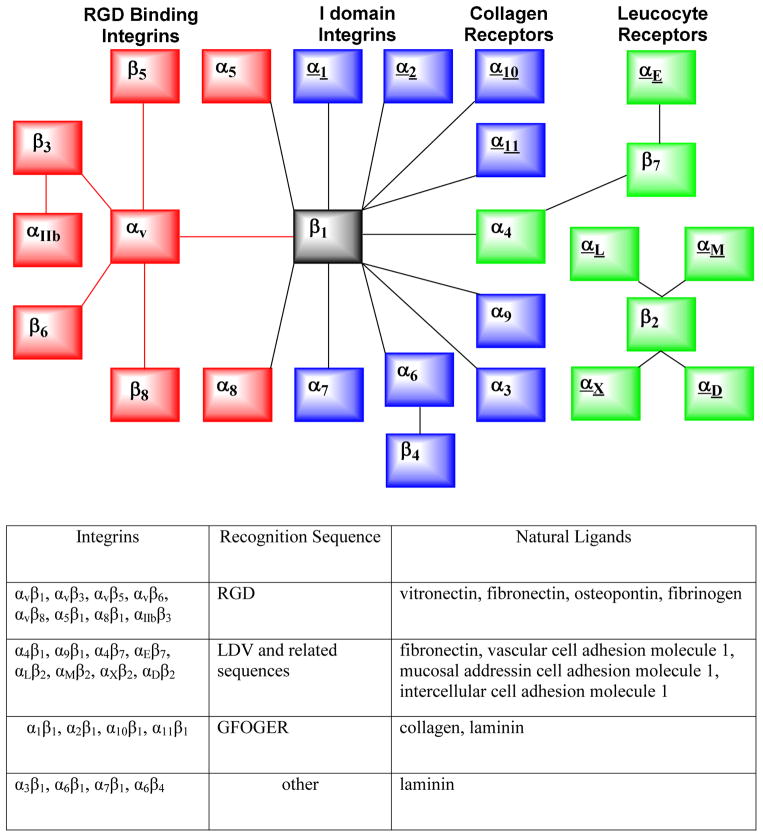
Tumors produce many angiogenic factors, which are able to activate endothelial cells in the established blood vessels and induce endothelial proliferation, migration, and new vessel formation (angiogenesis) through a series of sequential but partially overlapping steps. Angiogenesis is a requirement for tumor growth and metastasis.98–101 The angiogenic process depends on the vascular endothelial cell migration and invasion, and is regulated by various cell adhesion receptors. Integrins are such a family of receptors that facilitate cellular adhesion to and migration on extracellular matrix proteins in the intercellular spaces and basement membranes, and regulate the entry and withdraw from the cell cycle. The integrin family comprises 24 transmembrane receptors (Figure 3).98–101 Integrins possess redundancy in ligand recognition, adhesion and signaling. Their main function is to integrate cell adhesion and interaction with the extracellular microenvironment with the intracellular signaling and cytoskeletal rearrangement through transmitting signals across the cell membrane upon ligand binding. Many integrins are crucial to tumor growth and metastasis. They also contribute to the pathological events such as thrombosis,102 atherosclerosis,103 infection caused by pathogenic microorganisms,104,105 and immune dysfunction.106 Among the 24 members of integrin family, the αvβ3 is studied most extensively for its role in tumor angiogenesis and metastasis.98–101,107,108 It is not surprising that radiolabeled cyclic RGD peptides are often called “αvβ3–targeted” radiotracers in most of the current literature.11–65
Figure 3.
Top: Combinations of integrin subunits that form the 24 human receptors. Bottom: Natural integrin ligands and their corresponding recognition peptide sequences. Both the schematic illustration and table were adapted from reference 98.
The changes in the αvβ3 expression levels and activation state have been well-documented during tumor growth and metastasis.98–101,107,108 The αvβ3 is expressed at relatively low levels on the epithelial cells and mature endothelial cells, but it is highly expressed in solid tumors, including osteosarcomas, glioblastoma, melanomas, and carcinomas of lung and breast.107–124 Studies show that the αvβ3 is over-expressed on both tumor cells and activated endothelial cells of tumor neovasculature.44,47,115 It is believed that the αvβ3 on endothelial cells modulate the cell adhesion and migration during tumor angiogenesis, while the αvβ3 on carcinoma cells potentiate metastasis by facilitating the invasion and movement of tumor cells across blood vessels.119–124 It has also been shown that the αvβ3 expression levels correlate well with the metastatic potential and aggressiveness of solid tumors.106–108,122–124 The αvβ3 is an important biological target for development of antiangiogenic drugs,125–135 and molecular imaging probes for diagnosis of αvβ3-positive tumors.11–65
Cyclic RGD Peptides as Targeting Biomolecules
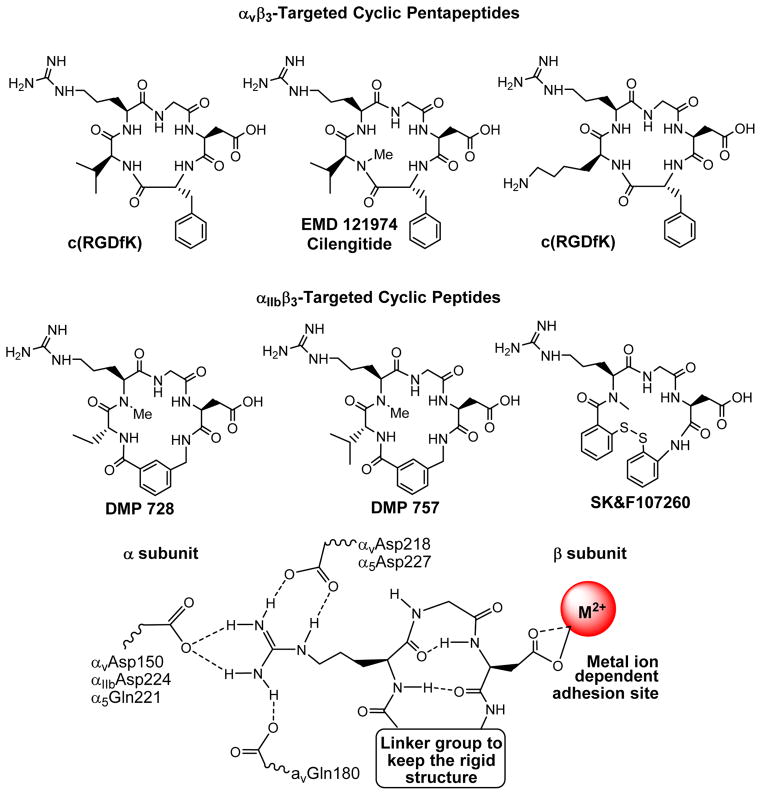
The αvβ3 is a receptor for extracellular matrix proteins with the exposed RGD tripeptide sequence. Theoretically, both linear and cyclic RGD peptides can be used as targeting biomolecules to develop αvβ3–targeted radiotracers. The drawback associated with the linear RGD peptides is their low binding affinity (IC50 > 100 nM), lack of specificity (αvβ3 vs. αIIBβ3, αvβ5, α5β1, α6β4, α4β1 or αvβ6), and rapid degradation by proteases in serum.130,134 It was shown that cyclization of RGD peptides via linkers, such as S-S disulfide, thioether, and aromatic rings, leads to the increased integrin binding affinity. The integrin selectivity (αvβ3 vs. αIIbβ3) could be achieved by altering the cyclic RGD peptide backbone (Figure 4). Incorporation of the RGD tripeptide sequence into a cyclic pentapeptide (Figure 4: c(RGDfV) and EMD121974 or Cilengitide) significantly increases the binding affinity and selectivity of αvβ3/αvβ5 over αIIbβ3.128–134 Many cyclic RGD peptides have been evaluated as αvβ3/αvβ5 antagonists for treatment of cancer. The results from structure-activity studies indicate that the amino acid residue in position 5 has little impact on the αvβ3/αvβ5 binding affinity.128–134 The valine (V) residue in c(RGDfV) can be replaced by lysine (K) or glutamic acid (E) to afford c(RGDfK) and c(RGDfE), respectively, without significantly changing their αvβ3/αvβ5 binding affinity. Cilengitide is currently under phase III clinical investigations as an “orphan drug” for treatment of glioblastoma and other cancer types either stand-alone or in combination with radiation therapy.135–142 It seems that the αIIBβ3 is less sensitive to variations in the peptide backbone and can accommodate a larger distance than both αvβ3 and αvβ5.66,129 The addition of a rigid aromatic ring into a cyclic peptide structure (Figure 4: DMP728 and DMP757) enhance the binding affinity and selectivity of αIIBβ3 over αvβ3/αvβ5. DMP728 and DMP757 were originally developed by DuPont Pharma as anti-thrombosis agents.143–145 We have been using the 99mTc-labeled DMP757 derivatives as SPECT radiotracers for thrombosis imaging.146–154
Figure 4.
Examples of cyclic RGD peptides and key features at the binding site between the cyclic RGD peptide and integrins (particularly αvβ3, αvβ5 and αIIbβ3). The integrin selectivity can be achieved by altering the linker group in the cyclic peptide backbone. Cyclic pentapeptides are highly selective for αvβ3 and αvβ5 whereas the cyclic peptides with rigid aromatic rings show very high selectivity for αIIBβ3 over αvβ3, αvβ5 and α5β1.
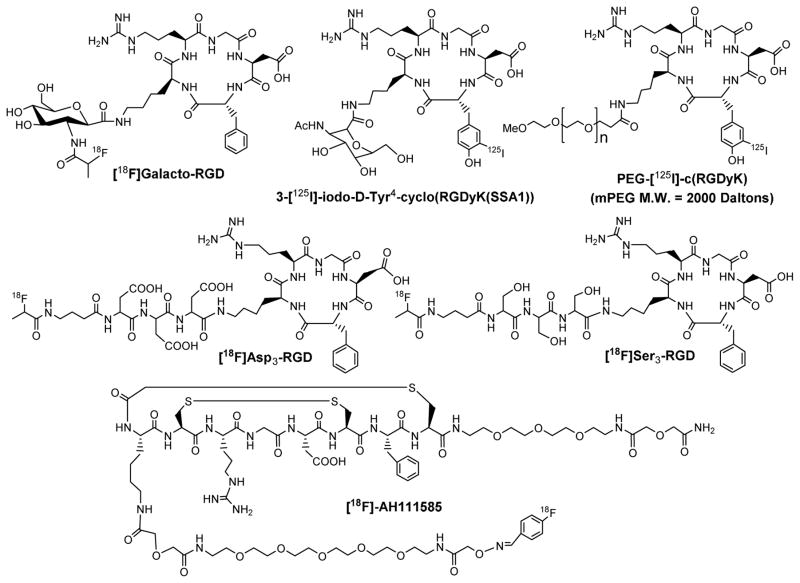
Figure 5 displays examples of monomeric cyclic RGD peptides. Among the radiotracers evaluated in various pre-clinical tumor-bearing animal models, 18F-Galacto-RGD was the first PET radiotracer under clinical investigation for visualizing the αvβ3 expression in cancer patients.[18F]AH111585 is another αvβ3-targeted PET radiotracer under clinical evaluation for tumor imaging.159 The imaging studies in cancer patients showed that there was sufficient αvβ3 expression for PET imaging, and the tumor uptake of 18F-Galacto-RGD correlates well with the αvβ3 expression level.157,158 However, the radiotracers derived from monomeric cyclic RGD peptides all have relatively low tumor uptake and T/B ratios because of their low αvβ3 binding affinity and fast dissociation kinetics.
Figure 5.
Examples of the radiolabeled monomeric cyclic RGD peptides as radiotracers. The D3 (Asp-Asp-Asp), S3 (Ser-Ser-Ser), PEG (polyethylene glycol) and sugar linkers are used to enhance radiotracer excretion kinetics from non-cancerous organs. [18F]Galacto-RGD was the first PET radiotracer under clinical investigation for visualization of αvβ3 expression in cancer patients. [18F]AH111585 was developed as a new PET radiotracer for tumor imaging.
It is important to note that cyclic RGD peptides bind not only αvβ3 but also αvβ5, α5β1, α6β4, α4β1 and αvβ6 integrins regardless of their multiplicity because they all share similar features at the RGD-binding sites (Figure 4). While αvβ3 plays a pivotal role in tumor growth, progression and metastasis, αIIBβ3 is critical for platelet aggregation during thrombosis formation. It was believed that the interaction between αvβ3 and αIIbβ3 facilitates the adhesion of tumor cells to tumor vasculature, which often leads to metastasis.160,161 The αvβ5 is very similar to αvβ3 in the ligand binding region (Figure 4), and they share a similar expression pattern and biological function. Both αvβ5 and αvβ3 are highly expressed on the activated endothelial cells and have similar roles in tumor angiogenesis, promoting angiogenic response to growth factors. The αvβ5 is overexpressed on many tumor types in both cell lines and clinical material.162 A number of tumors co-express αvβ3 and αvβ5,163–169 because they both engage the same extracellular matrix ligands and activate cell signaling pathways to promote tumor progression.168,169 It was reported that the expression of αvβ3, αvβ5, α5β1, α6β4, α4β1 and αvβ6 on the tumor cells is well correlated with the tumor progression.98,164,165 Structures of other RGD-binding integrins (e.g. αvβ6, αvβ8, αvβ1 and α8β1) have not yet been studied in details. However, it is well-known that the α4β1, α9β1, α4β7, αEβ7, αLβ2, αMβ2, αXβ2 and αDβ2 integrins all recognize the LDV, LDT and IDS tripeptide sequences.98 In contrast the collagen and laminin-binding integrins (α1β1, α2β1, α10β1 and α11β1) recognize the GFOGER hexapeptide sequence.169
There has been a continuing debate on whether one should separate αvβ3 from αvβ5, α5β1, α6β4, α4β1 and αvβ6. The answer to this question lies in the purpose of PET or SPECT imaging studies. If the purpose is to detect the presence of tumor or to distinguish between the benign and malignant tumors, there will be no need to separate them. The presence of multiple integrins results in a larger “receptor population”. Actually, the capability of radiolabeled cyclic RGD peptides to target multiple integrins is expected to improve their tumor uptake because larger receptor population than αvβ3 alone. Studies on the compounds highly active against αvβ3 reveals that they also possess similar activity against αvβ5.170 Dual antagonists have been deliberately prepared and were found to have very similar affinity for αvβ3 and αvβ5.170,171 As long as the biomolecule contains one or more RGD tripeptide sequence, it will bind αvβ3, αvβ5, α5β1, α6β4, α4β1 and αvβ6 regardless of peptide multiplicity. If the purpose of PET or SPECT study is to screen the appropriate patients for a specific therapy with αvβ3 antibodies or small-molecule αvβ3 antagonists, the separation of αvβ3 from αvβ5, α5β1, α6β4, α4β1 and αvβ6 may become necessary because the αvβ3-specificity is more important in this situation. However, radiolabeled cyclic RGD peptides may still be useful for this purpose if the expression of αvβ3 is linearly correlated to the expression levels of αvβ5, α5β1, α6β4, α4β1 and/or αvβ6.
Maximizing Binding Affinity via Multimerization
Multimerization of Cyclic RGD Peptides
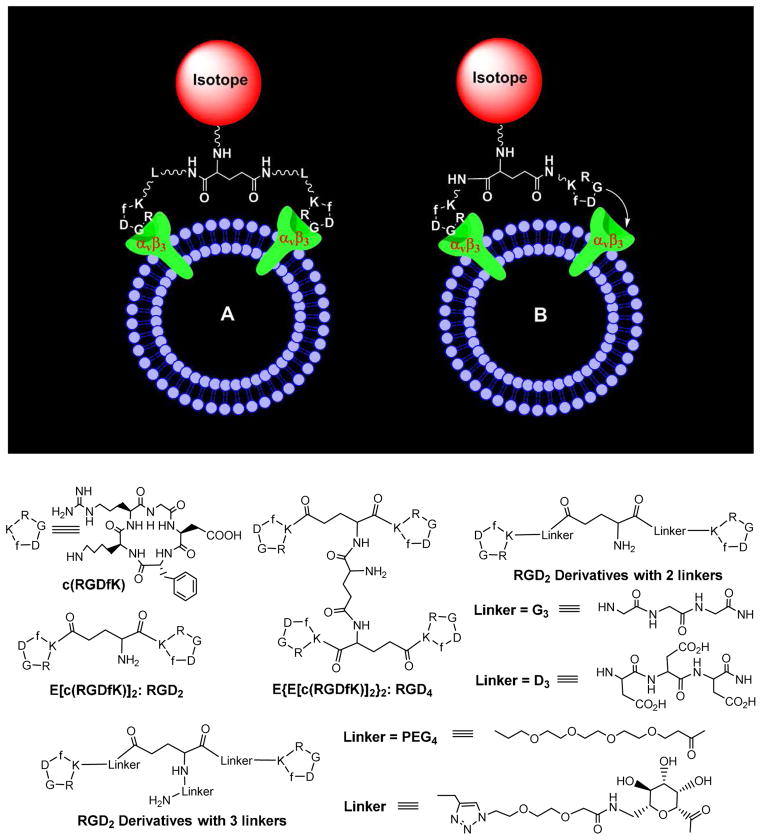
Multivalent interactions have been used in such a way that the weak interactions may become biological relevant.172–174 The multivalence concept has been used to develop αvβ3-targted radiotracers.11–65 It is believed that multimeric RGD peptides could provide more potent antagonists with better tumor-targeting capability. RGD2 (Figure 6) was the first cyclic RGD dimer for development of diagnostic (99mTc and 64Cu) and therapeutic (90Y and 177Lu) radiotracers.175–179 RGD4 (Figure 6) was also used to develop SPECT and PET radiotracers.19,21,30 The in vitro assays and biodistribution data clearly showed that the radiolabeled dimeric and tetrameric cyclic RGD peptides have higher αvβ3 binding affinity and better tumor uptake than their corresponding monomeric analogs.19,21,30,34–50
Figure 6.
Top: Schematic illustration of the interactions between a dimeric cyclic RGD peptide and αvβ3. A: The distance between two RGD motifs is long due to the presence of two linkers (L). As a result, the cyclic RGD dimer is able to bind αvβ3 in a “bivalent” fashion. B: The distance between two RGD motifs is not long enough for simultaneous αvβ3 binding. However, the RGD concentration is “locally enriched” in the vicinity of neighboring αvβ3 sites once the first RGD motif is bound. In both cases, the end-result would be higher αvβ3 binding affinity for dimeric cyclic RGD peptides. Bottom: Examples of cyclic RGD dimers and tetramers for development of αvβ3–targeted radiotracers. The D3, G3, PEG2, PEG4 and sugar linkers are used to increase the distance between two cyclic RGD motifs and to improve radiotracer excretion kinetics from non-cancerous organs.
Bivalency and Locally Enhanced RGD Concentration
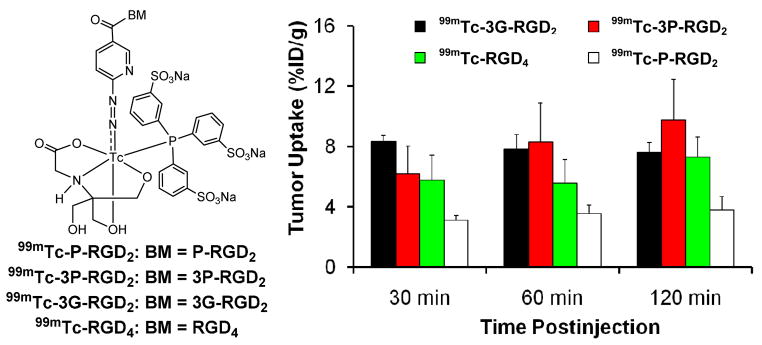
Figure 6 illustrates the interactions between a dimeric RGD peptide and αvβ3. Two important factors (Figure 6A: bivalency and enhanced RGD concentration) contribute to the high αvβ3 binding affinity of multimeric cyclic RGD peptides (Table 1).52,55,61 The concentration factor exists in all multimeric cyclic RGD peptides regardless of the linker length between two RGD motifs. The key to achieve bivalency is the distance between two cyclic RGD motifs. Given the short distance (6 bonds excluding the side-arms of K-residues) between two RGD motifs in RGD2 it is unlikely that they bind to two adjacent αvβ3 sites simultaneously. However, the binding of one RGD motif to αvβ3 will increase the local concentration of second RGD motif in the vicinity of neighboring αvβ3 sites (Figure 6A). The concentration factor may explain the higher tumor uptake of radiolabeled RGD2 than that of its monomeric derivatives (Table 1).175–179 The distance between two cyclic RGD motifs is 38 bonds in 3P-RGD2, and 26 bonds 3G-RGD2, which are definitely long enough for 3P-RGD2 and 3G-RGD2 to achieve bivalency. As a result, HYNIC-3P-RGD2 (IC50 = 60 ± 3 nM) and HYNIC-3G-RGD2 (IC50 = 59 ± 3 nM) have higher αvβ3 binding affinity than HYNIC-P-RGD2 (IC50 = 89 ± 7 nM).34,35 99mTc-3P-RGD2 and 99mTc-3G-RGD2 had higher tumor uptake than 99mTc-P-RGD2 (Figure 7). The concentration factor is likely responsible for the higher αvβ3 binding affinity of HYNIC-RGD4 (IC50 = 7 ± 2 nM) than that of HYNIC-3P-RGD2 and HYNIC-3G-RGD2. The fact that the tumor uptake of 99mTc-3G-RGD2 and 99mTc-3P-RGD2 is well-comparable to that of 99mTc-RGD4 (Figure 7) within experimental errors suggests that multimeric cyclic RGD peptides are not necessarily multivalent in binding to integrins, and the contribution from the concentration factor is not as much as that from the bivalency factor.39,41 In addition, the capability of a multimeric cyclic RGD peptide to achieve bivalency also depends on the αvβ3 density in tumor tissues. If the tumor αvβ3 density is high, the distance between two neighboring αvβ3 sites will be short. As a result, it is easier for the multimeric cyclic RGD peptide to achieve bivalency. If the tumor αvβ3 density is low, the distance between two neighboring αvβ3 sites will be long. In this case, it will be much more difficult for the same multimeric cyclic RGD peptide to achieve simultaneous αvβ3 binding.
Table 1.
The IC50 values of selected cyclic peptides and their corresponding DOTA, FITC, HYNIC and NOTA conjugates.
| Peptide/Conjugate | IC50 (nM)* | Radiotracer | Ref. |
|---|---|---|---|
| c(RGDyK) | 458 ± 45 | 34, 35 | |
| HYNIC-G-RGD | 358 ± 8 | 99mTc-G-RGD | 34 |
| HYNIC-P-RGD | 452 ± 11 | 99mTc-P-RGD | 35 |
| HYNIC-RGD2 | 112 ± 21 | 99mTc-RGD2 | 35 |
| HYNIC-P-RGD2 | 84 ± 7 | 99mTc-P-RGD2 | 35 |
| HYNIC-2G-RGD2 | 60 ± 4 | 99mTc-2G-RGD2 | 34 |
| HYNIC-2P-RGD2 | 52 ± 7 | 99mTc-2P-RGD2 | 35 |
| HYNIC-P2D-RGD2 | 61 ± 2 | 99mTc-P2D-RGD2 | 49 |
| HYNIC-P2G-RGD2 | 62 ± 5 | 99mTc-P2G-RGD2 | 49 |
| HYNIC-3G-RGD2 | 59 ± 3 | 99mTc-3G-RGD2 | 34 |
| HYNIC-3P-RGD2 | 60 ± 3 | 99mTc-3P-RGD2 | 35 |
| HYNIC-Galacto-RGD2 | 29 ± 5 | 99mTc-Galacto-RGD2 | 48 |
| HYNIC-RGD4 | 7 ± 2 | 99mTc-RGD4 | 35 |
| DOTA-RGD2 | 102 ± 5 | 64Cu-RGD2/111In-RGD2 | 33,39, 41, 42 |
| DOTA-3G-RGD2 | 74 ± 3 | 64Cu-3G-RGD2/111In-3G-RGD2 | 37,39, 41 |
| DOTA-3P-RGD2 | 62 ± 6 | 64Cu-3P-RGD2/111In-3P-RGD2 | 37,39, 42 |
| DOTA-3P-RGK2 | 596 ± 48 | 111In-Galacto-RGD2 | 50 |
| DOTA-Galacto-RGD2 | 27 ± 2 | 111In-Galacto-RGD2 | 50 |
| DOTA-RGD4 | 10 ± 2 | 64Cu-RGD4/111In-RGD4 | 20 |
| NOTA-RGD2 | 100 ± 3 | 68Ga(NOTA-RGD2) | 23 |
| NOTA-2G3-RGD2 | 66 ± 4 | 68Ga(NOTA-2G-RGD2) | 23 |
| NOTA-2P-RGD2 | 54 ± 2 | 68Ga(NOTA-2P-RGD2) | 23 |
| FITC-RGD2 | 89 ± 17 | 183 | |
| FITC-3P-RGD2 | 32 ± 7 | 183 | |
| FITC-Galacto-RGD2 | 28 ± 8 | 183 | |
| FITC-3P-RGK2 | 589 ± 73 | 183 |
The IC50 values depend largely on the radioligand and tumor cell lines. To keep the consistency and reproducibility, U87MG glioma cells (high αvβ3 and αvβ5 expression) have been used as the “host cells” and 125I-echistatin as the radioligand. Since the whole-cell displacement assay is operator-dependent, caution should be taken when comparing their IC50 values reported in the literature.
Figure 7.
Direct comparison of tumor uptake for 99mTc-P-RGD2, 99mTc-3G-RGD2, 99mTc-3P-RGD2 and 99mTc-RGD4 in athymic nude mice bearing the MDA-MB-435 human breast cancer xenografts. The biodistribution data were from references 30, 34 and 35.
Multimerization on Radiotracer Tumor Retention Time
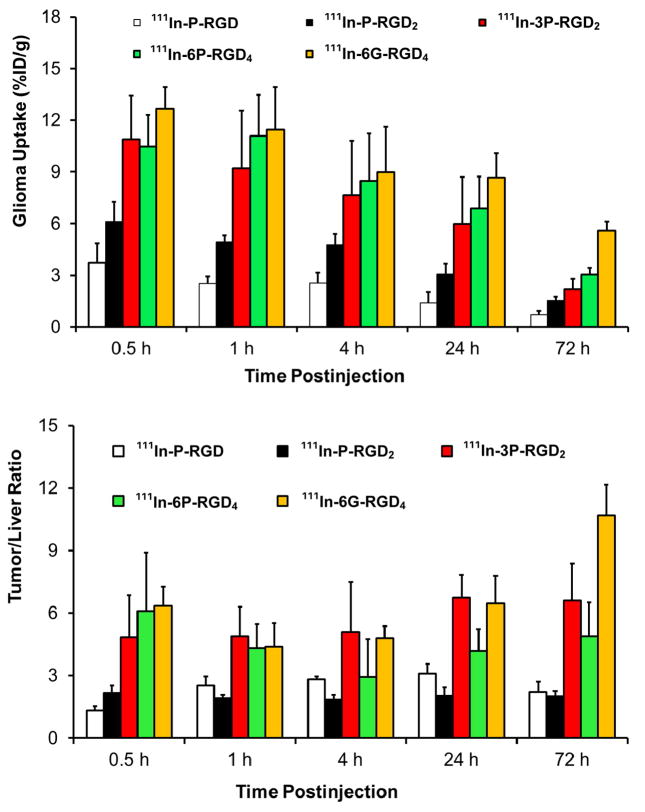
We examined the impact of multiplicity on the retention time of 111In-labeled cyclic RGD peptide DOTA conjugates (Figure 8) in the U87MG tumors.39,41,42 It was found that the glioma uptake (% ID/g at < 24 h p.i.) follow the general ranking order of 111In-6G-RGD4 ~ 111In-6P-RGD4 ~ 111In-3P-RGD2 ≫ 111In-P-RGD2 > 111In-P-RGD (Figure 9). Multimerization of significantly enhances the radiotracer tumor uptake. The tumor retention times follow the ranking order of 111In-6G-RGD4 (T1/2 > 72 h) > 111In-6P-RGD4 (T1/2 ~ 35 h) > 111In-3P-RGD2 > (T1/2 ~ 30 h) > 111In-P-RGD2 (T1/2 ~ 24 h) > 111In-P-RGD (T1/2 ~ 10 h).41 As a result of the increased peptide multiplicity, the tumor retention time of 111In-labeled cyclic RGD peptides increases and their dissociation rate from the tumor tissue decreases. The biodistribution data were consistent with the results from the whole-body planar imaging studies.39,41 The bivalency factor most likely contributes to the higher tumor uptake and longer tumor retention time of 111In-3P-RGD2 than that of 111In-P-RGD2. 111In-3P-RGD2 and 111In-6P-RGD4 share very similar (not identical) glioma tumor uptake values at <24 h p.i. Even though 111In-6P-RGD6 has a longer tumor retention time (as indicated by its higher tumor uptake at 72 h p.i.) than 111In-3P-RGD2, the tumor/liver ratios of 111In-3P-RGD2 are better than those of 111In-6P-RGD4.39,41 There is always a subtle balance between peptide multiplicity and tumor/background ratios. The combination of high tumor uptake and high tumor/liver ratios makes 111In-3P-RGD2 better suited for imaging than 111In-P-RGD, 111In-P-RGD2 and 111In-6P-RGD4.
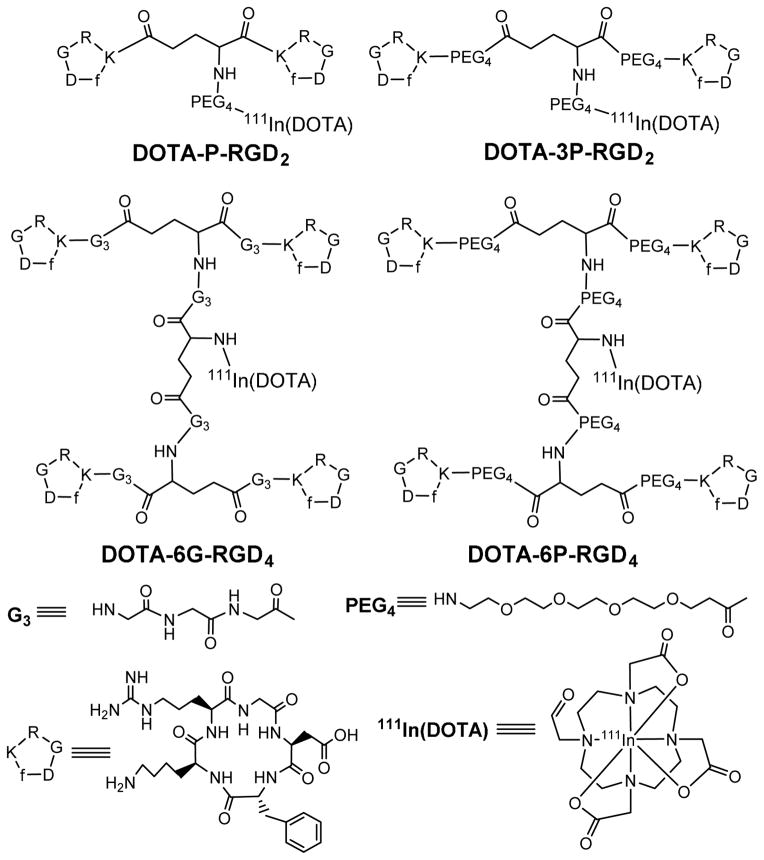
Figure 8.
Chemdraw structures of 111In-labeled cyclic RGD peptide DOTA-conjugates.
Figure 9.
Comparison of the glioma uptake (Top) and tumor/liver ratios (Bottom) of 111In-labeled cyclic RGD peptides in athymic nude mice bearing U87MG glioma xenografts at 0.5, 1, 4, 24 and 72 h after administration of 111In radiotracer. The biodistribution data were from references 41 and 42.
Impact of Linker Groups
As illustrated in Figure 6, the linker groups are used for two purposes: increasing the distance between two RGD motifs so that they are able to the αvβ3 in a bivalent fashion, and improving the radiotracer excretion kinetics. Different linkers (Figure 6) have been proposed to increase the distance between two RGD motifs and improve pharmacokinetics of radiotracers.38–42,47–50 The results from in vitro and in vivo assays showed that the linkers (G3 vs. D3 and PEG4 or PEG4 vs. SAA, 1,2,3-triazole and PEG2 moieties) have little impact on the αvβ3 binding affinity of HYNIC-conjugated dimeric cyclic RGD peptides (Table 1) as long as they are long enough for bivalency. However, the overall molecular charges may have significant impact on the radiotracer uptake in both tumors and normal organs. For example, HYNIC-3G-RGD2 and HYNIC-3P-RGD2 share almost identical αvβ3 binding affinity (Table 1). Since the G3 and PEG4 linkers are neutral, their corresponding radiotracers 99mTc-3G-RGD2 and 99mTc-3P-RGD2 have very similar glioma tumor uptake (Figure 7) and excretion kinetics from normal organs.34,35 Similar comparison can be made between 99mTc-3P-RGD2 and 99mTc-Galactor-RGD2.48 In contrast, the D3 linker is triply charged under physiological conditions. Even though HYNIC-P2D-RGD2 and HYNIC-P2G-RGD2 share almost identical αvβ3 binding affinity,49 the tumor uptake of 99mTc-P2D-RGD2 (2.20±0.42, 2.85±0.55, 3.11±0.47 and 2.45±0.90 %ID/g at 5, 30, 60 and 120 min p.i., respectively) was significantly lower (p < 0.01) than that of 99mTc-P2G-RGD2 (9.27±0.72, 8.85±0.67, 8.17±1.10 and 7.82±0.76 %ID/g at 5, 30, 60 and 120 min p.i., respectively) over the 2 h study period.49 In contrast, 99mTc-P2G-RGD2 and 99mTc-3P-RGD2 shared similar tumor uptake values (7.82 – 9.27 %ID/g for 99mTc-P2G-RGD2; and 7.24 – 8.72 %ID/g for 99mTc-3P-RGD2).49 However, 99mTc-P2D-RGD2 had the intestine uptake values of 5.86±1.37, 6.58±0.88, 7.08±0.92 and 4.74±0.33 %ID/g at 5, 30, 60 and 120 min p.i., respectively, which were much lower than those of 99mTc-P2G-RGD2 (11.72±2.01, 9.27±1.15, 6.17±1.55 and 4.74±1.09 %ID/g at 5, 30, 60 and 120 min p.i., respectively) over the 2 h study period. Therefore, the linker groups between the two cyclic RGD moieties have a significant impact on the blood clearance, tumor uptake and biodistribution properties of 99mTc-labeled dimeric cyclic RGD peptides.
Impact of 99mTc Chelates
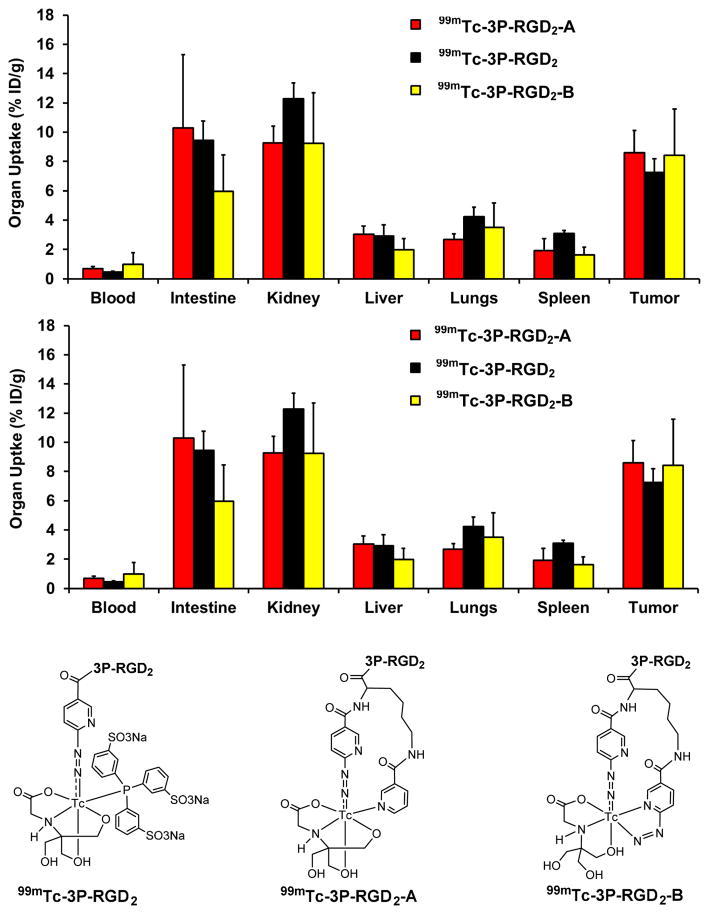
Figure 10 compares the 60-min biodistribution data of 99mTc-3P-RGD2, 99mTc-3P-RGD2-A and 99mTc-3P-RGD2-B in the U87MG glioma model.46,47 It was found that replacing the highly charged and bulky [99mTc(HYNIC)(tricine)(TPPTS)] (MW ~ 970 Daltons) with a smaller [99mTc(HYNIC-K(NIC))(tricine)] (MW ~ 650 Daltons) or [99mTc(K(HYNIC)2)(tricine)] (MW ~ 670 Daltons) had little impact on the tumor uptake,46,47 suggesting that changing the 99mTc chelates had no adverse effect on their tumor-targeting capability. However, the change in 99mTc chelates resulted in significant uptake differences in normal organs, (e.g. intestines, kidneys, lungs and spleen).46,47 Since [99mTc(HYNIC-K(NIC))(tricine)] is structurally similar to [99mTc(K(HYNIC)2)(tricine)] (Figure 10), it was not surprising that 99mTc-3P-RGD2-A and 99mTc-3P-RGD2-B shared almost identical biodistribution properties.47 A major advantage of using K(HYNIC)2 and HYNIC-K(NIC) as BFCs is the use of SnCl2 as a reducing agent during 99mTc-labeling because TPPTS can easily reduce the S-S disulfide bond, which is often important for small biomolecules to maintain their biological activity and tumor-targeting capability. The disadvantage of K(HYNIC)2 and HYNIC-K(NIC) is that there are more than two isomers in [99mTc(HYNIC-K(NIC))(tricine)] and [99mTc(K(HYNIC)2)(tricine)].46,47
Figure 10.
Direct comparison of the 60-min biodistribution data for 99mTc-3P-RGD2, 99mTc-3P-RGD2-A and 99mTc-3P-RGD2-B in the athymic nude mice bearing U87MG human glioma xenografts to show the impact of 99mTc chelates on biodistribution properties of 99mTc radiotracers. The biodistribution data were from references 46 and 47.
Impact of Radiometal Chelates
111In-3P-RGD2 and 64Cu-3P-RGD2 share the same DOTA-3P-RGD2 conjugate. In spite of the difference in their structures and overall molecular charge, the tumor uptake of 111In-3P-RGD2 (10.89 ± 2.55 and 7.65 ± 3.17 %ID/g at 30 and 240 min p.i., respectively) was very close to that of 64Cu-3P-RGD2 (8.23 ± 1.97 and 6.43 ± 1.22 %ID/g at 30 and 240 min p.i., respectively).37,39–42 They also shared very similar uptake in normal organs. For example, the kidney uptake of 111In-3P-RGD2 was 5.80 ± 0.95 at 30 min p.i. and 2.78 ± 0.20 %ID/g at 240 min p.i., and was well comparable to that of 64Cu-3P-RGD2 (6.59 ± 0.93 %ID/g at 30 min p.i. and 2.81 ± 0.36 % ID/g at 240 min p.i.). The liver uptake of 111In-3P-RGD2 is 2.52 ± 0.57 %ID/g at 30 min and 1.61 ± 0.06 %ID/g at 240 min p.i. while 64Cu-3P-RGD2 has the liver uptake of 2.80 ± 0.35 %ID/g at 30 min p.i. and 1.87 ± 0.51 %ID/g at 240 min p.i.37,39–42 Changing from 111In(DOTA) to 64Cu(DOTA) has minimal impact on the tumor uptake and excretion kinetics of the corresponding radiotracers. Similar conclusion can be made by comparing biodistribution properties of 64Cu-3P-RGD2 and 111In-3G-RGD2.37,41,42
Maximizing Radiotracer Uptake by Targeting Multiple Receptors
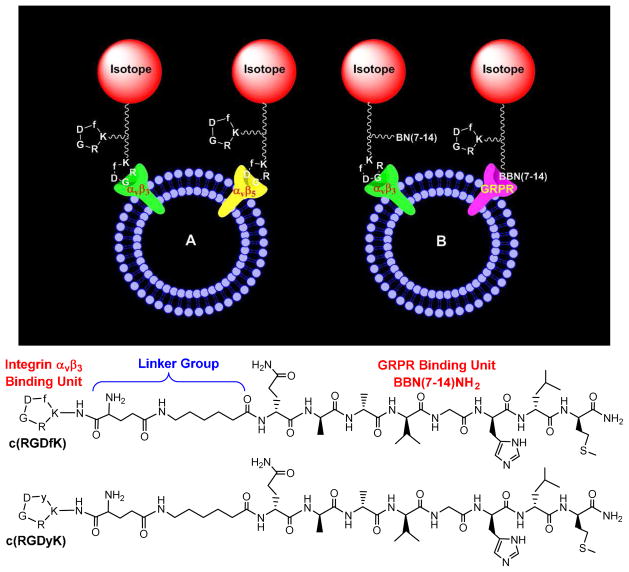
Both receptor binding affinity and receptor population are important for the tumor selectivity and tumor uptake of radiolabeled cyclic RGD peptides. There are two general approaches to maximize the “receptor population”. The first approach (Figure 11A) involves the use of same cyclic RGD peptide (monomeric and multimeric) to target two or more integrins (particularly αvβ3 and αvβ5) overexpressed on both tumor cells and activated endothelial cells of tumor neovasculature. Radiolabeled multimeric cyclic RGD peptides have advantages over those targeting only αvβ3 or αvβ5 because of their capability to target multiple integrins (e.g. αvβ3, αvβ5, α5β1, α6β4, α4β1 and αvβ6). Another approach involves the use of a bifunctional peptide (Figure 11B) that is able to target two different receptors, such as αvβ3 and gastrin-releasing peptide receptor (GRPR). By targeting two different receptors, the “bifunctional” radiotracer will have more opportunities to localize in the tumor tissue (including the tumor cells and tumor neovasculature) and are expected to have a slower dissociation rate from the tumor.
Figure 11.
A: Schematic presentation of a dimeric cyclic RGD peptide targeting two or more integrins (e.g. αvβ3, αvβ5, α5β1, α6β4, α4β1 and αvβ6). B: Schematic illustration of a bifunctional peptide targeting two different receptors (e.g. αvβ3 and GRPR). By targeting two different receptors, the radiotracer will have more opportunities to localize in tumor due to the increased receptor population. The two targeted receptors (e.g. αvβ3/αvβ5 or αvβ3/GRPR) must be co-localized and the distance between them must be short for the bifunctional radiotracer to achieve simultaneous receptor binding. Bottom: Examples of bifunctional peptides containing c(RGDfK)/c(RGDyK) and Aca-BBN(7–14)NH2 (ε-aminocaproic acid- Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2).
Bivalent heterodimers containing BBN(7-14) and c(RGDfK) or c(RGDyK) (Figure 11) have been used to target the αvβ3 and GRPR simultaneously.180–182 The xenografted PC-3 and MDA-MB-435 tumor-bearing animal models were used to evaluate their tumor-targeting capability. However, there is lack of concrete in vivo evidence to prove if c(RGDfK)-BBN(7-14) and c(RGDyK)-BBN(7-14) are indeed “bivalent”, and whether there is a “synergetic effect” between the cyclic RGD and BBN(7-14) peptides. It is also unknown about which receptor binding unit actually contributes to the tumor uptake of “bifunctional radiotracers”. Since the PC-3 tumors have low αvβ3 expression and the MDA-MB-435 tumors have little or no expression of GRPR,40,47,181,182 the xenografted PC-3 and MDA-MB-435 tumor-bearing models are not appropriate to prove the concept of “bivalent heterodimers” because the αvβ3 and GRPR receptors must be co-localized and the distance between them must be short in order for the bifunctional radiotracer to achieve bivalency. Otherwise, it is very difficult to observe the “synergetic effect” even if they are able to target individual receptors.
Important Biological Assays
Integrin Binding Assays
The integrin binding affinity of a cyclic RGD peptide can be determined using the immobilized αvβ3 assay or the whole-cell competitive displacement assay with 125I-echistatin or 125I-c(RGDyK) as the radioligand. The immobilized αvβ3 assay can provide the αvβ3 binding affinity of a cyclic RGD peptide with high specificity and selectivity. However, the IC50 (the concentration to achieve 50% displacement of the radioligand or fluorescent probe) values from the immobilized αvβ3 assays don’t reflect the contributions from its binding to other integrins (namely αvβ5, α5β1, α6β4, α4β1 and αvβ6), and are not reliable to predict the tumor uptake of radiolabeled cyclic RGD peptides since the αvβ3 density would never be as high as that used in the immobilized αvβ3 assay. In contrast, the whole-cell displacement assay will provide a measure of the capability of a specific cyclic RGD peptide in binding to all integrins (αvβ3, αvβ5, α5β1, α6β4, α4β1 and αvβ6) on tumor cells. However, the IC50 values depends largely on the radioligand (125I-c(RGDyK) vs. 125I-echistatin) and tumor cell lines (U87MG vs. MDA-MB-435) used in this assay. The IC50 values using 125I-echistatin as the radioligand are much higher than those with 125I-c(RGDyK) because of the higher integrin binding affinity of 125I-echistatin. To keep the consistency and reproducibility, we have been using U87MG glioma cells (high αvβ3 and αvβ5 expression) as the “host cells” and 125I-echistatin as the radioligand.34–50 Since the whole-cell displacement assay is operator-dependent, caution should be taken when comparing their IC50 values with those reported in the literature. Whenever possible, a “control compound”, such as c(RGDfK) or c(RGDyK) should be used in each experiment.
Blocking Experiments to Demonstrate Integrin Specificity
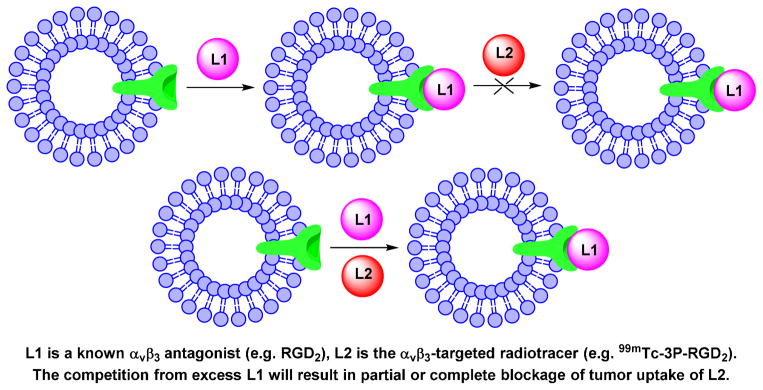
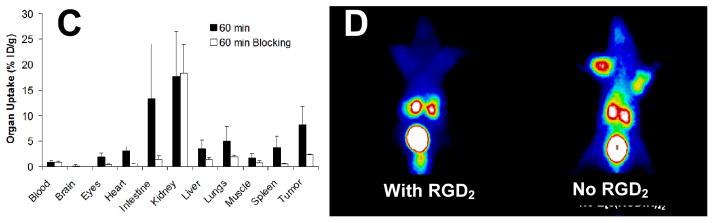
Blocking experiment (Chart II) is commonly used to demonstrate the αvβ3 specificity of radiolabeled cyclic RGD peptides with a known αvβ3 antagonist (e.g. c(RGDfK) or RGD2) as the blocking agent. The blocking experiment is often performed using the in vitro tissue (Figure 12A) and cellular (Figure 12B) IHC staining assays, or by exvivo biodistribution (Figure 12C) and/or in vivo imaging (Figure 12D). The blocking agent can be administered before injection of the radiotracer or co-administered with the radiotracer. Co-injection of excess blocking agent (e.g. c(RGDfK) or RGD2) will result in partial or complete blockage of the radiotracer tumor uptake (Figure 12C). There is also a significant reduction in radiotracer uptake in the αvβ3-positive organs (e.g. eyes, intestine, kidneys, lungs, liver, muscle and spleen).41
Chart 2.
Schematic Illustration of Blocking Experiments.
Figure 12.
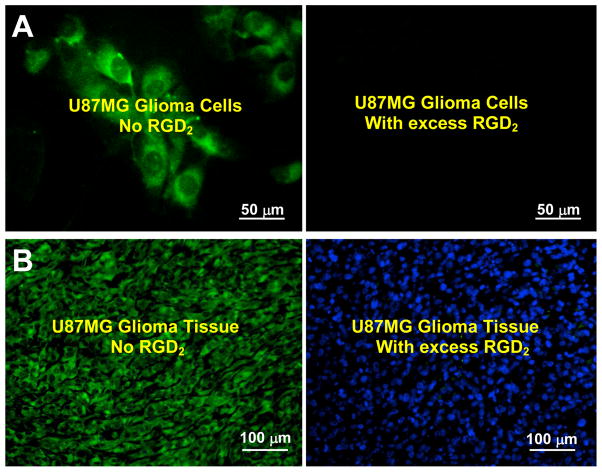
A: Selected microscopic images (Magnification: 400×) of living U87MG glioma cells stained with FITC-Galacto-RGD2 in the absence (left) and presence (right) of excess RGD2. B: Microscopic images (Magnification: 200×) of a tumor slice stained with FITC-Galacto-RGD2 in the absence (left) and presence (right) of excess RGD2. The cellular and tumor staining data were from reference 183. C: Comparison of organ uptake (%ID/g) for 99mTc-2P-RGD2 in the absence or presence of excess RGD2 at 60 min p.i. D: The 60-min planar images of the tumor-bearing mice administered with 99mTc-3P-RGD2 in the absence/presence of RGD2. Co-injection of excess RGD2 resulted in significant reduction in the uptake of 99mTc-3P-RGD2 in both tumor and normal organs. The biodistribution and imaging data were obtained from reference 35.
Nonsense Peptide to Demonstrate RGD Specificity
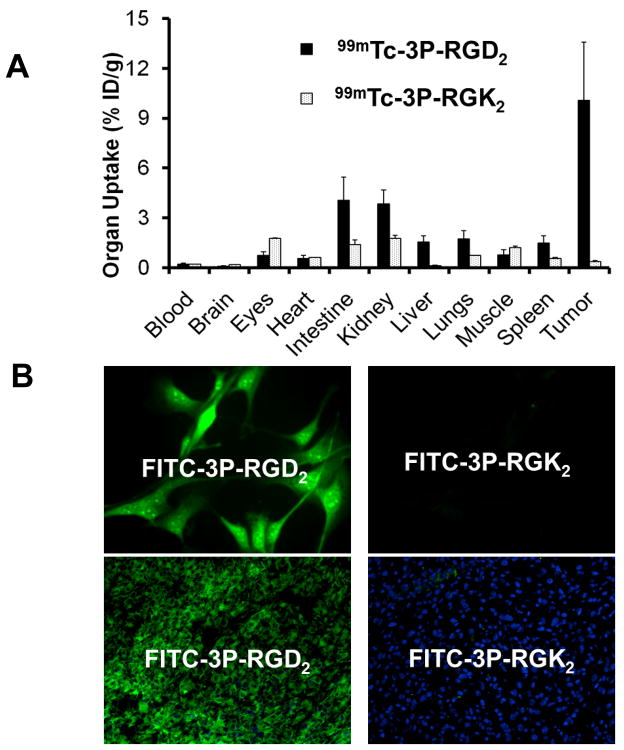
There are several ways to determine the RGD-specificity of cyclic RGD peptides. These include: (1) in vitro αvβ3 binding assay, (2) tissue or cellular IHC staining assay, (3) PET or SPECT imaging, and (4) ex-vivo biodistribution. In all cases, a “nonsense peptide” with the “scrambled sequence” will be used for compassion purposes. For example, 3P-RGK2 is a nonsense peptide with the chemical composition identical to that of 3P-RGD2. The αvβ3 binding affinity of DOTA-3P-RGK2 (IC50 = 596±48 nM) was >20x lower than that of DOTA-3P-RGD2 (IC50 = 29±4 nM). Similar results were also observed with FITC-3P-RGK2 (IC50 = 589±73 nM) and FITC-3P-RGD2 (IC50 = 32±7 nM). Due to the low αvβ3 affinity of DOTA-3P-RGK2,41,42 111In-3P-RGK2 had much lower uptake than 111In-3P-RGD2 in tumors and the αvβ3–positive organs, such as intestine, liver, lungs and spleen (Figure 13A).41,42 The U87MG glioma cells (Figure 13B) and tumor tissue (Figure 13C) could be stained with FITC-3P-RGD2, but not with FITC-3P-RGK2 under the same conditions.183 These results clearly show that the radiotracer uptake in tumors indeed is RGD–specific. It must be noted that using the “scrambled” peptides (e.g. 3P-RGK2) can significantly reduce the αvβ3 binding affinity, but this may not totally eliminate their capability for αvβ3 binding because the αvβ3 binding involves all three amino acid residues (Figure 4) in order to achieve maximal binding capability. Even if the αvβ3 binding capability can be totally eliminated, the scrambled peptides can still bind to other integrins (αvβ5, α5β1, α6β4, α4β1 and αvβ6) with low affinity.
Figure 13.
A: comparison of the 60-min biodistribution data of 99mTc-3P-RGD2 and 99mTc-3P-RGK2 in athymic nude mice bearing U87MG glioma xenografts. The low tumor uptake of 99mTc-3P-RGK2 indicates that the αvβ3-binding of radiolabeled dimeric cyclic peptides are RGD-specific. B: Selected microscopic images (magnification: 400x) of the acetone-fixed U87MG glioma cells stained with FITC-3P-RGD2 and FITC-3P-RGK2. C: Microscopic images (magnification: 200×) of the xenografted U87MG glioma tissue stained with FITC-3P-RGD2 and FITC-3P-RGK2. Blue color indicates the presence of nuclei stained with DAPI. The cellular and tissue staining data were from reference 183.
Pharmacokinetics and Metabolism
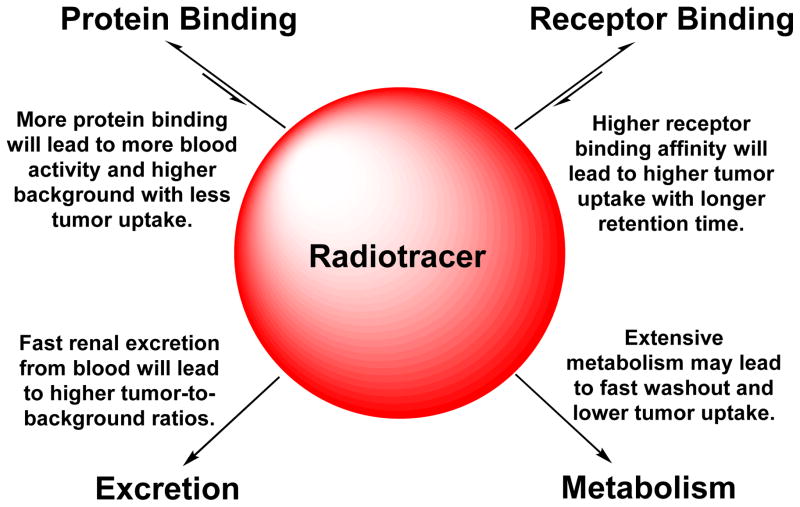
Pharmacokinetics describes how the body affects a specific drug after administration through the mechanisms of absorption and distribution, as well as the chemical changes of drug substance in the body. There are two important biological interactions (Chart III: receptor binding and protein binding) once the radiotracer is injected into blood circulation. Receptor binding is necessary for the radiotracer to localize in tumor selectively. Higher binding affinity will lead to more radiotracer tumor uptake.49 Protein bonding is generally detrimental because it will reduce the number of radiotracer molecules available for receptor binding, and result in more blood radioactivity (Chart III).49 Therefore, the protein bonding should be minimized. In addition, hydrophilic radiotracers tend to fast renal excretion, which will lead to lower background radioactivity in the blood pool and normal organs (such as liver, lungs and muscle) with better T/B ratios. In contrast, lipophilic radiotracers tend to excreted through hepatobiliary route with a higher degree of metabolism. High metabolic instability may result in lower tumor uptake, and higher background activity if the metabolite has longer body retention, which will definitely lead to poorer target-to-background ratios.
Chart 3.
Schematic Illustration of Biological Interactions, Elimination Routes and Metabolism of Target-Specific Radiotracers.
The tumor uptake and distribution properties of are normally determined by biodistribution at different time points after administration of the radiotracer. Even though PET has the capacity to quantify absolute radioactivity, the CT component must be included and co-registered during image acquisition and data processing. Otherwise, it would be very difficult to determine the volume and radioactivity concentration in each organ. Both PET and SPECT have the capability to determine the tumor uptake and washout kinetics via dynamic planar imaging. The tumor uptake is often expressed as the percentage of initial uptake. The blood clearance kinetics of a radiotracer can be measured by collecting blood samples at specific time points over a specific period of time.49 The blood radioactivity usually expressed as the percentage of its initial radioactivity will be plotted against time. The most important parameter for kinetic studies is the rate of changes in the radiotracer organ uptake. The metabolic stability is determined by analyzing both urine and feces samples at a specified time point. In certain situations, tissue samples are harvested to determine the in vivo stability of radiotracers in the tumor, kidneys, liver and lungs. It is important to note that the radiotracer uptake in a specific organ represents only a small portion of the total radioactivity injected into each animal. A major portion of the injected radiotracer and its metabolites has been excreted via both renal and hepatobiliary routes. Thus, the assays of radioactivity in urine and fasces samples are more reliable for determination of the metabolic stability of the radiotracer.
Monitoring Tumor Response to Antiangiogenic Therapy
Clinical Need for Biomarkers to Monitor Antiangiogenic Therapy
Inhibiting angiogenesis is a promising strategy for cancer therapy. As antiangiogenic therapies have become a common practice in clinics, finding suitable biomarkers for modulation of the tumor vasculature has become important. An ideal biomarker for monitoring antiangiogenic therapy should have the capacity to track biological changes in the tumor vasculature during and after antiangiogenic treatment. Microvessel density has been proposed as the prognostic indicator of the progression, overall survival, and disease-free survival in cancer patients. Evaluation of microvessel density is often performed by immunostaining endothelial cells in the tumor tissues and counting the number of vessels. However, this approach is not practical for routine monitoring of antiangiogenic therapy mainly due to invasive nature of the biopsy procedure. DCE-MRI were used to measure the tumor perfusion properties;184–187 but it is technically challenging and standardization is very complicated. The MRI techniques have little or no capability to monitor biological changes in the tumor vasculature during and after antiangiogenic treatment. 18F-FDG PET has been used to monitor the antiangiogenic linifanib therapy by determining the reduced metabolic activity in the treated tumors.188 However, glucose metabolism may not be an ideal biomarker for monitoring antiangiogenic therapy because most of the metabolic activities take place in tumor cells rather than tumor vasculature.
Linear Relationship between Tumor Uptake and αvβ3 Expression
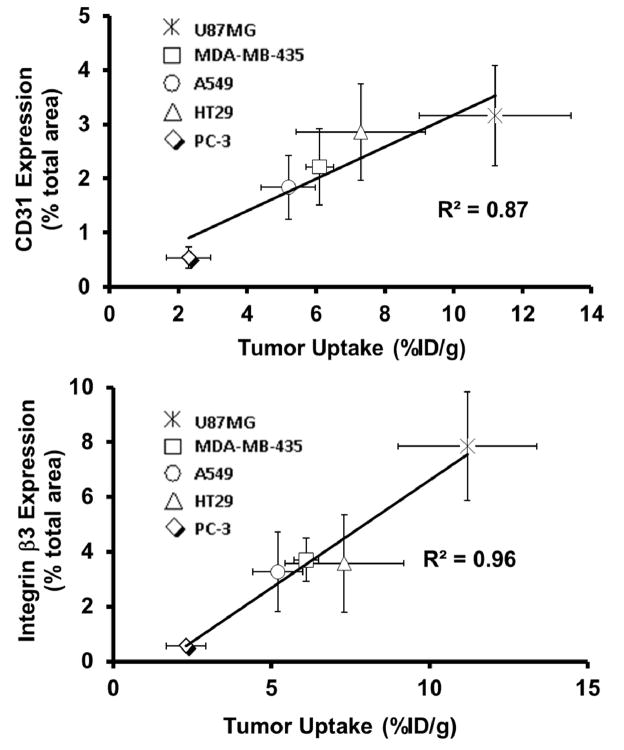
It is well-established that the total αvβ3 expression on both tumor cells and activated endothelial cells of the tumor neovasculature contributes to the tumor uptake of a αvβ3-targeted radiotracer regardless of peptide multiplicity.44,115 If the αvβ3–targeted radiotracer is used for accurate measurement of αvβ3 expression, there must be a linear relationship between its tumor uptake and αvβ3 expression levels. However, there were a few reports to describe this relationship in the literature.20,155–158 99mTc-3P-RGD2 was studied for its capability to monitor the αvβ3 expression levels.44 IHC was performed to determine the β3 levels in the xenografted U87MG, MDA-MB-435, A549, HT29 and PC-3 tumors.44 An excellent relationship (Figure 14A) was observed between the tumor uptake of 99mTc-3P-RGD2 and the αvβ3 expression levels.44 There is also an excellent relationship between its tumor uptake and CD31 expression levels (Figure 14B). These linear relationships suggest that 99mTc-3P-RGD2 is useful to monitor the αvβ3 and CD31 expression in cancer patients, to select most appropriate cancer patients who will benefit most from antiangiogenic therapy, and to monitor the tumor response to the antiangiogenic treatment.
Figure 14.
Relationship between the tumor uptake (%ID/g: radioactivity density) and relative β3 (top) CD31 (bottom) expression levels (fluorescence density) in five different xenografted tumors (U87MG, MDA-MB-435, A549, HT29 and PC-3). The total β3 expression was represented by the percentage of red area over total area in each slice of tumor tissue. Each data point was derived from at least 15 different areas of same tissue (100X magnification). The experimental data were from reference 40.
Monitoring Antiangiogenic Therapy with αvβ3–Targeted Radiotracers
The cross-talk between integrins and receptor tyrosine kinases (e.g. VEGFR and PDGFR) is crucial for many cellular functions.189–194 The αvβ3 and PDGFR-β co-localize and PDGFR activation increases the endothelial cell migration and proliferation. The functional association between αvβ3 and VEGFR2/PDGFR is of reciprocal nature since each is able to promote the activation of its counterpart. This mutually beneficial relationship provides the conceptual basis to use radiolabeled cyclic RGD peptides for monitoring antiangiogenic therapy.195–198 It was reported that the tumor uptake of [18F]AH111585 was significantly reduced 2 days after sunitinib treatment, and [18F]AH111585 was better than 18F-FDG for monitoring angiogenesis therapy.196 64Cu-DOTA-RGD was used to monitor dasatinib therapy.198 It was found that dasatinib treatment significantly reduced the 64Cu-DOTA-RGD uptake in treated tumors. A significant challenge for 18F PET radiotracers, such as [18F]-Galacto-RGD and [18F]AH111585, to assume widespread clinical utility is their poor availability at high cost.
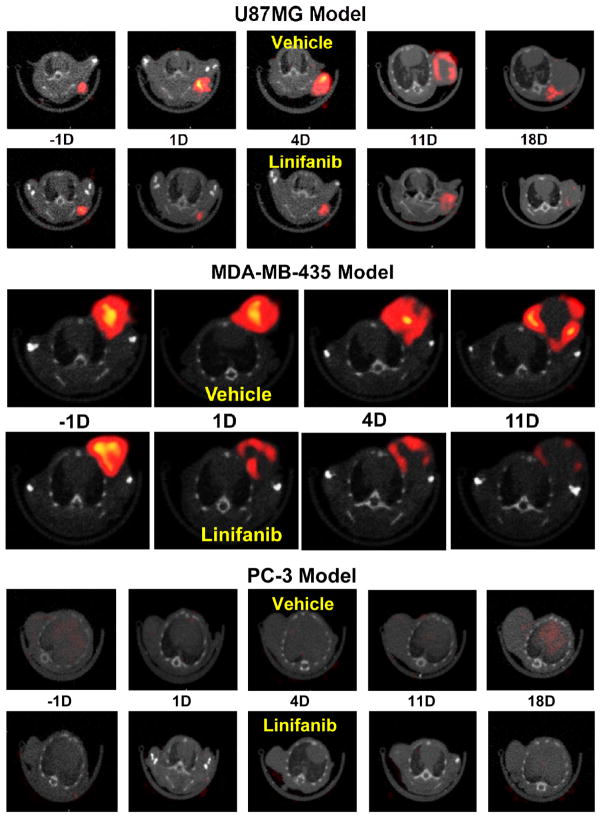
99mTc-3P-RGD2 was used to monitor the tumor response to linifanib treatment.199,200 Linifanib a multi-targeted receptor tyrosine kinase inhibitor targeting VEGF and PDGF receptors.201–206 It was found that there was a significant decrease in its tumor uptake and T/M ratios in the xenografted U87MG model while no significant changes in tumor uptake of 99mTc-3P-RGD2 were seen in the PC-3 model after linifanib therapy.199 The uptake changes in MDA-MB-435 tumors were between those observed in the U87MG and PC-3 models (Figure 15).200 This is consistent with the αvβ3 expression levels on three xenografted tumors.44,47 Highly vascularized U87MG tumors with high levels of αvβ3 and CD31 have better response to linifanib therapy than poorly vascularized PC-3 tumors with low levels of αvβ3 and CD31 (Figure 15). Thus, 99mTc-3P-RGD2 has the potential as the pre-treatment screening tool to select appropriate patients who will benefits most the antiangiogenic treatment. If the tumor in cancer patient has a high αvβ3 expression, as indicated by high tumor uptake of 99mTc-3P-RGD2 in SPECT/CT images at the time of diagnosis, antiangiogenic therapy would more likely be effective. If the tumor has little αvβ3 expression, as indicated by the low tumor uptake of 99mTc-3P-RGD2, antiangiogenic therapy would not be effective regardless the amount of antiangiogenic drug administered into each patient.
Figure 15.
The transverse views of representative SPECT/CT images for athymic nude mice bearing U87MG glioma (top), MDA-MB-435 breast cancer (middle) and PC-3 prostate (bottom) cancer xenografts at −1, 1, 4, 11 and 18 days for the vehicle and linifanib-treated groups. The imaging data were from references 199 and 217.
Monitoring Tumor Metastasis
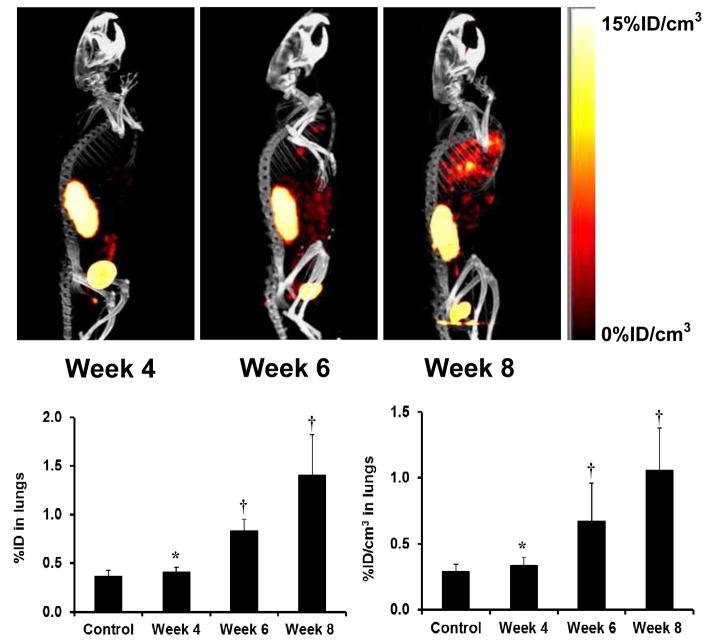
Recently, we used 99mTc-3P-RGD2 for noninvasive monitoring of tumor growth and progression of breast cancer metastasis.207,208 As illustrated in Figure 16A, there were no metastatic tumors detectable in the lungs at week 4 after tumor cell inoculation. By week 6, small lesions started to appear in the mediastinum and lungs. At week 8, SPECT/CT images revealed many metastatic tumors in both lungs. Figure 16B compares the %ID (left) and %ID/cm3 (right) uptake values of 99mTc-3P-RGD2 in the lungs. Even though the lung uptake of 99mTc-3P-RGD2 (0.41 ± 0.05 %ID) at week 4 slightly higher than that in the control animals (0.36 ± 0.06 %ID), this difference was not significant (p > 0.05) within experimental errors. At week 6, the tumor burden in lungs became significant (Figure 16B). The lung uptake of 99mTc-3P-RGD2 was higher (0.89 ± 0.12 %ID; p < 0.01) than that in the control group.208 By week 8, the lung uptake of 99mTc-3P-RGD2 increased to 1.40 ± 0.42 %ID. In all cases, the lung size remained relatively unchanged (1.21 – 1.32 cm3) during the 8-week study period. It is clear that 99mTc-3P-RGD2 SPECT is an excellent molecular imaging tool to monitor the tumor micrometastasis and metastatic tumor burden in a noninvasive fashion.
Figure 16.
Top: The 3D views of SPECT/CT images of an athymic nude mouse at week 4, 6 and 8 after tail-vein injection of 1.0 × 106 MDA-MB-231 cells. Bottom: The %ID (left) and %ID/cm3 (right) uptake values of 99mTc-3P-RGD2 in the lungs obtained from SPECT/CT quantification in the athymic nude mice (n = 8) at week 4, 6 and 8 after tail-vein injection of 1.0 × 106 MDA-MB-231 cells. Normal animals (n = 4) were used in the control group. †: p < 0.05, significantly different from the control group; *: p > 0.05, no significant difference from the control group. The imaging and SPECT quantification data were from reference 200.
Clinical Experiences with 99mTc-3P-RGD2
99mTc-3P-RGD2 is under clinical investigation as a new radiotracer for tumor imaging. In the first-in-human study, 99mTc-3P-RGD2 was investigated for its capability to differentiate solitary pulmonary nodules (SPNs).209 Among the 21 patients with SPNs, 15 (71%) were diagnosed as malignant, and 6 (29%) were benign. The sensitivities for CT and 99mTc-3P-RGD2 SPECT were 80% and 100%, respectively. All SPNs undetected by CT can be accurately diagnosed by 99mTc-3P-RGD2 SPECT. These results demonstrated the utility of 99mTc-3P-RGD2 SPECT in differentiating SPNs.209 A multicenter study was performed in 70 patients with suspected lung cancer.210 It was found that 99mTc-3P-RGD2 SPECT was effective in detecting lung malignancies. Planar imaging and chest SPECT are complementary for detection of primary tumors and metastasis.210 In a recently study, 99mTc-3P-RGD2 SPECT was used to detect the radioactive iodine-refractory differentiated thyroid carcinoma (DTC).211 It was found that 99mTc-3P-RGD2 SPECT was able to identify all the DTC lesions. There was a significant correlation between the T/B ratios and growth rates of DTC lesions. It was concluded that 99mTc-3P-RGD2 SPECT is useful for the diagnosis of DTC lesions.211 99mTc-3P-RGD2 SPECT was also evaluated for its capability to assess the breast cancer lessons.212 It was found that the mean target/non-target ratio of 99mTc-3P-RGD2 in malignant lesions was significantly higher than that in benign lesions (3.54±1.51 vs. 1.83±0.98, p < 0.001). The sensitivity, specificity, and accuracy of 99mTc-3P-RGD2 were 89.3%, 90.9% and 89.7%, respectively, with the target/non-target cut-off value of 2.40.213 The mean target/non-target ratio of 99mTc-MIBI in malignant lesions was also significantly higher than that in the benign lesions (2.86±0.99 vs. 1.51±0.61, p < 0.001). The sensitivity, specificity and accuracy of 99mTc-MIBI were 87.5%, 72.7% and 82.1%, respectively, with the target/non-target cut-off value of 1.45. The area under the curve for 99mTc-3P-RGD2 was higher than that for 99mTc-MIBI, but this difference was not statistically significant, most likely because of the limited number of patients.
Clinical Experiences with 18F-Alfatide-I
[18F]AlF(NOTA-P-RGD2) (denoted as 18F-Alfatide-I) is the 18F-labeled cyclic RGD dimer P-RGD2, the 99mTc and 111In analogs of which have been evaluated as SPECT radiotracers for tumor imaging.38,41 The Al(NOTA) chelate was used platform for 18F-labeling of P-RGD2 to avoid the multistep and time-consuming radiosynthesis.214 Under optimized conditions, 18F-Alfatide-I was prepared in high yield (~42%) with >95% radiochemical purity. However, chromatographic purification is still needed after 18F-labeling. It took about 20 min to complete both radiosynthesis and post-labeling filtration. Nine patients with lung cancer were examined with 18F-Alfatide-I PET, and 1 tuberculosis patient was also investigated using 18F-Alfatide-I and 18F-FDG PET. It was found that 18F-Alfatide-I PET could identify all the tumors with the mean uptake of 2.90 ± 0.10. The tumor/muscle and tumor/blood ratios were 5.87 ± 2.02 and 2.71 ± 0.92, respectively. It was concluded that 18F-Alfatide-I PET allows specific imaging of the αvβ3 expression in lung cancer patients.214 18F-Alfatide-I was also compared to 18F-FDG in detection of DTC lymph node metastasis.215 Twenty DTC patients with presumptive lymph node metastasis were examined with 18F-Alfatide-I and 18F-FDG PET/CT. Sixteen patients were evaluated by cytology results. A total of 39 presumptive lymph node metastases were clearly visualized on PET/CT images, and 35 lesions were confirmed as malignant tumor by biopsy and other clinical findings. It was also found that 15 DTC lesions with the diameter >1.5 cm had higher 18F-Alfatide-I uptake than the lesions <1.5 cm. Although most DTC lymph node metastases showed abnormal uptake of 18F-Alfatide-I, its diagnostic value was not as good as that of 18F-FDG.215
Clinical Experiences with 18F-Alfatide-II
[18F]AlF(NOTA-2P-RGD2) (denoted as 18F-Alfatide-II) is another 18F-labeled dimeric cyclic RGD peptide 2P-RGD2, which was first developed by Dr. Liu’s group at Purdue University for preparation of 99mTc-2P-RGD2.35,41 Dr. Chen’s group at the National Institute of Biomedical Imaging and Bioengineering (NIBIB) recently reported the use of 18F-Alfatide-II and 18F-FDG for monitoring the tumor responses to doxorubicin therapy in xenografted U87MG and MDA-MB-435 models.216 It was found that there were substantial differences in the 18F-Alfatide-II binding potential and 18F-FDG influx rate 3 days after doxorubicin treatment.216 It was also found that injection of [18F]Alfatide-II was well tolerated in all healthy volunteers.217 18F-Alfatide-II showed a rapid clearance from the blood pool and kidneys. Nine patients with 20 brain metastatic lesions identified by MRI and/or CT were enrolled in this study. All 20 brain lesions were visualized by 18F-Alfatide-II PET, while only ten lesions were visualized by 18 F-FDG, and 13 by CT.217 It was concluded that 18F-Alfatide-II is valuable biomarker of angiogenesis in finding brain metastases of different cancers.
New Opportunities and Challenges
Advantages of Multimeric Cyclic RGD Peptides
Tumor imaging with radiolabeled cyclic RGD peptides depends on their αvβ3 binding affinity and the total αvβ3 population. The advantage of multimeric cyclic RGD peptides is their higher αvβ3 binding affinity than that of their monomeric analogs, and their capability to target multiple integrins in tumor tissues. The multi-integrin targeting capability may contribute to the fact that they are able to localize in human carcinomas of different origin. Our studies show that radiolabeled tetrameric cyclic RGD peptides have higher uptake than their dimeric analogs in both tumors and αvβ3-positive organs.33,50 High tumor uptake is important for the sensitivity, but their potential as tumor imaging agents will rely upon the contrast between tumor and normal tissues. There is always a subtle balance between the tumor uptake and T/B ratios.
Other Applications and Limitations
The success of radiolabeled cyclic RGD peptides as PET or SPECT radiotracers can be attributed to their capability to target a large population of cancer patients with carcinomas of breast, lung and prostate. It is important to note that αvβ3 is also overexpressed on activated endothelial cells during wound healing and post-infarct remodeling, in rheumatoid arthritis and atherosclerotic plaque.218–221 Thus, αvβ3 is a biomarker for inflammation and angiogenesis.221 The αvβ3–targeted radiotracers have been used for imaging myocardial angiogenesis,222,223 inflammatory diseases,224 hindlimb ischemia,225 and plaque vulnerability.226,227 It was reported that the 111In-labeled αvβ3 antagonist was able to image angiogenesis after myocardial infarction.222 18F-Galacto-RGD has been used to distinguish between the acute and chronic phases of T-cell mediated immune responses,228 and to image the αvβ3 expression in human carotid atherosclerosis.229 These promising results suggest that αvβ3–targeted radiotracers might be valuable for imaging angiogenesis after ischemic injury, myocardial infarction and inflammation. These broad applications of αvβ3–targeted radiotracers also raise significant questions related to their tumor specificity. It important to note that radiolabeled RGD peptides are αvβ3–targeted radiotracers. As long as the diseased tissue has the αvβ3 over-expression, it will have radiotracer uptake. In this situation, the differentiation between tumors and other diseased tissues will depend on their difference in αvβ3 expression levels. For example, the mean T/B ratio of 99mTc-3P-RGD2 in malignant breast cancer lesions was 3.54±1.51, which was significantly (p < 0.001) higher than that in the inflammatory benign lesions (1.83±0.98).213 In this respect, radiolabeled cyclic RGD peptides are very similar to 18F-FDG since the metabolic activity is elevated during tumor growth, wound healing, post-infarct remodeling, and in rheumatoid arthritis and atherosclerotic plaque.
Integrin αvβ3 Expression Heterogeneity
Because of variations in the tumor vasculature, different tumor tissues often have a large difference in αvβ3 expression levels. Recently, we evaluated FITC-labeled dimeric RGD peptides (e.g. FITC-Galacto-RGD2) for their potential as fluorescent probes to quantify the total αvβ3 expression levels in both the xenografted tumors and human carcinoma tissues.183 It was found that the total αvβ3 levels followed a general order: colon cancer > pancreatic cancer > lung adenocarcinoma ≈ squamous cell lung cancer ≫ gastric cancer ≈ esophageal cancer. The same conclusion was also made using the fluorescence-labeled integrin β3 antibody. For the xenografted tumors (cancer cells of human origin and tumor vasculature of murine origin), the αvβ3 expression levels followed the general trend: U87MG (high αvβ3 expression on both glioma cells and tumor vasculature) > MDA-MB-435 (moderately high αvβ3 expression on breast tumor cells and tumor vasculature) ~ A549 (moderately high αvβ3 expression on lung tumor cells and tumor vasculature) ~ HT29 (high αvβ3 expression on the tumor vasculature, but low αvβ3 expression on tumor cells) > PC-3 (low αvβ3 expression on both prostate cancer cells and tumor vasculature).44,46,183 It has been estimated that the percentage of contribution from the tumor neovasculature to the total αvβ3 expression level and tumor uptake of 99mTc-3P-RGD2 is ~60% in the xenografted U87MG glioma model.199 In case of the xenografted HT29 tumors, the main contribution to the tumor uptake of 99mTc-3P-RGD2 is from the αvβ3 on neovasculature.44,46 Furthermore, the αvβ3 expression level may change with tumor metastatic status. For example, the αvβ3 expression level may be low in primary tumors. However, it could be very high once the αvβ3 is activated and the tumor becomes highly metastatic. Therefore, caution must be taken in generalizing the αvβ3-expression levels in different cancer types.
Comparison with 18F-FDG
18F-FDG is the gold-standard in diagnostic oncology. It is not surprising that 18F-FDG is often utilized as the positive control in evaluations of αvβ3–targeted radiotracers. However, this comparison may not be fair because they are targeting two different biological processes. 18F-FDG is used to detect the metabolic activity while the RGD-based radiotracers target tumor αvβ3 expression. Clinical data from cancer patients suggest that perfusion, αvβ3 expression and glucose metabolism in active tumor areas are independent variables of the tumor biology.230,231 There is no direct relationship between the tumor metabolism and αvβ3 expression.230,231 Molecular imaging with 18F-FDG and αvβ3-targeted radiotracers can provide complementary information and might help to evaluate both tumor metabolism and angiogenesis in-vivo in its full complexity.
Challenges in Radiotracer Development
The discovery of highly novel targeting biomolecules (such as multimeric cyclic RGD peptides) requires intensive efforts from researchers and resources from their institutions. It is easy to use known cyclic RGD peptides from the literature to develop αvβ3–targeted radiotracers by simply changing the radioisotope (99mTc vs. 18F or 68Ga) or radiometal chelate. However, the novelty of radiotracers developed from this approach is highly questionable. No industrial partners would commit millions of dollars to develop a new radiotracer without the intellectual proprietary position for commercialization. It must be emphasized that discovery of new multimeric cyclic RGD peptides is only the first step of a long process of radiotracer development. For diagnostic radiopharmaceuticals, the development times are generally 8 – 10 years and the total development costs are between $100 million and $200 million.232 For research purposes, it is common to use post-labeling chromatography to improve radiotracer purity and specific activity before being used for pre-clinical animal studies. In clinical settings, chromatographic purification is not practical. Thus, development of efficient radiolabeling techniques is very important regardless of radioisotope. HYNIC and MAG2 are useful for routine 99mTc-labeling of small biomolecules using kit formulation,46,47,68–70 while DOTA and NOTA derivatives are better suited for chelation of 64Cu and 68Ga.7,67,68,71,72 Successful development of Al(NOTA) as a prosthetic group for 18F-labeling of biomolecules represents a milestone for routine radiosynthesis of receptor-based target-specific 18F radiotracers.90–97 Another significant challenge that many academic researchers have to face is how to translate the results from bench discovery and preclinical evaluations in small animal models into actual clinical practice. Academic researchers often consider clinical translation to be the first use of a new radiotracer in humans.232 From the industry point of view, clinical translation means sustained sales and an impact of a new radiotracer on patient care.232 The costs to develop a new radiotracer into a successful “diagnostic drug” routinely used in nuclear medicine clinics are much more than those to test a new radiotracer in the physician-sponsored clinical trials. Successful development of a new radiotracer requires teamwork from several different disciplines and strong commitment from radiopharmaceutical and pharmaceutical industry. Otherwise, clinical translation will remain as “an elusive goal” for many, including academic researchers, industrial drug developer, clinicians and cancer patients.
Potential Impact of Future SPECT Scanners
The success of a new SPECT radiotracer also depends on availability of high quality SPECT scanners. It is well-accepted that conventional dual-head SPECT scanners currently available in nuclear medicine clinics have significant drawbacks in radioactivity quantification, speed of dynamic imaging, spatial resolution and tissue attenuation. If the industry is willing to devote the resources for development of new SPECT cameras as much as those for PET cameras, most of these challenges can be overcome. The development of ultrafast SPECT scanners (e.g. D-SPECT from Spectrum Dynamics, IQ SPECT® developed by Siemens Medical Solutions and CardiArc® manufactured by CardiArc Inc.) represents an excellent examples of future SPECT cameras with the capability for quantification of organ uptake and fast speed of dynamic imaging with good spatial resolution.233–240 The larger radiation collection angles along with the use of many stationary solid state CZT (cadmium zinc telluride) detectors in a D-SPECT scanner provide 10x more efficient photon collection, leading to improved count statistics and higher quality images. Furthermore, development of the CZT-based whole-body SPECT/CT scanners and new computer software with the capability for attenuation and photon-scattering correction will help to achieve full clinical potentials of the αvβ3–targeted SPECT radiotracers.
Conclusions
Radiolabeled cyclic RGD peptides are called “αvβ3–targeted” radiotracers because αvβ3 is studied most extensively for its role in tumor angiogenesis and metastasis. The capability of radiolabeled cyclic RGD peptides to target multiple integrins will improve their tumor uptake due to the increased receptor population. Multimerization of cyclic RGD peptides increases the tumor uptake and retention times of their radiotracers. Two most important factors (bivalency and locally enhanced RGD concentration) contribute to the higher αvβ3 binding affinity of multimeric cyclic RGD peptides than their corresponding monomeric analogs. The concentration factor exists in all multimeric cyclic RGD peptides regardless of the linker length between two cyclic RGD motifs. The key to achieve bivalency is the distance between two cyclic RGD motifs. Among the dimeric and tetrameric cyclic RGD peptides evaluated in our laboratories, 2P-RGD2, 3P-RGD2, 2G-RGD2, 3G-RGD2 and Galacto-RGD2 show the most promising results with respect to T/B ratios of their radiotracers.23,24,34–50 In the literature, a few 68Ga-labeled trimeric RGD peptides were used as PET radiotracers.11,14 It would be interesting to compare them with their dimeric and tetrameric analogs with respect to the tumor uptake and T/B ratios of 99mTc, 111In, 64Cu and 68Ga radiotracers in the same model, and determine the optimal multiplicity for development of αvβ3-targeted cyclic RGD peptides.
99mTc-3P-RGD2, 18F-Alfatide-I and 18F-Alfatide-II are currently evaluated as new radiotracers for early cancer detection in clinics. Since 99mTc-3P-RGD2 is prepared in >95% purity without purification, it offers significant advantages over the corresponding 18F radiotracers, which are often requires post-labeling chromatographic purification. It would be difficult for 18F radiotracers to assume widespread clinical acceptance if they have a poor clinical availability. Their high cost may also prove to be too much for a large population of cancer patients to afford the prescribed procedures. The beauty of new radiotracer development lies in science; but the success of new radiotracer relies on its clinical availability at low cost and the easiness of its routine radiosynthesis. The ultimate goal is to improve the quality of life for cancer patients with αvβ3–targeted radiotracers.
Acknowledgments
The author would like to thank all his graduate students, postdoctoral fellows and visiting scholars over last 10 years for their contributions to the development of dimeric and tetrameric cyclic RGD peptides, and their 18F, 99mTc, 111In, 64Cu and 68Ga radiotracers. This work was supported by Purdue University and research grants: R01 CA115883 (S.L.) from the National Cancer Institute (NCI) and R21 EB017237-01 (S.L.) from the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
ABBREVIATIONS
General terms
- BFC
bifunctional coupling agent
- CT
X-ray computed tomography
- DCE-MRI
dynamic contrast-enhanced magnetic resonance imaging
- dasatinib
N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide
- 18F-FDG
2-deoxy-2-(18F)fluoro-D-glucose
- FITC
Fluorescein isothiocyanate isomer I
- IHC
immunohistochemistry
- MRI
magnetic resonance imaging
- MW
molecular weight
- PET
positron emission tomography
- PDGFR
Platelet-derived growth factor receptors
- RGD
arginine-glycine-aspartic (Arg-Gly-Asp)
- SPECT
single photon emission computed tomography
- sunitinib
hydroxy-(2S)-compound with N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide; and
- VEGFR
vascular endothelial growth factor receptors
Chelators
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetracetic acid
- HYNIC
6-hydazinonicotinic acid; and
- NOTA
1,4,7-tritazacyclononane-1,4,7-triacetic acid
Cyclic Peptides
- P-RGD
PEG4-c(RGDfK) = cyclo(Arg-Gly-Asp-D-Phe-Lys(PEG4)) (PEG4 = 15-amino-4,7,10,13-tetraoxapentadecanoic acid)
- RGD2
E[c(RGDfK)]2 = Glu[cyclo(Arg-Gly-Asp-D-Phe-Lys)]2
- Galacto-RGD2
Glu[cyclo[Arg-Gly-Asp-D-Phe-Lys(SAA-PEG2-(1,2,3-triazole)-1-yl-4-methylamide)]]2 (SAA = 7-amino-L-glycero-L-galacto-2,6-anhydro-7-deoxyheptanamide, and PEG2 = 3,6-dioxaoctanoic acid)
- P-RGD2
PEG4-E[c(RGDfK)]2 = PEG4-Glu[cyclo(Arg-Gly-Asp-D-Phe-Lys)]2
- P2D-RGD2
PEG4-E[D3-c(RGDfK)]2 = PEG4-Glu[cyclo[Arg-Gly-Asp-D-Phe-Lys(D3)]]2 (D3 = Asp-Asp-Asp)
- P2G-RGD2
PEG4-E[G3-c(RGDfK)]2 = PEG4-Glu[cyclo[Arg-Gly-Asp-D-Phe-Lys(G3)]]2 (G3 = Gly-Gly-Gly)
- 2G-RGD2
E[G3-c(RGDfK)]2 = Glu[cyclo[Arg-Gly-Asp-D-Phe-Lys(G3)]]2
- 2P-RGD2
E[PEG4-c(RGDfK)]2 = Glu[cyclo[Arg-Gly-Asp-D-Phe-Lys(PEG4)]]2
- 3G-RGD2
G3-E[G3-c(RGDfK)]2 = G3-Glu[cyclo[Arg-Gly-Asp-D-Phe-Lys(G3)]]2
- 3P-RGD2
PEG4-E[PEG4-c(RGDfK)]2 = PEG4-Glu[cyclo[Arg-Gly-Asp-D-Phe-Lys(PEG4)]]2
- 3P-RGK2
PEG4-E[PEG4-c(RGKfD)]2 = PEG4-Glu[cyclo[Arg-Gly-Lys(PEG4)-D-Phe-Asp]]2)
- RGD4
E{E[c(RGDfK)]2}2 = Glu{Glu[cyclo(Arg-Gly-Asp-D-Phe-Lys)]2}2
- 6G-RGD4
E{G3-E[G3-c(RGDfK)]2}2 = Glu{G3-Glu[cyclo(Lys(G3)-Arg-Gly-Asp-D-Phe)]-cyclo(Lys(G3)-Arg-Gly-Asp-D-Phe)}-{PEG4-Glu[cyclo(Lys(G3)-Arg-Gly-Asp-D-Phe)]-cyclo(Lys(G3)-Arg-Gly-Asp-D-Phe)}; and
- 6P-RGD4
E{PEG4-E[PEG4-c(RGDfK)]2}2 = Glu{PEG4-Glu[cyclo(Lys(PEG4)-Arg-Gly-Asp-D-Phe)]-cyclo(Lys(PEG4)-Arg-Gly-Asp-D-Phe)}-{PEG4-Glu[cyclo(Lys(PEG4)-Arg-Gly-Asp-D-Phe)]-cyclo(Lys(PEG4)-Arg-Gly-Asp-D-Phe)}
Cyclic Peptide Bioconjugates
- DOTA-RGD
DOTA-c(RGDfK)
- DOTA-P-RGD
DOTA-PEG4-c(RGDfK)
- DOTA-RGD2
DOTA-E[c(RGDfK)]2
- DOTA-P-RGD2
DOTA-PEG4-E[c(RGDfK)]2
- DOTA-2G-RGD2
DOTA-E[G3-c(RGDfK)]2
- DOTA-2P-RGD2
DOTA-E[PEG4-c(RGDfK)]2
- DOTA-3G-RGD2
DOTA-G3-E[G3-c(RGDfK)]2
- DOTA-3P-RGD2
DOTA-PEG4-E[PEG4-c(RGDfK)]2
- DOTA-3P-RGK2
DOTA-PEG4-E[PEG4-c(RGKfD)]2
- DOTA-Galacto-RGD2
DOTA-Glu[c[RGDfK(SAA-PEG2-(1,2,3-triazole)-1-yl-4-methylamide)]]2
- DOTA-RGD4
DOTA-E{E[c(RGDfK)]2}2
- DOTA-6G-RGD4
DOTA- E{G3-E[G3-c(RGDfK)]2}2
- DOTA-6P-RGD4
E{PEG4-E[PEG4-c(RGDfK)]2}2
- FITC-3P-RGD2
FITC-PEG4-E[PEG4-c(RGDfK)]2
- FITC-3P-RGK2
FITC-PEG4-E[PEG4-c(RGKfD)]2
- FITC-Galacto-RGD2
FITC-Glu[c[RGDfK(SAA-PEG2-(1,2,3-triazole)-1-yl-4-methylamide)]]2
- HYNIC-RGD2
HYNIC-E[c(RGDfK)]2
- HYNIC-P-RGD2
HYNIC-PEG4-E[c(RGDfK)]2
- HYNIC-P2D-RGD2
HYNIC-PEG4-E[D3-c(RGDfK)]2
- HYNIC-P2G-RGD2
HYNIC-PEG4-E[G3-c(RGDfK)]2
- HYNIC-2G-RGD2
HYNIC-E[G3-c(RGDfK)]2
- HYNIC-2P-RGD2
HYNIC-E[PEG4-c(RGDfK)]2
- HYNIC-3G-RGD2
HYNIC-G3-E[G3-c(RGDfK)]2
- HYNIC-3P-RGD2
HYNIC-PEG4-E[PEG4-c(RGDfK)]2
- HYNIC-K(NIC)-3P-RGD2
HYNIC-K(NIC)-PEG4-E[PEG4-c(RGDfK)]2 (NIC = nicotinyl)
- K(HYNIC)2-3P-RGD2
K(HYNIC)2-PEG4-E[PEG4-c(RGDfK)]2
- HYNIC-Galacto-RGD2
HYNIC-E[c[RGDfK(SAA-PEG2-(1,2,3-triazole)-1-yl-4-methylamide)]]2
- HYNIC-RGD4
HYNIC-E{E[c(RGDfK)]2}2
- NOTA-P-RGD2
NOTA-PEG4-E[c(RGDfK)]2
- NOTA-2G-RGD2
NOTA-E[G3-c(RGDfK)]2
- NOTA-2P-RGD2
NOTA-E[PEG4-c(RGDfK)]2
- NOTA-3G-RGD2
NOTA-G3-E[G3-c(RGDfK)]2; and
- NOTA-3P-RGD2
NOTA-PEG4-E[PEG4-c(RGDfK)]2
Radiolabeled Cyclic RGD Peptides
- 18F-Alfatide-I
[18F]AlF(NOTA-P-RGD2)
- 18F-Alfatide-II
[18F]AlF(NOTA-2P-RGD2)
- 18F-Galacto-RGD
(2-[18F]fluoropropanamide c(RGDfK(SAA))
- 64Cu-P-RGD2
64Cu(DOTA-P-RGD2)
- 64Cu-2G-RGD2
64Cu(DOTA-2G-RGD2)
- 64Cu-2P-RGD2
64Cu(DOTA-2P-RGD2)
- 64Cu-3G-RGD2
64Cu(DOTA-3G-RGD2)
- 64Cu-3P-RGD2
64Cu(DOTA-3P-RGD2)
- 68Ga-3G-RGD2
68Ga(DOTA-3G-RGD2)
- 68Ga-3P-RGD2
68Ga(DOTA-3P-RGD2)
- 111In-P-RGD
111In(DOTA-P-RGD)
- 111In-P-RGD2
111In(DOTA-P-RGD2)
- 111In-3G-RGD2
111In(DOTA-3G-RGD2)
- 111In-3P-RGD2
111In(DOTA-3P-RGD2)
- 111In-Galacto-RGD2
111In(DOTA-Galacto-RGD2)
- 111In-6G-RGD4
111In(DOTA-6G-RGD4)
- 111In-6P-RGD4
111In(DOTA-6P-RGD4)
- 99mTc-Galacto-RGD2
[99mTc(HYNIC-Galacto-RGD2)(tricine)(TPPTS)])
- 99mTc-RGD2
[99mTc(HYNIC-RGD2)(tricine)(TPPTS)])
- 99mTc-P-RGD2
[99mTc(HYNIC-P-RGD2)(tricine)(TPPTS)]
- 99mTc-P2D-RGD2
[99mTc(HYNIC-P2D-RGD2)(tricine)(TPPTS)]
- 99mTc-P2G-RGD2
[99mTc(HYNIC-P2G-RGD2)(tricine)(TPPTS)]
- 99mTc-2G-RGD2
[99mTc(HYNIC-2G-RGD2)(tricine)(TPPTS)]
- 99mTc-2P-RGD2
[99mTc(HYNIC-2P-RGD2)(tricine)(TPPTS)]
- 99mTc-3G-RGD2
[99mTc(HYNIC-3G-RGD2)(tricine)(TPPTS)]
- 99mTc-3P-RGD2
[99mTc(HYNIC-3P-RGD2)(tricine)(TPPTS)]
- 99mTc-3P-RGD2-A
[99mTc(HYNIC-K(NIC)-3P-RGD2)(tricine)]
- 99mTc-3P-RGD2-B
[99mTc(K(HYNIC)2-3P-RGD2)(tricine)]; and
- 99mTc-RGD4
[99mTc(HYNIC-RGD4)(tricine)(TPPTS)])
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Mankoff DA, Link JM, Linden HM, Sundararajan L, Krohn KA. Tumor receptor imaging. J Nucl Med. 2008;49:149S–163S. doi: 10.2967/jnumed.107.045963. [DOI] [PubMed] [Google Scholar]
- 3.Tweedle MF. Peptide-targeted diagnostics and radiotherapeutics. Acc Chem Res. 2009;42:958–968. doi: 10.1021/ar800215p. [DOI] [PubMed] [Google Scholar]
- 4.Fani M, Maecke HR. Radiopharmaceutical development of radiolabelled peptides. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S11–S30. doi: 10.1007/s00259-011-2001-z. [DOI] [PubMed] [Google Scholar]
- 5.Fani M, Maecke HR, Okarvi SM. Radiolabeled peptides: valuable tools for the detection and treatment of cancer. Theranostics. 2012;2:481–501. doi: 10.7150/thno.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaertner FC, Kessler H, Wester HJ, Schwaiger M, Beer AJ. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S126–138. doi: 10.1007/s00259-011-2028-1. [DOI] [PubMed] [Google Scholar]
- 7.Laverman P, Sosabowski JK, Boerman OC, Oyen WJG. Radiolabelled peptides for oncological diagnosis. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S78–S92. doi: 10.1007/s00259-011-2014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamous M, Haberkorn U, Mier W. Synthesis of peptide radiopharmaceuticals for the therapy and diagnosis of tumor diseases. Molecules. 2013;18:3379–3409. doi: 10.3390/molecules18033379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shokeen M, Anderson CJ. Molecular imaging of cancer with copper-64 radiopharmaceuticals and positron emission tomography (PET) Acc Chem Res. 2009;42:832–841. doi: 10.1021/ar800255q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correia JDG, Paulo A, Raposinho PD, Santos I. Radiometallated peptides for molecular imaging and targeted therapy. Dalton Trans. 2012;40:6144–6167. doi: 10.1039/c0dt01599g. [DOI] [PubMed] [Google Scholar]
- 11.Kubasa H, Schäfera M, Bauder-Wüsta U, Edera M, Oltmannsa D, Haberkornb U, Mierb W, Eisenhut M. Multivalent cyclic RGD ligands: influence of linker lengths on receptor binding. Nucl Med Biol. 2010;37:885–891. doi: 10.1016/j.nucmedbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Šiměcek J, Hermann P, Havíčková J, Herdtweck E, Kapp TG, Engelbogen N, Kessler H, Wester HJ, Notni J. Cyclen-based tetraphosphinate chelator for the preparation of radiolabeled tetrameric bioconjugates. Chem Eur J. 2013;19:7748–7757. doi: 10.1002/chem.201300338. [DOI] [PubMed] [Google Scholar]
- 13.Dumont RA, Deininger F, Haubner R, Maecke HR, Weber WA, Fani M. Novel 64Cu- and 68Ga-labeled RGD conjugates show improved PET imaging of αvβ3 integrin expression and facile radiosynthesis. J Nucl Med. 2011;52:1276–1284. doi: 10.2967/jnumed.111.087700. [DOI] [PubMed] [Google Scholar]
- 14.Knetsch PA, Zhai C, Rangger C, Blatzer M, Haas H, Kaeopookum P, Haubner R, Decristoforo C. [68Ga]FSC-(RGD)3 a trimeric RGD peptide for imaging αvβ3 integrin expression based on a novel siderophore derived chelating scaffold-synthesis and evaluation. Nucl Med Biol. 2015;42:115–122. doi: 10.1016/j.nucmedbio.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ. 68Ga-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol. 2012;39:777–784. doi: 10.1016/j.nucmedbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Guo J, Tang S, Lang L, Chen X, Perrin DM. One-step and one-pot-two-step radiosynthesis of cyclo-RGD-18F-aryltrifluoroborate conjugates for functional imaging. Am J Nucl Med Mol Imaging. 2013;3:44–56. [PMC free article] [PubMed] [Google Scholar]
- 17.Tsiapa I, Loudos G, Varvarigou A, Fragogeorgi E, Psimadas D, Tsotakos T, Xanthopoulos S, Mihailidis D, Bouziotis P, Nikiforidis GC, et al. Biological evaluation of an ornithine-modified 99mTc-labeled RGD peptide as an angiogenesis imaging agent. Nucl Med Biol. 2013;40:262–272. doi: 10.1016/j.nucmedbio.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Maschauer S, Haubner R, Kuwert T, Prante O. 18F-Glyco-RGD peptides for PET imaging of integrin expression: efficient radiosynthesis by click chemistry and modulation of biodistribution by glycosylation. Mol Pharm. 2014;11:505–515. doi: 10.1021/mp4004817. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. MicroPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 20.Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS, Chen X. Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FRGD2. J Nucl Med. 2005;47:113–121. [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Li Z, Chen K, Cai W, He L, Chin FT, Li F, Chen X. MicroPET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18FFPRGD4) J Nucl Med 2005. 2005;48:1536–1544. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li ZB, Chen K, Chen X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin αvβ3 expression. Eur J Nucl Med Mol Imaging. 2008;35:1100–1108. doi: 10.1007/s00259-007-0692-y. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Niu G, Shi J, Liu S, Wang F, Chen X. 68Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin αvβ3 PET imaging. Eur J Nucl Med Mol Imaging. 2009;36:947–957. doi: 10.1007/s00259-008-1045-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Liu S, Wang F, Chen X. Noninvasive imaging of tumor integrin expression using 18F-labeled RGD dimer peptide with PEG4 linkers. Eur J Nucl Med Mol Imaging. 2009;36:1296–1307. doi: 10.1007/s00259-009-1112-2. [DOI] [PubMed] [Google Scholar]
- 25.Dijkgraaf I, Liu S, Kruijtzer JA, Soede AC, Oyen WJG, Liskamp RM, Corstens FH, Boerman OC. Effect of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD Peptide. Nucl Med Biol. 2007;34:29–35. doi: 10.1016/j.nucmedbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Dijkgraaf I, Kruijtzer JA, Liu S, Soede AC, Oyen WJG, Corstens FH, Liskamp RM, Boerman OC. Improved targeting of the αvβ3 integrin by multimerization of RGD peptides. Eur J Nucl Med Mol Imaging. 2007;34:267–273. doi: 10.1007/s00259-006-0180-9. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Hsieh WY, Kim YS, Mohammed SI. Effect of coligands on biodistribution characteristics of ternary ligand 99mTc complexes of a HYNIC-conjugated cyclic RGDfK dimer. Bioconj Chem. 2005;16:1580–1588. doi: 10.1021/bc0501653. [DOI] [PubMed] [Google Scholar]
- 28.Jia B, Shi J, Yang Z, Xu B, Liu Z, Zhao H, Liu S, Wang F. 99mTc-labeled cyclic RGDfK dimer: initial evaluation for SPECT imaging of glioma integrin αvβ3 expression. Bioconj Chem. 2006;17:1069–1076. doi: 10.1021/bc060055b. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, He ZJ, Hsieh WY, Kim YS, Jiang Y. Impact of PKM linkers on biodistribution characteristics of the 99mTc-labeled cyclic RGDfK dimer. Bioconj Chem. 2006;17:1499–1507. doi: 10.1021/bc060235l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Hsieh WY, Jiang Y, Kim YS, Sreerama SG, Chen X, Jia B, Wang F. Evaluation of a 99mTc-labeled cyclic RGD tetramer for noninvasive imaging integrin αvβ3-positive breast cancer. Bioconj Chem. 2007;18:438–446. doi: 10.1021/bc0603081. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Kim YS, Hsieh WY, Sreerama SG. Coligand effects on solution stability, biodistribution and metabolism of 99mTc-labeled cyclic RGDfK tetramer. Nucl Med Biol. 2008;35:111–121. doi: 10.1016/j.nucmedbio.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia B, Liu Z, Shi J, Yu ZL, Yang Z, Zhao HY, He ZJ, Liu S, Wang F. Linker effects on biological properties of 111In-labeled DTPA conjugates of a cyclic RGDfK dimer. Bioconj Chem. 2008;19:201–210. doi: 10.1021/bc7002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JJ, Kim YS, He ZJ, Liu S. 99mTc-labeling of HYNIC-conjugated cyclic RGDfK dimer and tetramer using EDDA as coligand. Bioconj Chem. 2008;19:634–642. doi: 10.1021/bc7004208. [DOI] [PubMed] [Google Scholar]
- 34.Shi J, Wang L, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic Arginine-Glycine-Aspartic (RGD) dimers with triglycine linkers. J Med Chem. 2008;51:7980–7990. doi: 10.1021/jm801134k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Shi J, Kim YS, Zhai S, Jia B, Zhao H, Liu Z, Wang F, Chen X, Liu S. Improving tumor targeting capability and pharmacokinetics of 99mTc-labeled cyclic RGD dimers with PEG4 linkers. Mol Pharm. 6:231–245. doi: 10.1021/mp800150r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi J, Wang L, Kim YS, Zhai S, Jia B, Wang F, Liu S. 99mTcO(MAG2-3G3-dimer): A new integrin αvβ3-targeted radiotracer with high tumor uptake and favorable pharmacokinetics. Eur J Nucl Med Mol Imaging. 2009;36:1874–1884. doi: 10.1007/s00259-009-1166-1. [DOI] [PubMed] [Google Scholar]
- 37.Shi J, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD dimers with triglycine and PEG4 linkers. Bioconj Chem. 2009;20:750–759. doi: 10.1021/bc800455p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi J, Kim YS, Chakraborty S, Jia B, Wang F, Liu S. 2-Mercaptoacetylglycylglycyl (MAG2) as a bifunctional chelator for 99mTc-labeling of cyclic RGD dimers: effects of technetium chelate on tumor uptake and pharmacokinetics. Bioconj Chem. 2009;20:1559–1568. doi: 10.1021/bc9001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty S, Liu S, Kim YS, Shi J, Zhou Y, Wang F. Evaluation of 111In-labeled cyclic RGD peptides: tetrameric not tetravalent. Bioconj Chem. 2011;21:969–978. doi: 10.1021/bc900555q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Kim YS, Chakraborty S, Shi J, Gao H, Liu S. 99mTc-Labeled cyclic RGD peptides for noninvasive monitoring of tumor integrin αvβ3 expression. Mol Imaging. 2011;10:386–97. doi: 10.2310/7290.2011.00006. [DOI] [PubMed] [Google Scholar]
- 41.Shi J, Zhou Y, Chakraborty S, Kim YS, Jia B, Wang F, Liu S. Evaluation of 111In-labeled cyclic RGD peptides: effects of peptide and PEG4 multiplicity on their tumor uptake, excretion kinetics and metabolic stability. Theranostics. 2011;1:322–340. doi: 10.7150/thno/v01p0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi J, Jia B, Kim YS, Chakraborty S, Zhou Y, Wang F, Liu S. Impact of bifunctional chelators on biological properties of 111In-labeled cyclic peptide RGD dimers. Amino Acids. 2011;41:1059–1070. doi: 10.1007/s00726-009-0439-0. [DOI] [PubMed] [Google Scholar]
- 43.Jia B, Liu Z, Zhu Z, Shi J, Jin X, Zhao H, Li F, Liu S, Wang F. Blood clearance kinetics, biodistribution and radiation dosimetry of a kit-formulated integrin αvβ3-selective radiotracer 99mTc-3PRGD2 in non-human primates. Mol Imaging Biol. 2011;13:730–736. doi: 10.1007/s11307-010-0385-y. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Kim YS, Chakraborty S, Shi J, Gao H, Liu S. 99mTc-labeled cyclic RGD peptides for noninvasive monitoring of tumor integrin αvβ3 expression. Mol Imaging. 2011;10:386–397. doi: 10.2310/7290.2011.00006. [DOI] [PubMed] [Google Scholar]
- 45.Jacobson O, Zhu L, Niu G, Szajek L, Ma Y, Sun X, Yan Y, Kiesewetter DO, Liu S, Chen X. MicroPET imaging of integrin αvβ3 expressing tumors using 89Zr-RGD peptides. Mol Imaging Biol. 2011;13:1224–1233. doi: 10.1007/s11307-010-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Kim YS, Lu X, Liu S. Evaluation of 99mTc-labeled cyclic RGD dimers: impact of cyclic RGD peptides and 99mTc chelates on biological properties. Bioconj Chem. 2012;23:586–595. doi: 10.1021/bc200631g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji S, Zhou Y, Shao G, Liu S. (HYNIC)2K: A bifunctional chelator useful for 99mTc-labeling of small biomolecules. Bioconj Chem. 2013;24:701–711. doi: 10.1021/bc3006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji S, Czerwinski A, Zhou Y, Shao G, Valenzuela F, Sowiński P, Chauhan S, Pennington M, Liu S. 99mTc-Galacto-RGD2: a 99mTc-labeled cyclic RGD peptide dimer useful for tumor imaging. Mol Pharm. 2013;10:3304–3314. doi: 10.1021/mp400085d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Ji S, Liu S. Impact of multiple negative charges on blood clearance and biodistribution characteristics of 99mTc-labeled dimeric cyclic RGD peptides. Bioconj Chem. 2014;25:1720–1729. doi: 10.1021/bc500309r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y, Ji S, Yang Y, Tomaselli E, Liu S. 111In-Labeled cyclic RGD peptides useful as integrin αvβ3-targeted radiotracers for breast tumor imaging. Nucl Med Biol. 2015;42:137–145. doi: 10.1016/j.nucmedbio.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Meyer A, Auremheimer J, Modlinger A, Kessler H. Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Design. 2006;12:2723–2747. doi: 10.2174/138161206777947740. [DOI] [PubMed] [Google Scholar]
- 52.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3-targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 53.Beer AJ, Schwaiger M. Imaging of integrin αvβ3 expression. Cancer Metastasis Rev. 2008;27:631–644. doi: 10.1007/s10555-008-9158-3. [DOI] [PubMed] [Google Scholar]
- 54.Liu Z, Wang F, Chen X. Integrin αvβ3-targeted cancer therapy. Drug Dev Res. 2008;69:329–339. doi: 10.1002/ddr.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S. Radiolabeled RGD peptides as integrin αvβ3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconj Chem. 2009;20:2199–2213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stollman TH, Ruers TJM, Oyen WJG, Boerman OC. New targeted probes for radioimaging of angiogenesis. Methods. 2009;48:188–192. doi: 10.1016/j.ymeth.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Haubner R, Beer AJ, Wang H, Chen X. Positron emission tomography tracers for imaging angiogenesis. Eur J Nucl Med Mol Imaging. 2010;37(Suppl 1):S86–103. doi: 10.1007/s00259-010-1503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beer AJ, Chen X. Imaging of angiogenesis: from morphology to molecules and from bench to bedside. Eur J Nucl Med Mol Imaging. 2010;37(Suppl 1):S1–3. doi: 10.1007/s00259-010-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dijkgraaf I, Boerman OC. Molecular imaging of angiogenesis with SPECT. Eur J Nucl Med Mol Imaging. 2010;37(Suppl 1):S104–S113. doi: 10.1007/s00259-010-1499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakraborty S, Liu S. 99mTc and 111In-labeling of small biomolecules: bifunctional chelators and related coordination chemistry. Cur Top Med Chem. 2010;10:1113–1134. doi: 10.2174/156802610791384243. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Y, Chakraborty S, Liu S. Radiolabeled cyclic RGD peptides as radiotracers for imaging tumors and thrombosis by SPECT. Theranostics. 2011;1:58–82. doi: 10.7150/thno/v01p0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michalski MH, Chen X. Molecular imaging in cancer treatment. Eur J Nucl Med Mol Imaging. 2011;38:358–377. doi: 10.1007/s00259-010-1569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beer AJ, Kessler H, Wester HJ, Schwaiger M. PET Imaging of integrin αVβ3 expression. Theranostics. 2011;1:48–57. doi: 10.7150/thno/v01p0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danhier F, Le Breton A, Préat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–2973. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- 65.Tateishi U, Oka T, Inoue T. Radiolabeled RGD Peptides as integrin αvβ3–targeted PET tracers. Curr Med Chem. 2012;19:3301–3309. doi: 10.2174/092986712801215937. [DOI] [PubMed] [Google Scholar]
- 66.Daghestani HN, Day BW. Theory and applications of surface plasmon resonance, resonant Mirror, resonant waveguide grating, and dual polarization interferometry biosensors. Sensors 2010. 2010;10:9630–9646. doi: 10.3390/s101109630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wienken CJ, Baaske P, Rothbauer U, Dieter Brau ND, Duhr S. Protein-binding assays in biological liquid using microscale thermoresis. Nature Communications. 2010;1:100. doi: 10.1038/ncomms1093. [DOI] [PubMed] [Google Scholar]
- 68.Liu S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv Drug Delivery Rev. 2008;60:1347–70. doi: 10.1016/j.addr.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu S, Chakraborty S. 99mTc-centered one-pot synthesis for preparation of 99mTc radiotracers. Dalton Trans. 2011;40:6077–6086. doi: 10.1039/c0dt01462a. [DOI] [PubMed] [Google Scholar]
- 70.Liu S. 6-Hydrazinonicotinamide derivatives as bifunctional coupling agents for 99mTclabeling of small biomolecules. Top Curr Chem. 2005;252:193–216. [Google Scholar]
- 71.Anderson CJ, Green MA, Yashi YF. Chemistry of copper radionuclides and radiopharmaceutical products. In: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. John Wiley & Sons; New York: 2003. pp. 402–422. [Google Scholar]
- 72.Maecke H, Hofmann M, Haberkorn U. 68Ga-labeled peptides in tumor imaging. J Nucl Med. 2005;46:172S–178S. [PubMed] [Google Scholar]
- 73.Koukouraki S, Strauss LG, Georgoulias V, Schuhmacher J, Haberkorn U, Karkavitsas N, Dimitrakopoulou-Strauss A. Evaluation of the pharmacokinetics of 68Ga-DOTATOC in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging. 2006;33:460–466. doi: 10.1007/s00259-005-0006-1. [DOI] [PubMed] [Google Scholar]
- 74.Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S, Schuhmacher J, Strauss LG, Doll J, Mäcke HR, Eisenhut M, Debus J, Haberkorn U. Characterization of (68Ga)-DOTA-D-Phe1-Tyr3-octreotide (DOTATOC) kinetics in patients with meningiomas. J Nucl Med. 2005;46:763–769. [PubMed] [Google Scholar]
- 75.Koukouraki S, Strauss LG, Georgoulias V, Eisenhut M, Haberkorn U, Dimitrakopoulou-Strauss A. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F]FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging. 2006;33:1115–1122. doi: 10.1007/s00259-006-0110-x. [DOI] [PubMed] [Google Scholar]
- 76.Vaidyanathan G, White BJ, Zalutsky MR. Propargyl 4-[18F]fluorobenzoate: a putatively more stable prosthetic group for the fluorine-18 labeling of biomolecules via click chemistry. Cur Radiopharm. 2009;2:63–74. doi: 10.2174/1874471010902010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu S, Liu Z, Chen K, Yan Y, Watzlowik P, Wester HJ, Chin FT, Chen X. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol Imaging Biol. 2010;12:530–538. doi: 10.1007/s11307-009-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacobson O, Zhu L, Ma Y, Weiss ID, Sun X, Niu G, Kiesewetter DO, Chen X. Rapid and simple one-step F-18 labeling of peptides. Bioconj Chem. 2011;22:422–428. doi: 10.1021/bc100437q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glaser M, Morrison M, Solbakken M, Arukwe J, Karlsen H, Wiggen U, Champion S, Kindberg GM, Cuthbertson A. Radiosynthesis and biodistribution of cyclic RGD peptides conjugated with novel [18F]fluorinated aldehydecontaining prosthetic groups. Bioconj Chem. 2008;19:951–957. doi: 10.1021/bc700472w. [DOI] [PubMed] [Google Scholar]
- 80.Li ZB, Wu Z, Chen K, Chin FT, Chen X. Click chemistry for 18F-labeling of RGD peptides and microPET imaging of tumor integrin αvβ3 expression. Bioconj Chem. 2007;18:1987–1994. doi: 10.1021/bc700226v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hausner SH, Marik J, Gagnon MK, Sutcliffe JL. In vivo positron emission tomography (PET) imaging with an αvβ6 specific peptide radiolabeled using 18F-“click” chemistry: evaluation and comparison with the corresponding 4-[18F]fluorobenzoyl- and 2-[18F]fluoropropionyl-peptides. J Med Chem. 2008;51:5901–5904. doi: 10.1021/jm800608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X, Link JM, Stekhova S, Yagle KJ, Smith C, Krohn KA, Tait JF. Site-specific labeling of annexin V with F-18 for apoptosis imaging. Bioconj Chem. 2008;19:1684–1688. doi: 10.1021/bc800164d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Becaud J, Mu L, Karramkam M, Schubiger PA, Ametamey SM, Graham K, Stellfeld T, Lehmann L, Borkowski S, Berndorff D, et al. Direct one-step 18F-labeling of peptides via nucleophilic aromatic substitution. Bioconj Chem. 2009;20:2254–2261. doi: 10.1021/bc900240z. [DOI] [PubMed] [Google Scholar]
- 84.Hohne A, Mu L, Honer M, Schubiger PA, Ametamey SM, Graham K, Stellfeld T, Borkowski S, Berndorff D, Klar U, et al. Synthesis, 18F-labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconj Chem. 2008;19:1871–1879. doi: 10.1021/bc800157h. [DOI] [PubMed] [Google Scholar]
- 85.Mu L, Hohne A, Schubiger PA, Ametamey SM, Graham K, Cyr JE, Dinkelborg L, Stellfeld T, Srinivasan A, Voigtmann U, et al. Silicon-based building blocks for onestep 18F-radiolabeling of peptides for PET imaging. Angew Chem Int Ed Eng. 2008;47:4922–4925. doi: 10.1002/anie.200705854. [DOI] [PubMed] [Google Scholar]
- 86.Namavari M, Cheng Z, Zhang R, De A, Levi J, Hoerner JK, Yaghoubi SS, Syud FA, Gambhir SS. A novel method for direct site-specific radiolabeling of peptides using [18F]FDG. Bioconj Chem. 2009;20:432–436. doi: 10.1021/bc800422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wangler C, Schirrmacher R, Bartenstein P, Wangler B. Click-chemistry reactions in radiopharmaceutical chemistry: fast and easy introduction of radiolabels into biomolecules for in vivo imaging. Cur Med Chem. 2010;17:1092–1116. doi: 10.2174/092986710790820615. [DOI] [PubMed] [Google Scholar]
- 88.Schirrmacher R, Wängler C, Schirrmacher E. Recent developments and trends in 18F-radiochemistry: syntheses and applications. Mini-Rev Org Chem. 2007;4:317–329. [Google Scholar]
- 89.Jacobson O, Chen X. PET designated flouride-18 production and chemistry. Cur Top Med Chem. 2010;10:1048–1059. doi: 10.2174/156802610791384298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McBride WJ, Sharkey RM, Karacay H, D’Souza CA, Rossi EA, Laverman P, Chang CH, Boerman OC, Goldenberg DM. A novel method of 18F radiolabeling for PET. J Nucl Med. 2009;50:991–998. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 91.McBride WJ, D’Souza CA, Sharkey RM, Karacay H, Rossi EA, Chang CH, Goldenberg DM. Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex. Bioconj Chem. 2010;21:1331–1340. doi: 10.1021/bc100137x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laverman P, McBride WJ, Sharkey RM, Eek A, Joosten L, Oyen WJG, Goldenberg DM, Boerman OC. A novel facile method of labeling octreotide with 18F-fluorine. J Nucl Med. 2010;51:454–461. doi: 10.2967/jnumed.109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu S, Liu H, Jiang H, Xu Y, Zhang H, Cheng Z. One-step radiosynthesis of 18F-AlF-NOTA-RGD2 for tumor angiogenesis PET imaging. Eur J Nucl Med Mol Imaging. 2011;38:1732–1741. doi: 10.1007/s00259-011-1847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Souza CA, McBride WJ, Sharkey RM, Todaro LJ, Goldenberg DM. High-yielding aqueous 18F-labeling of peptides via Al18F chelation. Bioconj Chem. 2011;22:1793–1803. doi: 10.1021/bc200175c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lang L, Li W, Guo N, Ma Y, Zhu L, Kiesewetter DO, Shen B, Niu G, Chen X. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconj Chem. 2011;22:2415–2422. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McBride WJ, D’Souza CA, Karacay H, Sharkey RM, Goldenberg DM. New lyophilized kit for rapid radiofluorination of peptides. Bioconj Chem. 2012;23:538–547. doi: 10.1021/bc200608e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laverman P, D’Souza CA, Eek A, McBride WJ, Sharkey RM, Oyen WJG, Goldenberg DM, Boerman OC. Optimized labeling of NOTA-conjugated octreotide with F-18. Tumor Biol. 2012;33:427–434. doi: 10.1007/s13277-011-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheldrake HM, Patterson LP. Strategies to inhibit tumor associated integrin receptors: rationale for dual and multi-antagonists. J Med Chem. 2014;57:6301–6315. doi: 10.1021/jm5000547. [DOI] [PubMed] [Google Scholar]
- 99.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodivala-Dilke K. αvβ3 integrin and angiogenesis: a moody integrin in a changing Environment. Curr Opin Cell Biol. 2008;20:514–519. doi: 10.1016/j.ceb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chao JT, Meininger GA, Patterson JL, Jones SA, Partridge CR, Neiger JD, Williams ES, Kaufman SJ, Ramos KS, Wilson E. Regulation of α7-integrin expression in vascular smooth muscle by injury-induced atherosclerosis. Am J Physiol Heart Circ Physiol. 2004;287:H381–389. doi: 10.1152/ajpheart.00939.2003. [DOI] [PubMed] [Google Scholar]
- 103.Antonov AS, Kolodgie FD, Munn DH, Gerrity RG. Regulation of macrophage foam cell formation by αvβ3 integrin: potential role in human atherosclerosis. Am J Pathol. 2004;165:247–58. doi: 10.1016/s0002-9440(10)63293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zecchinon L, Fett T, Baise E, Desmecht D. Characterization of the caprine (Capra hircus) beta-2 integrin CD18-encoding cDNA and identification of mutations potentially responsible for the ruminant-specific virulence of Mannheimia haemolytica. Mol Membr Biol. 2004;21:289–95. doi: 10.1080/09687680412331282785. [DOI] [PubMed] [Google Scholar]
- 105.Isberg RR, Van Nhieu GT. The mechanism of phagocytic uptake promoted by invasin-integrin interaction. Trends Cell Biol. 1995;5:120–4. doi: 10.1016/s0962-8924(00)88962-x. [DOI] [PubMed] [Google Scholar]
- 106.Morris MA, Ley K. Trafficking of natural killer cells. Curr Mol Med. 2004;4:431–438. doi: 10.2174/1566524043360609. [DOI] [PubMed] [Google Scholar]
- 107.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, Clément-Lacroix P, Clézardin P. Tumor αvβ3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 109.Trikha M, Timar J, Zacharek A, Nemeth JA, Cai Y, Dome B, Somlai B, Raso E, Ladanyi A, Honn KV. Role for β3 integrins in human melanoma growth and survival. Int J Cancer. 2002;101:156–167. doi: 10.1002/ijc.10521. [DOI] [PubMed] [Google Scholar]
- 110.Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black PM. αvβ3 and αvβ5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–390. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 111.Gasparini G, Brooks PC, Biganzoli E, Vermeulen PB, Bonoldi E, Dirix LY, Ranieri G, Miceli R, Cheresh DA. Vascular integrin αvβ3: a new prognostic indicator in breast cancer. Clin Cancer Res. 1998;4:2625–2634. [PubMed] [Google Scholar]
- 112.Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck CA. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 113.Falcioni R, Cimino L, Gentileschi MP, D’Agnano I, Zupi G, Kennel SJ, Sacchi A. Expression of β1, β3, β4, and β5 integrins by human lung carcinoma cells of different histotypes. Exp Cell Res. 1994;210:113–122. doi: 10.1006/excr.1994.1017. [DOI] [PubMed] [Google Scholar]
- 114.Sengupta S, Chattopadhyay N, Mitra A, Ray S, Dasgupta S, Chatterjee A. Role of αvβ3 integrin receptors in breast tumor. J Exp Clin Cancer Res. 20:585–590. [PubMed] [Google Scholar]
- 115.Zitzmann S, Ehemann V, Schwab M. Arginine-Glycine-Aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res. 2002;62:5139–43. [PubMed] [Google Scholar]
- 116.Taherian A, Li X, Liu Y, Haas TA. Differences in integrin expression and signalling within human breast cancer cells. BMC Cancer. 2011;11:293. doi: 10.1186/1471-2407-11-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gupta A, Cao W, Chellaiah MA. Integrin αvβ3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-κB ligand signaling axis. Mol Cancer. 2012;11:66. doi: 10.1186/1476-4598-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cooper CR, Chay CH, Pienta KJ. The role of αvβ3 in prostate cancer progression. Neoplasia. 2002;4:191–194. doi: 10.1038/sj.neo.7900224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pilch J, Habermann R, Felding-Habermann B. Unique ability of integrin αvβ3 to support tumor cell arrest under dynamic flow conditions. J Biol Chem. 2002;277:21930–21938. doi: 10.1074/jbc.M201630200. [DOI] [PubMed] [Google Scholar]
- 120.Dittmar T, Heyder C, Gloria-Maercker E, Hatzmann W, Zänker KS. Adhesion molecules and chemokines: the navigation system for circulating tumor (stem) cells to metastasize in an organ-specific manner. Clin Exp Metastasis. 2008;25:11–32. doi: 10.1007/s10585-007-9095-5. [DOI] [PubMed] [Google Scholar]
- 121.Omar O, Lennerås M, Svensson S, Suska F, Emanuelsson L, Hall J, Nannmark U, Thomsen P. Integrin and chemokine receptor gene expression in implant-adherent cells during early osseointegration. J Mater Sci Mater Med. 2010;21:969–980. doi: 10.1007/s10856-009-3915-x. [DOI] [PubMed] [Google Scholar]
- 122.Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, Ponomarev V, Gerald WL, Blasberg R, Massagué J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin αvβ3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A. 2009;106:10666–10671. doi: 10.1073/pnas.0903035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gottschalk KE, Kessler H. The structures of integrins and integrin-ligand complexes: Implications for drug design and signal transduction. Angew Chem Int Ed Engl. 2002;41:3767–3774. doi: 10.1002/1521-3773(20021018)41:20<3767::AID-ANIE3767>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 126.Kumar CC. Integrin αvβ3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4:123–131. doi: 10.2174/1389450033346830. [DOI] [PubMed] [Google Scholar]
- 127.D’Andrea LD, Del Gatto A, Pedone C, Benedetti E. Peptide-based molecules in angiogenesis. Chem Biol Drug Des. 2006;67:115–26. doi: 10.1111/j.1747-0285.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 128.Gottschalk KE, Kessler H. The structures of integrins and integrin-ligand complexes: Implications for drug design and signal transduction. Angew Chem Int Ed Engl. 2002;41:3767–3774. doi: 10.1002/1521-3773(20021018)41:20<3767::AID-ANIE3767>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 129.Pfaff M, Tangemann K, Müller B, Gurrath M, Muller G, Kessler H, Timpl R, Engel J. Selective recognition of cyclic RGD peptides of NMR defined conformation by αIIbβ3, αvβ3, and α5β1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- 130.Gurrath M, Müller G, Kessler H, Aumailley M, Timpl R. Conformation/activity studies of rationally designed potent anti-adhesive RGD peptides. Eur J Biochem. 1992;210:911–921. doi: 10.1111/j.1432-1033.1992.tb17495.x. [DOI] [PubMed] [Google Scholar]
- 131.Muller G, Gurrath M, Kessler H, Timpl R. Dynamic forcing, a method for evaluating activity and selectivity profiles of RGD (Arg-Gly-Asp) peptides. Angew Chem Int Ed Engl. 1992;31:326–8. [Google Scholar]
- 132.Haübner R, Gratias R, Diefenbach B, Goodman SL, Jonczyk A, Kessler H. Structural and functional aspect of RGD-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonist. J Am Chem Soc. 1996;118:7461–7472. [Google Scholar]
- 133.Giannis A, Rubsam F. Integrin antagonists and other low molecular weight compounds as inhibitors of angiogenesis: new drugs in cancer therapy. Angew Chem Int Ed Engl. 1997;36:588–590. [Google Scholar]
- 134.Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the αvβ3 integrin for a new cancer therapy. Angew Chem Int Ed Engl. 1997;36:1375–1389. [Google Scholar]
- 135.Alghisi GC, Ponsonnet L, Rüegg C. The integrin antagonist cilengitide activates αvβ3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS One. 2009;4:e4449. doi: 10.1371/journal.pone.0004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamada S, Bu XY, Khankaldyyan V, Gonzales-Gomez I, McComb JG, Laug WE. Effect of the angiogenesis inhibitor Cilengitide (EMD 121974) on glioblastoma growth in nude mice. Neurosurgery. 2006;59:1304–1312. doi: 10.1227/01.NEU.0000245622.70344.BE. [DOI] [PubMed] [Google Scholar]
- 137.Bello L, Lucini V, Giussani C, Carrabba G, Pluderi M, Scaglione F, Tomei G, Villani R, Black PM, Bikfalvi A, et al. IS20I, a specific αvβ3 integrin inhibitor, reduces glioma growth in vivo. Neurosurgery. 2003;52:177–185. doi: 10.1097/00006123-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 138.Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. Cilengitide targeting of αvβ3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 2002;62:4263–4272. [PubMed] [Google Scholar]
- 139.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, et al. Phase I and correlative biology study of Cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ, Rich KM, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 141.Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, Hossfeld DK, Stöger H, Neyns B, Herzog P, et al. A randomized multicenter phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6:285. doi: 10.1186/1471-2407-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Exp Opin Investig Drugs. 2008;17:1225–1235. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bach AC, Espina JR, Jackson SA, Stouten PFW, Duke JL, Mousa SA, DeGrado WF. Type II′ to type Iβ-turn swap changes specificity for integrins. J Am Chem Soc. 1996;118:293–294. [Google Scholar]
- 144.Harlow RL. The structure of water as organized in an RGD peptide crystal at −80 °C. J Am Chem Soc. 1993;115:9838–9839. [Google Scholar]
- 145.Saitoh H, Aungst BJ. Prodrug and analog approaches to improving the intestinal absorption of a cyclic peptide, GPIIb/IIIa receptor antagonist. Pharm Res. 1997;14:1026–9. doi: 10.1023/a:1012149227756. [DOI] [PubMed] [Google Scholar]
- 146.Edwards DS, Liu S, Barrett JA, Harris AR, Looby RJ, Ziegler MC, Heminway SJ, Carroll TR. New and versatile ternary ligand system for technetium radiopharmaceuticals: water soluble phosphines and tricine as coligands in labeling a hydrazinonicotinamide-modified cyclic glycoprotein IIb/IIIa receptor antagonist with 99mTc. Bioconj Chem. 1997;8:146–154. doi: 10.1021/bc970002h. [DOI] [PubMed] [Google Scholar]
- 147.Oyen WJG, Boerman OC, Brouwers FM, Barrett JA, Verheugt FW, Ruiter DJ, Corstens FH, van der Meer JW. Scintigraphic detection of acute experimental endocarditis with the technetium-99m labelled glycoprotein IIb/IIIa receptor antagonist DMP444. Eur J Nucl Med. 2000;27:392–399. doi: 10.1007/s002590050521. [DOI] [PubMed] [Google Scholar]
- 148.Mitchel J, Waters D, Lai T, White M, Alberghini T, Salloum A, Knibbs D, Li D, Heller GV. Identification of coronary thrombus with a IIb/IIIa platelet inhibitor radiopharmaceutical, technetium-99m DMP-444: a canine model. Circulation. 2000;101:1643–1646. doi: 10.1161/01.cir.101.14.1643. [DOI] [PubMed] [Google Scholar]
- 149.Scharn DM, Oyen WJG, Klemm PL, Wijnen MH, vanderVliet JA. Assessment of prosthetic vascular graft thrombogenicity using the technetium-99m labeled glycoprotein IIb/IIIa receptor antagonist DMP444 in a dog model. Cardiovasc Surg. 2002;10:566– 569. doi: 10.1016/s0967-2109(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 150.Sakuma T, Sari I, Goodman CN, Lindner JR, Klibanov AL, Kaul S. Simultaneous integrin αvβ3 and glycoprotein IIb/IIIa inhibition causes reduction in infarct size in a model of acute coronary thrombosis and primary angioplasty. Cardiovasc Res. 2005;66:552–561. doi: 10.1016/j.cardiores.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 151.Sakuma T, Sklenar J, Leong-Poi H, Goodman NC, Glover DK, Kaul S. Molecular imaging identifies regions with microthromboemboli during primary angioplasty in acute coronary thrombosis. J Nucl Med. 2004;45:1194–1200. [PubMed] [Google Scholar]
- 152.Klem JA, Schaffer JV, Crane PD, Barrett JA, Henry GA, Canestri L, Ezekowitz MD. Detection of deep venous thrombosis by DMP 444, a platelet IIb/IIIa antagonist: a preliminary report. J Nucl Cardiol. 2000;7:359–364. doi: 10.1067/mnc.2000.106967. [DOI] [PubMed] [Google Scholar]
- 153.Brouwers FM, Oyen WJJ, Boerman OC, Barrett JA, Verheugt FW, Corstens FH, Van der Meer JW. Evaluation of Tc-99m-labeled glycoprotein IIb/IIIa receptor antagonist DMP444 SPECT in patients with infective endocarditis. Clin Nucl Med. 2003;28:480–484. doi: 10.1097/01.RLU.0000067508.82824.75. [DOI] [PubMed] [Google Scholar]
- 154.Fang W, He J, Kim YS, Zhou Y, Liu S. Evaluation of 99mTc-labeled cyclic RGD peptide with a PEG4 linker for thrombosis imaging: comparison with DMP444. Bioconj Chem. 2011;22:1715–1722. doi: 10.1021/bc2003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, Weber WA, Schwaiger M. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-Galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–1341. [PubMed] [Google Scholar]
- 156.Haubner R, Weber WA, Beer AJ, Vabulience E, Reim D, Sarbia M, Becker KF, Goebel M, Hein R, Wester HJ, et al. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLOS Medicine. 2005;2:e70, 244–252. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I, Watzlowik P, Wester HJ, Haubner R, Schwaiger M. [18F]Galacto-RGD positron emission tomography for imaging of αvβ3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6616. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]
- 158.Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nahrig J, Watzlowik P, Wester HJ, Harbeck N, Schwaiger M. Patterns of αvβ3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med. 2008;49:255–259. doi: 10.2967/jnumed.107.045526. [DOI] [PubMed] [Google Scholar]
- 159.Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, McParland B, Cohen PS, Hui A, Palmieri C, et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 160.Felding-Habermann B, Habermann R, Saldivar E, Ruggeri ZM. Role of β3 integrins in melanoma cell adhesion to activated platelets under flow. J Biol Chem. 1996;271:5892–900. doi: 10.1074/jbc.271.10.5892. [DOI] [PubMed] [Google Scholar]
- 161.Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, et al. Platelet and osteoclast β3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goodman SL, Grote HJ, Wilm C. Matched rabbit monoclonal antibodies against αv-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol Open. 2012;1:329–340. doi: 10.1242/bio.2012364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Böger C, Kalthoff H, Goodman SL, Behrens HM, Röcken C. Integrins and their ligands are expressed in non-small cell lung cancer but not correlated with parameters of disease progression. Virchows Arch. 2014;464:69–78. doi: 10.1007/s00428-013-1506-1. [DOI] [PubMed] [Google Scholar]
- 164.Roth P, Silginer M, Goodman SL, Hasenbach K, Thies S, Maurer G, Schraml P, Tabatabai G, Moch H, Tritschler I, et al. Integrin control of the transforming growth factor-β pathway in glioblastoma. Brain. 2013;136:564–576. doi: 10.1093/brain/aws351. [DOI] [PubMed] [Google Scholar]
- 165.Vogetseder A, Thies S, Ingold B, Roth P, Weller M, Schraml P, Goodman SL, Moch H. av-Integrin isoform expression in primary human tumors and brain metastases. Int J Cancer. 2013;133:2362–2371. doi: 10.1002/ijc.28267. [DOI] [PubMed] [Google Scholar]
- 166.Monferran S, Skuli N, Delmas C, Favre G, Bonnet J, Cohen-Jonathan-Moyal E, Toulas C. αvβ3 and αvβ5 Integrins control glioma cell response to ionizing radiation through ILK and RhoB. Int J Cancer. 2008;123:357–364. doi: 10.1002/ijc.23498. [DOI] [PubMed] [Google Scholar]
- 167.Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrin β5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene. 2013;32:3049–3058. doi: 10.1038/onc.2012.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sung V, Stubbs JTI, Fisher L, Aaron AD, Thompson EW. Bone sialoprotein supports breast cancer cell adhesion proliferation and migration through differential usage of the αvβ3 and αvβ5 integrins. J Cell Physiol. 1998;176:482–494. doi: 10.1002/(SICI)1097-4652(199809)176:3<482::AID-JCP5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 169.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.De Corte BL, Kinney WA, Liu L, Ghosh S, Brunner L, Hoekstra WJ, Santulli RJ, Tuman RW, Baker J, Burns C, et al. Piperidine-containing β-arylpropionic acids as potent antagonists of αvβ3/αvβ5 integrins. Bioorg Med Chem Lett. 2004;14:5227–5232. doi: 10.1016/j.bmcl.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 171.Letourneau JJ, Liu J, Ohlmeyer MHJ, Riviello C, Rong Y, Li H, Appell KC, Bansal S, Jacob B, Wong A, et al. Synthesis and initial evaluation of novel, nonpeptidic antagonists of the αv-integrins αvβ3 and αvβ5. Bioorg Med Chem Lett. 2009;19:352–355. doi: 10.1016/j.bmcl.2008.11.074. [DOI] [PubMed] [Google Scholar]
- 172.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 173.Goel A, Baranowska-Kortylewicz J, Hinrichs SH, Wisecarver J, Pavlinkova G, Augustine S, Colcher D, Booth BJM, Batra SK. 99mTc-labeled divalent and tetravalent CC49 single-chain Fv’s: novel imaging agents for rapid in vivo localization of human colon carcinoma. J Nucl Med. 2001;42:1519–1527. [PubMed] [Google Scholar]
- 174.Viti F, Tarli L, Giovannoni L, Zardi L, Neri D. Increased binding affinity and valence of recombinant antibody fragments lead to improved targeting of tumoral angiogenesis. Cancer Res. 1999;59:347–352. [PubMed] [Google Scholar]
- 175.Liu S, Edwards DS, Ziegler MC, Harris AR, Hemingway SJ, Barrett JA. 99mTc-Labeling of a hydrazinonictotinamide-conjugated vitronectin receptor antagonist. Bioconj Chem. 2001;12:624–629. doi: 10.1021/bc010012p. [DOI] [PubMed] [Google Scholar]
- 176.Liu S, Cheung E, Rajopadhye M, Ziegler MC, Edwards DS. 90Y- and 177Lu-labeling of a DOTA-conjugated vitronectin receptor antagonist for tumor therapy. Bioconj Chem. 2001;12:559–568. doi: 10.1021/bc000146n. [DOI] [PubMed] [Google Scholar]
- 177.Janssen M, Oyen WJG, Massuger LFAG, Frielink C, Dijkgraaf I, Edwards DS, Rajopadhye M, Corsten FHM, Boerman OC. Comparison of a monomeric and dimeric radiolabeled RGD-peptide for tumor targeting. Cancer Biother Radiopharm. 2002;17:641–646. doi: 10.1089/108497802320970244. [DOI] [PubMed] [Google Scholar]
- 178.Janssen M, Oyen WJG, Dijkgraaf I, Massuger LFAG, Frielink C, Edwards DS, Rajopadyhe M, Boonstra H, Corsten FHM, Boerman OC. Tumor targeting with radiolabeled αvβ3 integrin binding peptides in a nude mice model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- 179.Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, Conti PS. MicroPET imaging of breast cancer αv-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imag Biol. 2004;6:350–359. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 180.Li Z, Wu Z, Chen K, Ryu EK, Chen X. 18F-Labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49:453–461. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 181.Liu Z, Yan Y, Liu S, Wang F, Chen X. 18F, 64Cu, and 68Ga labeled RGDbombesin heterodimeric peptides for PET imaging of breast cancer. Bioconj Chem. 2009;20:1016–1025. doi: 10.1021/bc9000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu Z, Yan Y, Chin FT, Wang F, Chen X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J Med Chem. 2009;52:425–432. doi: 10.1021/jm801285t. [DOI] [PubMed] [Google Scholar]
- 183.Zheng Y, Ji S, Czerwinski A, Valenzuela F, Pennington M, Liu S. FITC-conjugated dimeric cyclic RGD peptides as fluorescent probes for in vitro assays of integrin αvβ3. Bioconj Chem. 2014;25:1925–1941. doi: 10.1021/bc500452y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.O’Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96:189–195. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-oncology. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Foote RL, Weidner N, Harris J, Hammond E, Lewis JE, Vuong T, Ang KK, Fu KK. Evaluation of tumor angiogenesis measured with microvessel density (MVD) as a prognostic indicator in nasopharyngeal carcinoma: results of RTOG 9505. Int J Radiation Oncol Biol Phy. 2005;61:745–753. doi: 10.1016/j.ijrobp.2004.07.694. [DOI] [PubMed] [Google Scholar]
- 187.Bauerle T, Komljenovic D, Merz M, Berger MR, Goodman SL, Semmler W. Cilengitide inhibits progression of experimental breast cancer bone metastases as imaged noninvasively using VCT, MRI and DCE-MRI in a longitudinal in vivo study. Int J Cancer. 2011;128:2453–2462. doi: 10.1002/ijc.25563. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Y, Cole T, Tapang P, Luo F, Hradil V, Jiang F, Luo Y, Albert D, Fox GB. Total lesion glycolysis and FDG SUV as early readouts of tumor response to Linifanib (ABT-869) in SCID mice with HT1080 xenografts. J Nucl Med. 2010;51:111–111. [Google Scholar]
- 189.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophy Acta. 1998;1378:F79–113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 190.Woodard AS, Garcia-Cardena G, Leong M, Madri JA, Sessa WC, Languino LR. The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J Cell Sci. 1998;111:469–478. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]
- 191.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- 193.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circulation Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Somanath PR, Ciocea A, Byzova TV. Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem Biophys. 2009;53:53–64. doi: 10.1007/s12013-008-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Beer AJ, Schwaiger M. PET of αvβ3-integrin and αvβ5-integrin expression with 18F-fluciclatide for assessment of response to targeted therapy: ready for prime time? J Nucl Med. 2011;52:335–337. doi: 10.2967/jnumed.110.078568. [DOI] [PubMed] [Google Scholar]
- 196.Morrison MS, Ricketts SA, Barnett J, Cuthbertson A, Tessier J, Wedge SR. Use of a novel Arg-Gly-Asp radioligand, 18F-AH111585, to determine changes in tumor vascularity after antitumor therapy. J Nucl Med. 2009;50:116–122. doi: 10.2967/jnumed.108.056077. [DOI] [PubMed] [Google Scholar]
- 197.Battle MR, Goggi JL, Allen L, Barnett J, Morrison MS. Monitoring tumor response to antiangiogenic sunitinib therapy with 18F-fluciclatide, an 18F-labeled αvβ3-integrin and αvβ5-integrin imaging agent. J Nucl Med. 2011;52:424–430. doi: 10.2967/jnumed.110.077479. [DOI] [PubMed] [Google Scholar]
- 198.Dumont RA, Hildebrandt I, Su H, Haubner R, Reischl G, Czernin JG, Mischel PS, Weber WA. Noninvasive imaging of αvβ3 function as a predictor of the antimigratory and antiproliferative effects of dasatinib. Cancer Res. 2009;69:3173–3179. doi: 10.1158/0008-5472.CAN-08-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ji S, Zhou Y, Shao G, Liu S, Voorbach MJ, Luo Y, Cole T, Zhang Y, Albert DH, Mudd SR. Monitoring tumor response to linifanib therapy with SPECT/CT using the αvβ3-targeted radiotracer 99mTc-3P-RGD2. J Pharmacol Exp Ther. 2013;346:251–258. doi: 10.1124/jpet.112.202622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ji S, Zheng Y, Shao G, Zhou Y, Liu S. Integrin αvβ3–targeted radiotracer 99mTc-3P-RGD2 useful for noninvasive monitoring of breast tumor response to antiangiogenic linifanib therapy but not anti-integrin αvβ3 RGD2 therapy. Theranostics. 2013;3:816–830. doi: 10.7150/thno.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou J, Goh BC, Albert DH, Chen CS. ABT-869, a promising multi-targeted tyrosine kinase inhibitor: from bench to bedside. J Hematol Oncol. 2009;2:33–46. doi: 10.1186/1756-8722-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jiang F, Albert DH, Luo Y, Tapang P, Zhang K, Davidsen SK, Fox GB, Lesniewski R, McKeegan EM. ABT-869, a multitargeted receptor tyrosine kinase inhibitor, reduces tumor microvascularity and improves vascular wall integrity in preclinical tumor models. J Pharmacol Exp Ther. 2011;338:134–142. doi: 10.1124/jpet.110.178061. [DOI] [PubMed] [Google Scholar]
- 203.Hernandez-Davies JE, Zape JP, Landaw EM, Tan X, Presnell A, Griffith D, Heinrich MC, Glaser KB, Sakamoto KM. The multitargeted receptor tyrosine kinase inhibitor linifanib (ABT-869) induces apoptosis through an Akt and glycogen synthase kinase 3beta-dependent pathway. Mol Cancer Ther. 2011;10:949–959. doi: 10.1158/1535-7163.MCT-10-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Luo Y, Jiang F, Cole TB, Hradil VP, Reuter D, Chakravartty A, Albert DH, Davidsen SK, Cox BF, McKeegan EM, et al. A novel multi-targeted tyrosine kinase inhibitor, linifanib (ABT-869), produces functional and structural changes in tumor vasculature in an orthotopic rat glioma model. Cancer Chemother Pharmacol. 2012;69:911–921. doi: 10.1007/s00280-011-1740-7. [DOI] [PubMed] [Google Scholar]
- 205.Wong CI, Koh TS, Soo R, Hartono S, Thng CH, McKeegan E, Yong WP, Chen CS, Lee SC, Wong J, et al. Phase I and biomarker study of ABT-869, a multiple receptor tyrosine kinase inhibitor, in patients with refractory solid malignancies. J Clin Oncol. 2009;27:4718–4726. doi: 10.1200/JCO.2008.21.7125. [DOI] [PubMed] [Google Scholar]
- 206.Tannir NM, Wong YN, Kollmannsberger CK, Ernstoff MS, Perry DJ, Appleman LJ, Posadas EM, Cho D, Choueiri TK, Coates A, et al. Phase 2 trial of linifanib (ABT-869) in patients with advanced renal cell cancer after sunitinib failure. Eur J Cancer. 2011;47:2706–2714. doi: 10.1016/j.ejca.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shao G, Zhou Y, Liu S. Monitoring glioma growth and tumor necrosis with u-SPECT-II/CT for by targeting integrin αvβ3. Mol Imaging. 2013;12:39–48. [PubMed] [Google Scholar]
- 208.Zhou Y, Shao G, Wang F, Liu S. Imaging breast cancer lung metastasis by u-SPECT-II/CT with an integrin αvβ3-targeted radiotracer 99mTc-3P-RGD2. Theranostics. 2012;2:577–587. doi: 10.7150/thno.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ma Q, Ji B, Jia B, Gao S, Ji T, Wang X, Han Z, Zhao G. Differential diagnosis of solitary pulmonary nodules using 99mTc-3P-RGD scintigraphy. Eur J Nucl Med Mol Imaging. 2011;38:2145–52. doi: 10.1007/s00259-011-1901-2. [DOI] [PubMed] [Google Scholar]
- 210.Zhu Z, Miao W, Li Q, Dai H, Ma Q, Wang F, Yang A, Jia B, Jing X, Liu S, et al. 99mTc-3PRGD2 for integrin receptor imaging of lung cancer: a multicenter study. J Nucl Med. 2012;53:716–722. doi: 10.2967/jnumed.111.098988. [DOI] [PubMed] [Google Scholar]
- 211.Zhao D, Jin X, Li F, Liang J, Lin Y. Integrin αvβ3 imaging of radioactive iodine–refractory thyroid cancer using 99mTc-3PRGD2. J Nucl Med. 2012;53:1872–1877. doi: 10.2967/jnumed.112.107821. [DOI] [PubMed] [Google Scholar]
- 212.Ma Q, Chen B, Gao S, Ji T, Wen Q, Song Y, Zhu L, Xu Z, Liu L. 99mTc-3P4-RGD2 Scintimammography in the assessment of breast lesions: comparative study with 99mTc-MIBI. PLoS ONE. 2014;9:e108349. doi: 10.1371/journal.pone.0108349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu L, Song Y, Gao S, Ji T, Zhang H, Ji B, Chen B, Jia B, Wang F, Xu Z, Ma Q. 99mTc-3PRGD2 scintimammography in palpable and nonpalpable breast lesions. Mol Imaging. 2014;13:1–7. doi: 10.2310/7290.2014.00010. [DOI] [PubMed] [Google Scholar]
- 214.Wan W, Guo N, Pan D, Yu C, Weng Y, Luo S, Ding H, Xu Y, Wang L, Lang L, et al. First experience of 18F-Alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–698. doi: 10.2967/jnumed.112.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cheng W, Wu Z, Liang S, Fu H, Wu S, Tang Y, Ye Z, Wang H. Comparison of 18F-AIF-NOTA-PRGD2 and 18F-FDG uptake in lymph node metastasis of differentiated thyroid cancer. PLOS ONE. 2014;9:e100521. doi: 10.1371/journal.pone.0100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Guo J, Guo N, Lang L, Kiesewetter DO, Xie Q, Li Q, Eden HS, Niu G, Chen X. 18F-Alfatide II and 18F-FDG dual-tracer dynamic PET for parametric, early prediction of tumor response to therapy. J Nucl Med. 2014;55:154–160. doi: 10.2967/jnumed.113.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yu C, Pan D, Mi B, Xu Y, Lang L, Niu G, Yang M, Wan W, Chen X. 18F-Alfatide II PET/CT in healthy human volunteers and patients with brain metastases. Eur J Nucl Med Mol Imaging. 2015 doi: 10.1007/S00259-015-3118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hoshiga M, Alpers CE, Smith LL, Giachelli CM, Schwartz SM. αvβ3 integrin expression in normal and atherosclerotic artery. Circ Res. 1995;77:1129–1135. doi: 10.1161/01.res.77.6.1129. [DOI] [PubMed] [Google Scholar]
- 219.Antonov AS, Antonova GN, Munn DH, Mivechi N, Lucas R, Catravas JD, Verin AD. αvβ3 integrin regulates macrophage inflammatory responses via PI3 kinase/akt-dependent NF-κB activation. J Cell Physiol. 2011;226:469–476. doi: 10.1002/jcp.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Antonov AS, Kolodgie KD, Munn DH, Gerrity RG. Regulation of macrophage foam cell formation by αvβ3 integrin: potential role in human atherosclerosis. Am J Pathol. 2004;165:247–258. doi: 10.1016/s0002-9440(10)63293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Saraste A, Nekolla SG, Schwaiger M. Cardiovascular molecular imaging: an overview. Cardiovasc Res. 2009;83:643–652. doi: 10.1093/cvr/cvp209. [DOI] [PubMed] [Google Scholar]
- 222.Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, Su HL, Edwards DS, Liu S, Harris TD, et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113:1684–1691. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Choi H, Phi JH, Paeng JC, Kim SK, Lee YS, Jeong JM, Chung JK, Lee DS, Wang KC. Imaging of integrin αvβ3 expression using 68Ga-RGD positron emission tomography in pediatric cerebral infarct. Mol Imaging. 2013;12:213–217. [PubMed] [Google Scholar]
- 224.Razavian M, Marfatia R, Mongue-Din H, Tavakoli S, Sinusas AJ, Zhang J, Nie L, Sadeghi MM. Integrin-targeted imaging of inflammation in vascular remodeling. Arterioscler Thromb Vasc Biol. 2011;31:2820–2826. doi: 10.1161/ATVBAHA.111.231654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hua J, Dobrucki LW, Sadeghi MM, Zhang J, Bourke BN, Cavaliere P, Song J, Chow C, Jahanshad N, van Royen N, et al. Noninvasive imaging of angiogenesis with a 99mTc labeled peptide targeted at αvβ3 integrin after murine hindlimb ischemia. Circulation. 2005;111:3255–3260. doi: 10.1161/CIRCULATIONAHA.104.485029. [DOI] [PubMed] [Google Scholar]
- 226.Waldeck J, Häger F, Höltke C, Lanckohr C, von Wallbrunn A, Torsello G, Heindel W, Theilmeier G, Schäfers M, Bremer C. Fluorescent reflectance imaging of macrophage-rich atherosclerostic plaques using αvβ3 integrin-targeted fluorochrome. J Nucl Med. 2008;49:1845–1851. doi: 10.2967/jnumed.108.052514. [DOI] [PubMed] [Google Scholar]
- 227.Su H, Gorodny N, Gomez LF, Gangadharmath UB, Mu F, Chen G, Walsh JC, Szardenings K, Berman DS, Kolb HC, et al. Atherosclerotic plaque uptake of a novel integrin tracer 18F-Flotegatide in a mouse model of atherosclerosis. J Nucl Cardiol. 2014;21:553–62. doi: 10.1007/s12350-014-9879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pichler BJ, Kneilling M, Haubner R, Braumüller H, Schwaiger M, Röcken M, Weber WA. Imaging of delayed-type hypersensitivity reaction by PET and 18FGalacto-RGD. J Nucl Med. 2005;46:184–189. [PubMed] [Google Scholar]
- 229.Beer AJ, Pelisek J, Heider P, Saraste A, Reeps C, Metz S, Seidl S, Kessler H, Wester HJ, Eckstein HH, et al. PET/CT imaging of integrin αvβ3 expression in human carotid atherosclerosis. J Am Coll Cardiol Img. 2014;7:178–187. doi: 10.1016/j.jcmg.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 230.Beer AJ, Kessler H, Wester HJ, Schwaiger M. PET imaging of integrin αvβ3 expression. Theranostics. 2011;1:48–57. doi: 10.7150/thno/v01p0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester HJ, Peschel C, Lordick F, Schwaiger M. Comparison of integrin αvβ3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-Galacto-RGD and 18F-FDG. J Nucl Med. 2008;49:22–29. doi: 10.2967/jnumed.107.045864. [DOI] [PubMed] [Google Scholar]
- 232.Josephson L, Rudin M. Barriers to clinical translation with diagnostic drugs. J Nucl Med. 2013;54:329–332. doi: 10.2967/jnumed.112.107615. [DOI] [PubMed] [Google Scholar]
- 233.Esteves FP, Raggi P, Folks RD, Keidar Z, Askew JW, Rispler S, O’Connor MK, Verdes L, Garcia EV. Novel solid-state-detector dedicated cardiac camera for fast myocardial perfusion imaging: multicenter comparison with standard dual detector cameras. J Nucl Cardiol. 2009;16:927–934. doi: 10.1007/s12350-009-9137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Duvall WL, Croft LB, Godiwala T, Ginsberg E, George T, Henzlova MJ. Reduced isotope dose with rapid SPECT MPI imaging: initial experience with a CZT SPECT camera. J Nucl Cardiol. 2010;17:1009–1014. doi: 10.1007/s12350-010-9215-5. [DOI] [PubMed] [Google Scholar]
- 235.Pazhenkottil AP, Buechel RR, Herzog BA, Nkoulou RN, Valenta I, Fehlmann U, Ghadri JR, Wolfrum M, Husmann L, Kaufmann PA. Ultrafast assessment of left ventricular dyssynchrony from nuclear myocardial perfusion imaging on a new high-speed gamma camera. Eur J Nucl Med Mol Imaging. 2010;37:2086–2092. doi: 10.1007/s00259-010-1507-0. [DOI] [PubMed] [Google Scholar]
- 236.Schillaci O, Danieli R. Dedicated cardiac cameras: a new option for nuclear myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2010;37:1706–1709. doi: 10.1007/s00259-010-1526-x. [DOI] [PubMed] [Google Scholar]
- 237.Fiechter M, Ghadri JR, Kuest SM, Pazhenkottil AP, Wolfrum M, Nkoulou RN, Goetti R, Gaemperli O, Kaufmann PA. Nuclear myocardial perfusion imaging with a novel cadmium-zinc-telluride detector SPECT/CT device: first validation versus invasive coronary angiography. Eur J Nucl Med Mol Imaging. 2011;38:2025–2030. doi: 10.1007/s00259-011-1877-y. [DOI] [PubMed] [Google Scholar]
- 238.Gaemperli O, Kaufmann PA. Lower dose and shorter acquisition: pushing the boundaries of myocardial perfusion SPECT. J Nucl Cardiol. 2011;18:830–832. doi: 10.1007/s12350-011-9410-z. [DOI] [PubMed] [Google Scholar]
- 239.DePuey EG. Advances in SPECT camera software and hardware: Currently available and new on the horizon. J Nucl Cardiol. 2012;19:551–581. doi: 10.1007/s12350-012-9544-7. [DOI] [PubMed] [Google Scholar]
- 240.Bailey DL, Willowson KP. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med. 2013;54:83–89. doi: 10.2967/jnumed.112.111476. [DOI] [PubMed] [Google Scholar]