Abstract
Background:
The management of deep carious lesions can be done by various techniques but residual caries dilemma still persists and bacterial reduction in cavities treated by either partial or complete caries removal techniques is debatable. So the objective of the present randomized clinical trial was to compare microbial counts in cavities submitted to complete caries removal and partial caries removal using either hand instruments or burs before and after 3 weeks of restoration.
Materials and Methods:
Primary molars with acute carious lesions in inner half of dentine and vital pulp were randomly divided into three groups of 14 each: Group A: Partial caries removal using hand instruments atraumatic restorative treatment (ART) only; Group B: Partial caries removal using bur; Group C: Complete caries removal using bur and caries detector dye. Dentine sample obtained after caries removal and 3 weeks after restoration, were subjected to microbial culture and counting (colony-forming units [CFU]/mg of dentine) for total viable bacterial count, Streptococcus spp., mutans streptococci, Lactobacillus spp.
Results:
Three techniques of caries removal showed significant (P < 0.05) reduction in all microorganisms studied after 3 weeks of evaluation, but there was no statistically significant difference in percentage reduction of microbial count among three groups.
Conclusion:
Results suggest the use of partial caries removal in a single session as compared to complete caries removal as a part of treatment of deep lesions in deciduous teeth in order to reduce the risk of pulp exposure. Partial caries removal using ART can be preferred for community settings as public health procedure for caries management.
Keywords: Caries, clinical trial, complete, dental atraumatic restorative treatment, microflora, partial, randomized, removal
INTRODUCTION
Is it necessary to remove all carious tissue from lesions approaching the pulp? The management of deep carious lesions, approaching a healthy pulp, presents a significant challenge to the dental practitioner as deep caries may induce severe inflammatory reactions in the pulp and may cause pulpal necrosis. While excavating the deep caries lesions, there are chances that dentin barrier may be broken and the healing of the pulp impaired.[1,2]
The complete removal of carious tissue which is the traditional management of carious lesions of any kind, dictates the removal of all infected and affected dentin to prevent further cariogenic activity and provide a well mineralized base of dentin for restoration.[1] A caries detector dye can be used as an aid to indicate infected dentine during cavity preparation.[3,4] The success of restorative treatment, depending on the complete elimination of bacteria and complete caries removal, is still considered as the best conservative treatment, irrespective of the restorative material used.[5]
To minimize the risk of exposing or even breaching the pulp, several authors have investigated and proposed alternative approaches to the complete caries removal technique. One such less invasive approach is the partial removal of carious tissue that aimed at maintaining the deeper layer of partially infected carious dentin, which can remineralize.[6] Ricketts et al.,[7] after a systematic review, reported that partial caries removal technique is better in terms of carious lesion progression and longevity of the restorations, as well as the preservation of pulpal tissue.
Partial caries removal is preferable to complete caries removal in the deep lesions in order to reduce the risk of carious exposure. There is insufficient evidence, however, to know whether it is necessary to reopen and excavate further. Previous studies where cavities have not been reopened did not report adverse consequences.[7]
In developing countries, the prevalence of untreated dental caries is very high. Multitude of reasons for lack of utilization of dental care such as financial barriers, lack, and mal-distribution of oral health care personnel and equipments, pain and fear barriers, and dependency on conventional dentistry that require dental clinics or expensive portable dental equipment which uses electricity, have been observed.[8]
New techniques like atraumatic restorative treatment (ART), require the excavation to be performed with hand instruments only. Due to hand and wrist fatigue and limited visibility into the cavity during the excavation process, there is an increased risk of incomplete caries excavation.[9] It has been argued that since all carious dentine is not removed from the hand-prepared cavity, the caries process is soon resumed.[10] Considering ART as a type of partial caries removal method, further studies are needed to examine the numbers of bacteria left in the cavity after preparation, as well as the type of cultivable flora, to support or reject this argument.
Restorative dentistry aims to create a favorable environment to arrest caries with minimal operative intervention. However, the discussion about the minimal amount of dentine caries or number of microorganisms that can be left behind without danger of the lesion progression has not yet been settled.[4,11,12,13,14,15]
Several microbiological studies had reported the persistence of bacteria in dentin in cavities treated by either partial caries removal or complete caries removal techniques.[16,17] It is still unclear whether the microorganisms remaining after sealing of the cavity treated by complete caries removal proliferate or not.
Since viable bacteria may persist in the cavities regardless of the technique of caries removal used, this study was done with a hypothesis that there is no difference in bacterial growth under the restorations either after complete removal of carious tissue using bur and dye or after partial removal of carious tissue using bur/hand instruments ART after 3 weeks of sealing with restorative material. In the literature available, no previous study has been attempted with short duration of 3 weeks, comparing the microbial levels after partial caries removal using bur or ART technique with complete caries removal.
So the present randomized clinical trial was done with the aim to compare microbial counts between cavities submitted to complete removal of carious tissue and partial removal of carious tissue using either hand instruments (ART) or burs before and after 3 weeks of cavity sealing with restorative material.
MATERIALS AND METHODS
Study design and selection of sample
This three-armed, parallel-design, double-blind, and randomized clinical trial was done on 8-10-year-old school children in Udupi Taluk. The detailed information about study and procedure was given to the parents and school teachers. Only those children who fulfilled the inclusion criteria and whose parents signed the informed consent, permitting the participation of the children participated in the study. All children received dental care in a Mobile Dental Clinic and were instructed regarding oral hygiene maintenance. The permission to conduct the study was obtained from the Ethical Committee of the Manipal University (IEC-24/2011). The trial is registered in the Clinical Trial Registry-India (CTRI - REF/2015/05/008975).
Sample size calculation
The pilot study (involving 10 teeth) was done to calculate the sample size. The mean and standard deviation of the difference in bacterial count before and after cavity sealing in the complete removal of carious tissue and partial caries removal using either bur or hand instruments was used and a number of 12 were calculated for each group, after adjusting the power of the study at 80% and keeping a level of significance at 5%. Accordingly, the number of teeth per group was adjusted to 14, assuming the possible losses of 10% during the follow-up period.
Settings and participants
A random sample was selected among children from schools located in Udupi Taluk, between February 2011 and May 2011. A total of 220 children were examined for the presence of acute carious lesions in dentin, and 68 teeth were selected for radiographic examination. The sample consisted of 42 deciduous molars from 42 children aged 8-10 years who fulfilled the following criteria, modified by Ribeiro et al.:[18]
Patient – Healthy and with at least one carious primary molar.
Tooth – Primary molar presenting pulp vitality, without previous restoration and with no radiographic signs suggestive of pulp and/or periapical abnormalities.
Carious lesion – Active carious lesion in the inner half of dentin, with the buccal-lingual opening measuring at least 2 mm and involving the occlusal or occluso-proximal surface.
The sample was randomized using a table of random numbers by a person who did not belong to the research group, for a total of 14 teeth in each of the three groups. The examiner who was doing the treatment was given this information only when he was performing the clinical procedure.
Calibration of the examiner for both techniques of partial caries removal was performed on four extracted deciduous molars each, which were evaluated after treatment by a second examiner experienced in this procedure. A kappa value of 0.8 was obtained. The cavity classification (class I or II), dental arch involved (upper or lower), and age (8, 9, or 10 years) were considered to maintain the homogeneity of the groups.
Clinical procedures
The teeth fulfilling the inclusion criteria were isolated using a rubber dam and then submitted to the procedure of caries removal. They were randomly divided into three groups according to the technique used for caries excavation. Only superficial necrotic dentin was removed from the pulpal and axial walls in both techniques of partial caries removal.
Group A: Partial caries removal using hand instruments (atraumatic restorative treatment)
Initial access to the lesion was gained with the use of dental hatchet, following which, whole of the carious tissue involving the lateral walls and dentino-enamel junction was removed. Only superficial necrotic dentin was removed from the pulpal and axial walls using small (diameter -1 mm) and medium size excavators (diameter -1.5 mm).
Group B: Partial caries removal using the bur
Initial access to the lesion was gained with the use of bur, following which, whole of the carious tissue involving the lateral walls and dentino-enamel junction was removed. Only superficial necrotic dentin was removed from the pulpal and axial walls using low-speed round burs.
Group C: Complete caries removal using bur and dye
The cavity was accessed using a number 329 carbide bur at high rotation and carious tissue was completely removed with smooth spherical burs at low rotation, with the size of the burs being compatible with that of the lesion. To reduce examiner subjectivity, a caries detector dye (1% acid red 52 solutions in propylene glycol) was applied to dentin for 10 s, followed by washing. This procedure was repeated until the dentin was no longer stained, with this point being defined as complete caries removal.
Collection of remaining dentin samples
After caries removal using different techniques, a sample of remaining dentine was collected from the teeth of all three groups with a number 3 sterile bur at low rotation for the evaluation of dental tissue contamination. The bur containing the remaining dentin sample was immediately transferred to a sterilized bottle containing 2 ml thioglycolate transporting medium. This sterilized bottle was kept in the ice box and taken to the microbiology laboratory for processing, within an hour, by another examiner who was not known to the type of technique used for caries excavation. After dentin sample collection, the cavity was cleaned using wet cotton. All teeth in the three groups were protected with calcium hydroxide cement (Dycal, Dentsply), and restored with glass ionomer cement (GIC) (GC Asia Dental Pvt. Ltd, India) according to manufacturer instructions. To differentiate between restoration and dentin at the time of cavity re-opening, calcium hydroxide cement was used.
Dentin sample weight was calculated by measuring the difference between the weight of the whole set (sterilized bottle, transporting medium, and bur with dentin) and the previously determined weight of the set without dentin. Bur weight was measured before sterilization and recorded. The number of bacteria obtained for a given amount of dentin was used to estimate the number of bacteria present in 1 mg dentin (CFU/mg).
After 3 weeks of treatment, which is the minimum time duration required for reparative dentine formation, the teeth were submitted to clinical examinations to determine signs and symptoms of pulp vitality. The restorative material was removed in the three groups and a new dentin sample was collected from the same site. Reopening did not require complete removal of the restoration, only what was required to expose the dentin and permit microbiological collection. After dentin sample collection, the cavity was cleaned using wet cotton and then dried before restoration with GIC again.
Microbiological analysis
The sterilized bottles containing the dentin samples were shaken in a tube shaker for 30 s to disperse bacterial aggregates and decimal dilutions were then prepared in sterile saline (0.9% NaCl). Next, 0.1 ml aliquots of each dilution were spread, in duplicate, on the following solid media: Brain-heart infusion agar (EOS Laboratories, Mumbai, India) supplemented with 5% sheep blood for total viable microorganism count, Rogosa agar for Lactobacillus spp. count,[19] and mitis salivarius agar (MSA) (EOS Laboratories, Mumbai, India) for Streptococcus spp. Count and MSA supplemented with 20% sucrose, 0.2 units/ml bacitracin, and 1% potassium tellurite (mitis-salivarius bacitracin [MSB]) for mutans streptococci count.[20] The Rogosa and blood agar plates were incubated under anerobic conditions for 48 h,[21] whereas the MSA and MSB plates were incubated in an atmosphere of 5% CO2 for 48 h.[20] After incubation, microbial counts were performed with a digital colony counter (Himedia - LA660) by a single examiner, who was unaware of the treatment of the patients. Cell morphology was evaluated by Gram staining. The reproducibility of CFU per mg of dentine for duplicate samples was evaluated by comparison of total microorganism count on duplicate plates for each sample. There was a positive correlation between counts (r = 0.882; P < 0.001).
Statistical analysis
The statistician was blinded to type of technique used for caries excavation. The tooth was used as a parameter for analysis. After application of the Shapiro-Wilk test, data that showed nonnormal distribution were log 10 - transformed by the Box-Cox method. If fewer than 10 colonies were detected, a value of 2 (or log 10 100 CFU/ mg) was used for analysis. General linear model - repeated measure ANOVA was used to compare CFU counts between the groups subjected to three different techniques of caries removal. ANOVA test followed by post-hoc Tukey's test was used to compare mean reduction among the three groups. The distributions of the variables cavity classification, dental arch involved, and age were evaluated using Fisher's exact test. The analysis of the study was carried out using the Statistical Package for Social Sciences version 16.0 (SPSS version 16.0 Chicago, SPSS Inc.). The cut-off level for statistical significance was taken at 0.05.
RESULTS
Out of the 42 teeth included in this randomized clinical trial, six were lost during the 3 weeks follow-up period. In both Group A and Group B, one tooth with class II cavity was excluded because of loss of the restoration. One patient each in Group A and Group B and two patients in Group C were lost to follow-up. The final samples consisted of 36 teeth from 36 patients (12 teeth per group) [Figure 1].
Figure 1.
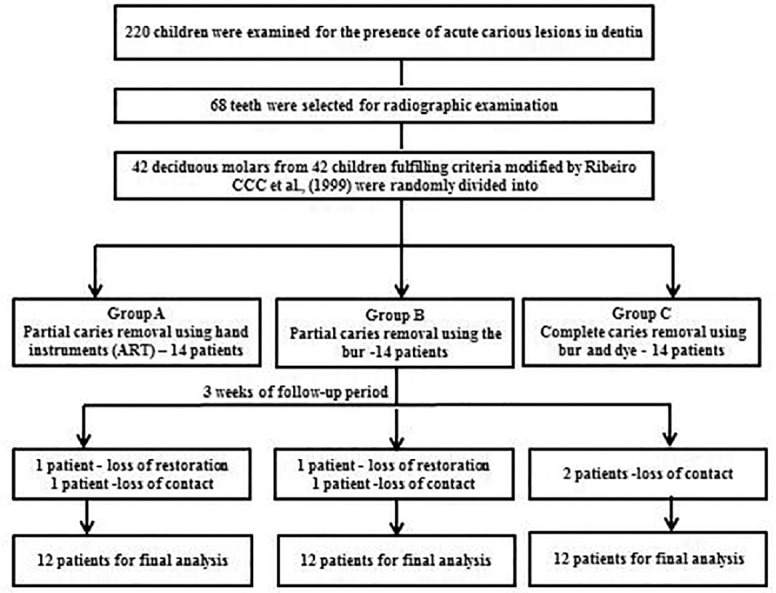
Clinical trial profile: Participants flow by the group.
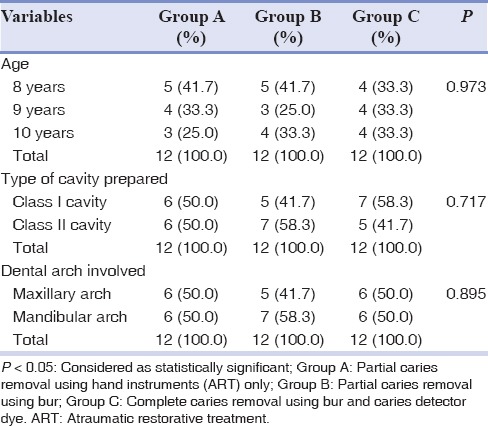
The characteristics of study participants in three groups were similar at baseline [Table 1]. There were no significant differences between the three groups in terms of age of the participants (P = 0.973), type of cavity prepared (P = 0.717), and dental arch involved (P = 0.895). No clinical signs of pulpal alterations were noted during 3 weeks of follow-up period.
Table 1.
Distribution of patients in three groups according to characteristic variables
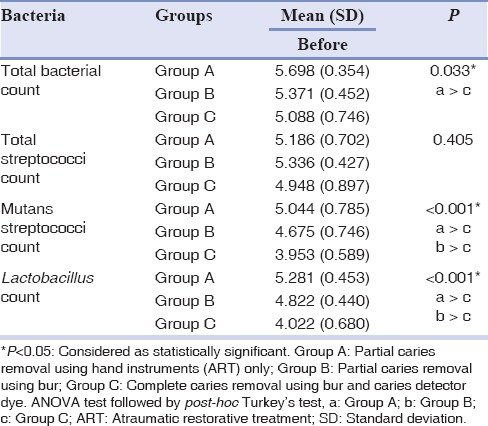
There was a statistically significant difference in microbial count between three groups of total viable bacterial, mutans streptococci, and Lactobacillus spp. count before restoration of cavity [Table 2]. Group A showed the highest microbial count followed by Group B and Group C for all the microorganisms studied. The total viable bacterial count was significantly higher in Group A as compared to Group C. Group C showed the lowest mutans streptococci and Lactobacillus spp. count (P < 0.001). The difference in microbial count between three groups decreased after 3 weeks for all of the microorganisms studied.
Table 2.
Comparison of microbial counts (mean [SD]) among the three groups before cavity restoration
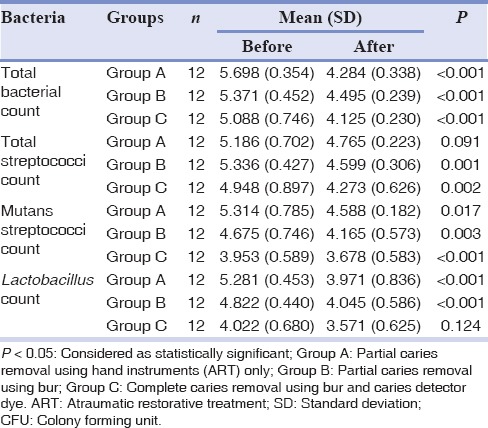
Comparison between counts before and after 3 weeks of cavity restoration among the three groups showed a significantly decreasing trend in all microorganisms except for total Streptococci spp. count in Group A (P = 0.091) and Lactobacillus spp. count in Group C (P = 0.124) [Table 3].
Table 3.
Mean (SD) number of microbial count reported as log10 (CFU) per mg of dentine before and after cavity restoration in patients submitted to three different techniques
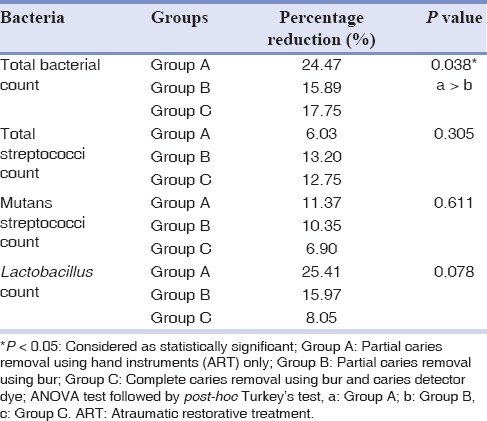
There was no significant difference in percentage reduction of all microorganism counts among the three groups after 3 weeks of restoration except for total viable bacterial count where a significant reduction was observed [Table 4]. Percentage reduction in the total viable bacterial count was significantly higher in Group A as compared to Group B. Group A showed higher percentage reduction in total viable bacterial count, mutans streptococci count, and Lactobacillus spp. count as compared to Group B and Group C. Group B and C showed an almost similar percentage reduction in total streptococci spp. count as compared to Group A.
Table 4.
Comparison of percentage reduction of microbial counts among the three groups after 3 weeks of restoration
DISCUSSION
This randomized clinical trial showed a larger number of microorganisms in dentin of teeth submitted to partial removal of carious tissue compared to teeth submitted to complete removal technique, before restoring the cavities. This finding is in concordance with the study done by Lula et al.,[22] when partial caries removal was done using the hand instruments ART, higher microbial count was found than that in partial caries removal using the bur. The difference in microbial count observed between the three groups at baseline disappeared at the end of the 3 weeks follow-up. These results support the importance of removal of the cariogenic biomass and of the superficial necrotic layer to arrest carious lesions.[15]
In both Group A and Group B, one tooth with class II cavity was excluded because of loss of the restoration. It might be because of poor patient compliance or failure of GIC restoration in class II cavity. One patient each in Group A and Group B and 2 patients in Group C were lost to follow-up as they failed to report on the day of follow-up examination.
The results of this trial showed a statistically significant decreasing trend in all the three groups for all of the microorganisms except for total streptococci spp. count in Group A and Lactobacillus spp. count in Group C. This reduction in the microbial count was also reported by Bjørndal et al.,[15] Fairbourn et al.,[23] Bjørndal and Larsen,[24] Bönecker et al.,[25] and Lula et al.[22]
A significant reduction of aciduric bacteria such as mutans streptococci and Lactobacillus spp. were observed in three groups after restoration. This finding suggested that there was absence of caries lesion activity, and the restored microenvironment was less acidic as compared to carious tissue environment. These findings are in agreement with the studies done by Lula et al.,[22] Mejàre et al.,[26] and Hojo et al.[27] The possible explanation for the reduction in microbial count could be due to restriction of exogenous nutrient supply by isolating the caries process from the oral cavity[28] or due to the cavity sealing with calcium hydroxide which has bactericidal and bacteriostatic properties and drastically reduce the presence of cariogenic bacteria.[22]
A quantitative reduction after sealing was observed in cavities submitted to different techniques of caries removal. The present randomized clinical trial did not show any statistically significant difference among the three groups for percentage reduction of total streptococci spp. count, mutans streptococci count, and Lactobacillus spp. count. Similar findings were reported by Lula et al.[22]
Partial caries removal using ART technique resulted in greater percentage reduction of total viable bacterial count, mutans streptococci count, and Lactobacillus spp. count as compared to partial caries removal using bur. The possible explanation for this difference between two techniques could be that during caries removal using bur, the microorganisms could be pushed deep inside the dentinal tubules due to nature of the procedure and it is possible that these microorganisms can remain viable for long time. During caries removal using ART technique, the caries is removed in en masse, and chances of residual carious tissue containing microorganism are low. These findings support the view that cavity sealing after partial removal of carious tissue may modify bacterial growth and may drastically reduce the presence of cariogenic bacteria.
It can be concluded that partial caries removal technique either using bur or hand instruments (ART) is associated with a marked reduction in bacterial growth. So partial caries removal technique, preferably using hand instruments (ART) can be used effectively for the management of deep caries in public health settings.
This study indicated that initial removal of the cariogenic biomass appears to be essential for control of caries progression. Disruption in microbial niche by partial dentine caries removal and tooth sealing arrests lesion progression, suggesting that complete dentine caries removal is not essential to control caries progression and caries management. This trial suggested that subsequent reopening of the cavity for removal of remnant infected dentin might be an unnecessary step. The use of less invasive technique of partial caries removal in a single session as part of treatment of deep caries lesions in deciduous teeth is recommended. This is relevant especially in view of the defined biological cycle of these teeth and the risk of pulp exposure associated with cavity reopening for removal of the entire infected dentin. However, further long-term studies are required to confirm the persistence of this reduction in microbial count.
Financial support and sponsorship
Nil.
Conflicts of interest:
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
ACKNOWLEDGMENTS
We are very grateful to the school teachers, parents, and children for their kind support, cooperation and participation in this trial, Dr. Mamatha Ballal and Dr. Sujata for assistance in Microbiology Lab, Dr. Asha Kamath for statistical analysis. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Thompson V, Craig RG, Curro FA, Green WS, Ship JA. Treatment of deep carious lesions by complete excavation or partial removal: A critical review. J Am Dent Assoc. 2008;139:705–12. doi: 10.14219/jada.archive.2008.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjørndal L, Reit C, Bruun G, Markvart M, Kjaeldgaard M, Näsman P, et al. Treatment of deep caries lesions in adults: Randomized clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur J Oral Sci. 2010;118:290–7. doi: 10.1111/j.1600-0722.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 3.Kidd EA, Joyston-Bechal S, Smith MM, Allan R, Howe L, Smith SR. The use of a caries detector dye in cavity preparation. Br Dent J. 1989;167:132–4. doi: 10.1038/sj.bdj.4806939. [DOI] [PubMed] [Google Scholar]
- 4.Kidd EA, Joyston-Bechal S, Beighton D. The use of a caries detector dye during cavity preparation: A microbiological assessment. Br Dent J. 1993;174:245–8. doi: 10.1038/sj.bdj.4808142. [DOI] [PubMed] [Google Scholar]
- 5.Weerheijm KL, Kreulen CM, de Soet JJ, Groen HJ, van Amerongen WE. Bacterial counts in carious dentine under restorations: 2-year in vivo effects. Caries Res. 1999;33:130–4. doi: 10.1159/000016506. [DOI] [PubMed] [Google Scholar]
- 6.Kato S, Fusayama T. Recalcification of artificially decalcified dentin in vivo. J Dent Res. 1970;49:1060–7. doi: 10.1177/00220345700490051001. [DOI] [PubMed] [Google Scholar]
- 7.Ricketts DN, Kidd EA, Innes N, Clarkson J. Complete or ultraconservative removal of decayed tissue in unfilled teeth. Cochrane Database Syst Rev. 2006;19:CD003808. doi: 10.1002/14651858.CD003808.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Frencken JE, Holmgren CJ. How effective is ART in the management of dental caries? Community Dent Oral Epidemiol. 1999;27:423–30. doi: 10.1111/j.1600-0528.1999.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Amerongen WE. Dental caries under glass ionomer restorations. J Public Health Dent. 1996;56:150–4. doi: 10.1111/j.1752-7325.1996.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 10.Toi CS, Bönecker M, Cleaton-Jones PE. Mutans streptococci strains prevalence before and after cavity preparation during Atraumatic Restorative Treatment. Oral Microbiol Immunol. 2003;18:160–4. doi: 10.1034/j.1399-302x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeronimus DJ, Jr, Till MJ, Sveen OB. Reduced viability of microorganisms under dental sealants. ASDC J Dent Child. 1975;42:275–80. [PubMed] [Google Scholar]
- 12.Going RE, Loesche WJ, Grainger DA, Syed SA. The viability of microorganisms in carious lesions five years after covering with a fissure sealant. J Am Dent Assoc. 1978;97:455–62. doi: 10.14219/jada.archive.1978.0327. [DOI] [PubMed] [Google Scholar]
- 13.Weerheijm KL, de Soet JJ, van Amerongen WE, de Graaff J. The effect of glass-ionomer cement on carious dentine: An in vivo study. Caries Res. 1993;27:417–23. doi: 10.1159/000261573. [DOI] [PubMed] [Google Scholar]
- 14.Eidelman E. Intentional sealing of occlusal dentin caries: A controversial issue. Pediatr Dent. 1993;15:312. [PubMed] [Google Scholar]
- 15.Bjørndal L, Larsen T, Thylstrup A. A clinical and microbiological study of deep carious lesions during stepwise excavation using long treatment intervals. Caries Res. 1997;31:411–7. doi: 10.1159/000262431. [DOI] [PubMed] [Google Scholar]
- 16.Lager A, Thornqvist E, Ericson D. Cultivatable bacteria in dentine after caries excavation using rose-bur or carisolv. Caries Res. 2003;37:206–11. doi: 10.1159/000070446. [DOI] [PubMed] [Google Scholar]
- 17.Orhan AI, Oz FT, Ozcelik B, Orhan K. A clinical and microbiological comparative study of deep carious lesion treatment in deciduous and young permanent molars. Clin Oral Investig. 2008;12:369–78. doi: 10.1007/s00784-008-0208-6. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro CC, Baratieri LN, Perdigão J, Baratieri NM, Ritter AV. A clinical, radiographic, and scanning electron microscopic evaluation of adhesive restorations on carious dentin in primary teeth. Quintessence Int. 1999;30:591–9. [PubMed] [Google Scholar]
- 19.Rogosa M, Mitchell JA, Wiseman RF. A selective medium for the isolation and enumeration of oral lactobacilli. J Dent Res. 1951;30:682–9. doi: 10.1177/00220345510300051201. [DOI] [PubMed] [Google Scholar]
- 20.Gold OG, Jordan HV, Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973;18:1357–64. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 21.Jurgensen CA, Jurgensen LD. Copper oxidation, an alternative method for obtaining anaerobiosis (in Portuguese) Rev Bras Patol Clin. 1982;18:58–63. [Google Scholar]
- 22.Lula EC, Monteiro-Neto V, Alves CM, Ribeiro CC. Microbiological analysis after complete or partial removal of carious dentin in primary teeth: A randomized clinical trial. Caries Res. 2009;43:354–8. doi: 10.1159/000231572. [DOI] [PubMed] [Google Scholar]
- 23.Fairbourn DR, Charbeneau GT, Loesche WJ. Effect of improved Dycal and IRM on bacteria in deep carious lesions. J Am Dent Assoc. 1980;100:547–52. doi: 10.14219/jada.archive.1980.0144. [DOI] [PubMed] [Google Scholar]
- 24.Bjørndal L, Larsen T. Changes in the cultivable flora in deep carious lesions following a stepwise excavation procedure. Caries Res. 2000;34:502–8. doi: 10.1159/000016631. [DOI] [PubMed] [Google Scholar]
- 25.Bönecker M, Toi C, Cleaton-Jones P. Mutans streptococci and lactobacilli in carious dentine before and after Atraumatic Restorative Treatment. J Dent. 2003;31:423–8. doi: 10.1016/s0300-5712(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 26.Mejàre B, Mejàre I, Edwardsson S. Bacteria beneath composite restorations — A culturing and histobacteriological study. Acta Odontol Scand. 1979;37:267–75. doi: 10.3109/00016357909004696. [DOI] [PubMed] [Google Scholar]
- 27.Hojo S, Komatsu M, Okuda R, Takahashi N, Yamada T. Acid profiles and pH of carious dentin in active and arrested lesions. J Dent Res. 1994;73:1853–7. doi: 10.1177/00220345940730121001. [DOI] [PubMed] [Google Scholar]
- 28.Bowden GH, Hamilton IR. Survival of oral bacteria. Crit Rev Oral Biol Med. 1998;9:54–85. doi: 10.1177/10454411980090010401. [DOI] [PubMed] [Google Scholar]


