Abstract
During the past century the prevalence of light at night has increased in parallel with obesity rates. Dim light at night (dLAN) increases body mass in male mice. However, the effects of light at night on female body mass remain unspecified. Thus, female mice were exposed to a standard light/dark (LD; 16h light at ~150 lux/8h dark at ~0 lux) cycle or to light/dim light at night (dLAN; 16h light at ~150 lux/8h dim light at ~5 lux) cycles for six weeks. Females exposed to dLAN increased the rate of change in body mass compared to LD mice despite reduced total food intake during weeks five and six, suggesting that dLAN disrupted circadian rhythms resulting in deranged metabolism.
Keywords: Circadian disruption, light pollution, light at night, body mass regulation
Introduction
The increase of light at night parallels the increasing rates of obesity and metabolic disorders throughout the world (Fonken & Nelson, 2011; Ogden, Carroll et al., 2014). Circadian regulation of energy homeostasis is controlled by an endogenous biological clock, located in the suprachiasmatic nuclei (SCN) of the hypothalamus. Light information travels directly from light-sensitive ganglion cells in the retina to the SCN which entrains individuals to the external day–night cycle (Hastings, Reddy et al., 2003; Burke, Markwald et al., 2013). Melatonin is produced only during the night in both diurnal and nocturnal animals and its production can be inhibited by exposure to nighttime light (Brainard, Richardson et al., 1982).
People in developed countries are exposed to some environmental light pollution, particularly those who participate in shift work. Shift work disrupts circadian clock function and is linked to circadian and metabolic consequences including sleep disturbances, elevated body mass index, altered plasma lipid metabolism and adiposity, and increased risk for cardiovascular disease (van Amelsvoort, Schouten et al., 1999; Stevens, Blask et al., 2007). Individuals with specific polymorphisms of the Clock gene are protected against obesity, suggesting a role for genetic variation in Clock in the development of metabolic syndrome, type 2 diabetes, and cardiovascular disease (Scott, Carter et al., 2008). Similarly, mice harboring a mutation in their Clock genes are susceptible to obesity and metabolic syndrome (Turek, Joshu et al., 2005). Dim light at night (dLAN) disrupts molecular circadian rhythms and increases body mass in male mice (Fonken, Aubrecht et al., 2013). Considered together, the endogenous biological clocks can be disrupted by light at night exposure and this can have adverse consequences on health. Obesity is more prevalent in women than men (Flegal, Carroll et al., 2010; Ogden, Carroll et al., 2014). Shift work is associated with increased risk for breast cancer and obesity in women (Schernhammer, Laden et al., 2001; Key, Appleby et al., 2003). Most research is focused on male rodents, which potentially misses critical sex differences. Here we expand on our previous work demonstrating that male mice exposed to dim light at night (dLAN) increase body mass (Fonken, Workman et al., 2010). We hypothesized that dLAN exposure in female mice also increases body mass. To assess the link between altered light cycles and metabolic disorder, we placed mice in either a standard light/dark cycle (LD; 16h light at ~150 lux/8h dark at ~0 lux) or exposed to cycles of light/dim light at night (dLAN; 16h light at ~150 lux/8h dim light at ~5 lux and measured weekly body mass, food intake, and home cage activity for 6 weeks.
Materials and Methods
Animals
Twenty female Swiss Webster mice were purchased at eight weeks of age from Charles River laboratories (Wilmington, MA, USA). Mice were individually housed in propylene cages (33 cm × 18 cm × 14 cm) at an ambient temperature of 22 ± 2 °C and relative humidity of 40% ± 10%. Mice were provided Harlan Teklad 8640 food (Madison, WI, USA) and filtered tap water ad libitum. Upon arrival mice were housed on a 16:8 light/dark cycle for one week to allow acclimation. After the acclimation period, mice were randomly assigned to exposure to dim light at night (dLAN, 16:8 light/dim light cycle at ~150 lux during the day and ~5 lux during the night; n = 11) or a traditional light:dark cycle (LD 16:8 light/dark cycle; ~150 lux during the day and ~0 lux at night; n=9) for 6 weeks. Food intake was measured daily before the onset of the dark period (15:00 EST). Body mass was measured weekly for the duration of the experiment before the onset of the dark period (15:00 EST). All procedures were approved by the Ohio State University Institutional Animal Care and Use Committee; all experiments conformed to international ethical standards for chronobiology research (Portaluppi, Smolensky et al., 2010).
Locomotor Activity
Home cage locomotor activity was monitored during the fifth and sixth weeks in experimental light conditions; each group was monitored for four consecutive days. Home cage activity was monitored by assessing interruptions of intersecting infrared beams placed around the cage. Activity was tracked in eight mice per group using OPTO M3 animal activity monitors (Columbus Instruments, Columbus, OH, USA) that continuously compiled data using MDI software (Toronto, Ontario, Canada). The total 24 h activity was averaged for the four days of home cage monitoring.
Statistics
Main effect of lighting (LD vs dLAN) was assessed in all analyses. Repeated measures ANOVA were used to analyze food intake, body mass, and change in body mass across weeks. A Greenhouse-Geisser correction was applied to body mass and change in body mass data because the assumption of sphericity was violated; only adjusted results are reported (Vasey & Thayer, 1987). A multivariate ANOVA was used to analyze food intake, body mass, and change in body mass differences for weeks one-six. A Univariate ANOVA was used to analyze average total activity. Due to unequal variance total food intake for the entire 6 weeks period was analyzed with a Kruskal-Wallis test. Statistics were performed using SPSS 22 for Windows (IBM, New York, NY, USA). Outliers determined by Z score (±2 SEM from mean) were removed from subsequent analysis, Mean differences were considered statistically significant when p was <0.05.
Results
Body Mass
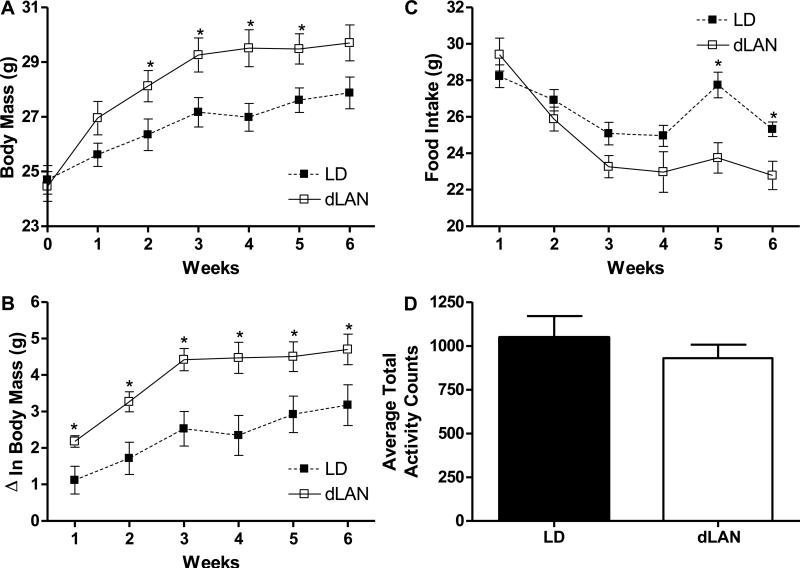
Body mass (F2.3,36.5=65.1, p<0.01; Fig 1A) and change in body mass (F2.7,46.6=33.2, p<0.01; Fig 1B) significantly increased across weeks in both groups. Lighting and body mass interacted across weeks (F2.3,36.5=5.5, p<0.01; Fig 1A) such that mice in dLAN gained more body mass than mice LD across the weeks of the experiment. dLAN mice and LD had similar body mass at baseline, week 1 and 6 (p> 0.05 in each case; Fig 1B), dLAN mice increased body mass weeks 2-5 (week 2 F1,16=4.6, p<0.05; week 3 F1,16=6.1, p<0.05; week 4 F1,16=8.2, p<0.05; week 5 F1,16=6.4, p<0.05; Fig 1A). One mouse in LD and one mouse in dLAN were removed from body mass analysis because they were outliers based on Z score analyses. Lighting and change in body mass did not interact across weeks (p>0.05), however, mice in dLAN had increased change in body mass compared to LD mice every week of the experiment (week 1 F1,17=7.17, p<0.05; week 2 F1,17=9.22, p<0.01; week 3 F1,17=11.8, p<0.01; week 4 F1,17=9.7, p<0.01;week 5 F1,17=6.09, p<0.05; week 6 F1,17=4.9, p<0.05; Fig 1B).
Figure 1. Female mice exposed to dim light at night (dLAN) had a greater change in body mass than mice exposed to dark nights (LD), despite decreased food intake in dLAN mice compared to LD mice in weeks five and six.
Body mass interacted with lighting across the six weeks of the experiment such that mice in dLAN (n=11) had a greater gain in body mass than mice in LD (n=9) (A). Change in body mass was increased in dLAN (n=11) mice compared to LD (n=9) mice across the six weeks of the experiment (B). Food intake across the six weeks of the experiment, LD (n=8) mice had increased food intake on weeks five and six compared to dLAN (n=11) exposed mice (C). Mice in LD (n=7) and dLAN (n=8) had similar amounts of 24 hour (h) activity averaged for the four days of home cage monitoring (D). Significant mean differences p<0.05 indicated by asterisk (*).
Food Intake
Food intake and lighting interacted across weeks (F5,85=3.74, p<0.01; Fig 1C) such that mice in the LD condition consumed more food than mice in dLAN during the fifth and sixth weeks (week 5 F1,17=12.1, p<0.01; week 6 F1,17=6.7, p<0.05; Fig 1C).
Locomotor Activity
Average total locomotor activity was similar between LD and dLAN mice (p>0.05; Fig 1D).
Discussion
Our previous work established that exposure to dLAN increased body mass and disrupted molecular circadian rhythms in males (Fonken, Aubrecht et al., 2013). We hypothesized that dLAN also elevates body mass in female mice. Female Swiss-Webster mice exposed to ~5 lux of nightly illumination increased body mass and rates of body mass change compared to mice exposed to dark nights despite reduced food intake. Increased food intake without increased change in body mass in female mice maintained under dark nights suggests a role for dLAN to dysregulate metabolic processes. Similarly, exposure of male mice to 5 lux of dLAN increased body mass and impaired glucose regulation (Fonken, Workman et al., 2010); LD and dLAN exposed mice had similar average total activity counts, suggesting that body mass differences are not due to lack of locomotion.
Removal of dLAN exposure can reverse metabolic disruption (Fonken, Weil et al., 2013). Dark nights restore nightly melatonin secretion (Brainard, Richardson et al., 1982). However, because the nightly melatonin signal is faint in this mouse strain, it seems more likely that the metabolic effects of dLAN are mediated via changes in core clock gene expression and protein production. For example, changes in phase and amplitude of several core clock genes occurred in response to dLAN in male mice that gained body mass (Fonken, Aubrecht et al., 2013). It appears from our data that no strong sex differences in the metabolic effects of dLAN exist in mice.
Light at night is increasingly pervasive in developed countries. Light at night disrupts regular circadian patterns communicated via the SCN (Brainard, Richardson et al., 1982; Hastings, Reddy et al., 2003). The consequences of light at night have profound implications for the population at large, but particularly shift workers. Shift work has been linked to increased body mass, as well as elevated rates of heart disease and cancer (van Amelsvoort, Schouten et al., 1999; Stevens, Blask et al., 2007). Collectively, our results suggest that dLAN has effects on body mass and likely metabolic function in both male and female mice that mimic health problems associated with shift work. Light at night appears to affect the health of shift workers which might be minimized by the use of long wavelength lighting that does not impair melatonin secretion (Lockley, Brainard et al., 2003).
Acknowledgements
TGA was supported by a NIDCR grant T32 DE014320.
Footnotes
Declaration of Interest
The authors have no interests to disclose.
References
- Brainard GC, Richardson BA, Petterborg LJ, Reiter RJ. The effect of different light intensities on pineal melatonin content. Brain Res. 1982;233:75–81. doi: 10.1016/0006-8993(82)90931-3. [DOI] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, Chinoy ED, Snider JA, Bessman SC, Jung CM, Wright KP., Jr. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep. 2013;36:1617–1624. doi: 10.5665/sleep.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Melendez-Fernandez OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms. 2013;28:262–271. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ. Illuminating the deleterious effects of light at night. F1000 Med Rep. 2011:3/18. doi: 10.3410/M3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Weil ZM, Nelson RJ. Dark nights reverse metabolic disruption caused by dim light at night. Obesity. 2013;21:1159–1164. doi: 10.1002/oby.20108. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Jr., Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Longcope C. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–1929. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes. 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, Rea MS, Reinlib L. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115:1357–1362. doi: 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsvoort LG, Schouten EG, Kok FJ. Duration of shiftwork related to body mass index and waist to hip ratio. Int J Obes Relat Metab Disord. 1999;23:973–978. doi: 10.1038/sj.ijo.0801028. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]