Abstract
This study investigated the effects of varicocele on semen parameters in infertile men based on the new 2010 World Health Organization laboratory manual for the examination of human semen. Semen analysis results (volume, sperm count, motility, and morphology) were the primary outcomes. An electronic search to collect the data was conducted using the Medline/PubMed, SJU discover, and Google Scholar databases. We searched articles published from 2010 to August 2015, i.e., after the publication of the 2010 WHO manual. We included only those studies that reported the actual semen parameters of adult infertile men diagnosed with clinical varicocele and contained a control group of either fertile men or normozoospermic men who were not diagnosed with varicocele. Ten studies were included in the meta-analysis, involving 1232 men. Varicocele was associated with reduced sperm count (mean difference: −44.48 × 106 ml−1; 95% CI: −61.45, −27.51 × 106 ml−1; P < 0.001), motility (mean difference: −26.67%; 95% CI: −34.27, −19.08; P < 0.001), and morphology (mean difference: −19.68%; 95% CI: −29.28, −10.07; P < 0.001) but not semen volume (mean difference: −0.23 ml; 95% CI: −0.64, 0.17). Subgroup analyses indicated that the magnitude of effect was influenced by control subtype but not WHO laboratory manual edition used for semen assessment. We conclude that varicocele is a significant risk factor that negatively affects semen quality, but the observed pooled effect size on semen parameters does not seem to be affected by the WHO laboratory manual edition. Given most of the studies published after 2010 still utilized the 1999 manual for semen analysis, further research is required to fully understand the clinical implication of the 2010 WHO laboratory manual on the association between varicocele and semen parameters.
Keywords: andrology laboratory, male infertility, meta-analysis, semen analysis, systematic review, varicocele, World Health Organization
INTRODUCTION
Clinical varicocele is defined as a palpable elongated, dilated and tortuous testicular pampiniform plexus of veins in the spermatic cord.1 From a pathophysiology standpoint, varicocele is a venous incompetence that allows reflux of blood into the internal spermatic vein.2 It is found in approximately 15%–20% of the normal adult male population and 35% of men with primary infertility.1,2 Although no mechanism has conclusively explained infertility in men with varicocele, a number of potential mediators have been suggested including scrotal hyperthermia, oxidative stress, hormonal disturbances, testicular hypoperfusion, testicular hypoxia, and backflow of toxic metabolites. It is also unknown why most men with varicocele retain their reproductive potential, but novel discoveries have suggested that variation in genetic transcriptional response to oxidative stress might confer sperm protection against damage.2
An early study conducted by the World Health Organization (WHO) involving 9034 men demonstrated that both sperm concentration and motility were lower in men with varicocele than in men without varicocele.3 This aforementioned study was conducted more than 20 years ago and, within this period, the WHO laboratory manual for the analysis of human semen has been updated 3 times. However, no study has critically examined the effect of varicocele on semen analysis results according to these various WHO criteria. This analysis is important because each new WHO laboratory manual edition not only changes the reference values for interpreting semen analysis results but also updates the methods for conducting such analyses.
In 2010, the WHO announced the first semen criteria based on a large study of fertile men across seven countries.4,5 In that updated fifth edition of the WHO manual, novel methods for measuring ejaculate volume by weight and assessing sperm morphology by strict criteria were incorporated.5 In addition, changes in the methods for assessing sperm count, sperm motility, and quality control routines were included. However, to our knowledge, no study has yet evaluated the effect of varicocele as a risk factor on semen parameters based on the latest WHO criteria.
Our objective was, therefore, to investigate the impact of varicocele on semen parameters as per the new WHO criteria. For this, we collected and summarized the studies that evaluated the effect of clinical varicocele on semen parameters of infertile men after the publication of the 2010 WHO laboratory manual for the examination of human semen.
MATERIALS AND METHODS
Search strategy
We conducted a systematic search using the Medline/PubMed, SJU Discover, and Google Scholar databases to identify all relevant studies published from 2010 (i.e., after the publication of the 2010 WHO laboratory manual for the examination of human semen) to August 2015. MESH search terms used were “varicocele” OR “semen analysis.” We limited the search to studies using human subjects and articles published in English. Hand searches were carried out on review articles and reference lists. Authors of unpublished or incomplete datasets were not contacted to provide information for this meta-analysis. This study was exempted from Institutional Review Board approval, given that it did not involve any human intervention. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement to report results.6
Eligibility criteria and data extraction
Studies were analyzed for inclusion independently by two of the authors (RS and SCE), and any discrepancies were resolved by discussion with the third author (AA). Five hundred sixty-two articles were identified. After removing duplicates, 375 articles were screened. This was reduced to 43 potentially suitable articles using abstract, largely due to the presence of risk factors other than varicocele and the inclusion of adolescent and pediatric varicocele, subclinical varicocele, and pre- and post-treatment (e.g., varicocele repair by surgery or embolization) outcomes. From these, 10 studies fulfilled all criteria and were included in the meta-analysis. The complete selection process is depicted in Figure 1.
Figure 1.
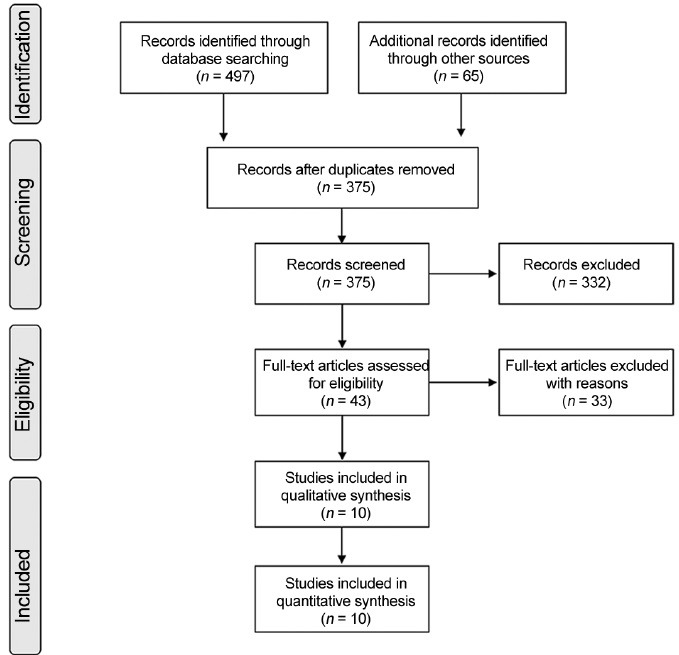
Flowchart for study identification and selection process.
Participants were infertile men aged 18 years and older regardless of origin and ethnicity. Clinical varicoceles were diagnosed based on the finding of varicose veins in the spermatic cord by either visual inspection or palpation with or without the aid of the Valsalva maneuver during a physical examination with the patient standing position. Only studies with a comparison control group of men with proven or unproven fertility or normozoospermia who were not diagnosed with varicocele were included. Semen analysis of all included subjects was based on the WHO laboratory manual (any edition) for the evaluation of human semen. Exclusion criteria included studies involving men with azoospermia, case reports, reviews, and experimental studies and studies with risk factors other than varicocele (multivariate analysis) (Table 1).
Table 1.
Selection criteria of included studies (PICOS)
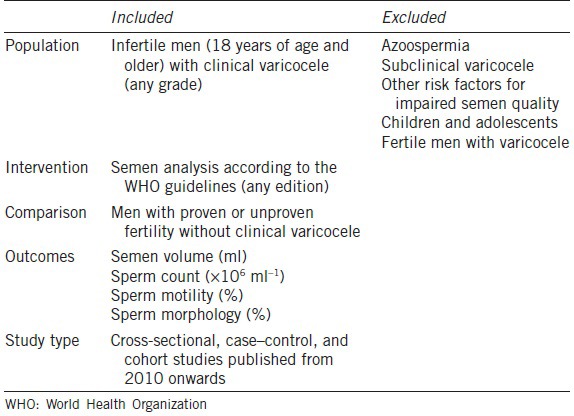
Outcome measures
We specified the primary outcome measures, a priori as semen volume (ml), sperm count (×106 ml−1), sperm motility (% motile sperm), and sperm morphology (% normal forms). In clinical settings, these parameters are the most frequent laboratory measures used for investigations of varicocele. Results are described as mean ± S.D. One exposure (risk factor) was compared at once (no multivariate analysis). Some of the studies provided data on all four of these outcome measures and others on just some of them. The following characteristics were assessed for each study: (i) Study design, (ii) Type of control, (iii) Semen analysis method (WHO laboratory manual edition used), and (iv) Sample size.
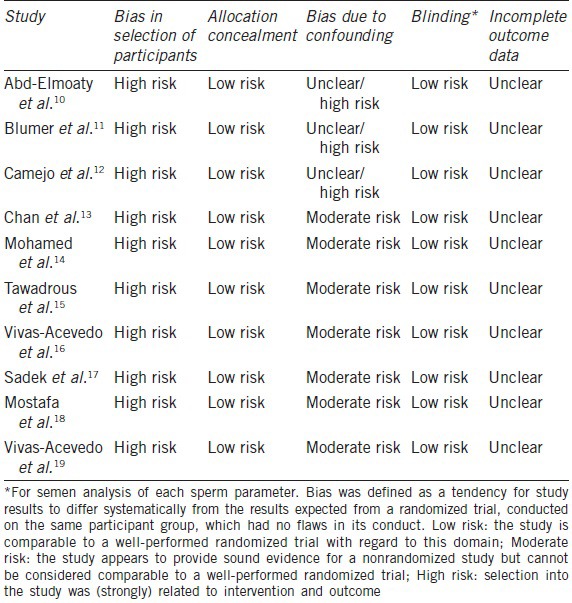
Risk of bias assessment
We followed the Cochrane Collaboration guidelines to assess the risk of bias in the included studies.7 We evaluated sequence generation, allocation concealment, bias from confounding, blinding, and incomplete outcome data. The quality assessment of the included trials is shown in Table 2. All included studies were nonrandomized trials; therefore, a high risk of bias (not random) was assumed. The risk of bias due to confounding was an issue for three studies that either selected volunteers or provided no detailed information about where the controls had been selected. Because the other studies had either matched a control group for age or utilized similar exclusion criteria, a grade of “moderate risk” was assumed. Important confounders such as smoking, infections, and toxic exposure were excluded. Observers performing semen analyses were not blinded to the groups analyzed, but we did not believe that this issue could introduce performance or detection bias. Missing data for each sperm parameter was not reported in individual studies, thus making the risk of bias due to incomplete outcome data unclear.
Table 2.
Quality assessment of included trials
Analysis
The Comprehensive Meta-analysis (CMA) software (Biostat version 3, Englewood, New Jersey, USA) was used to conduct the statistical analyses. Both fixed effects models (FEMs) and random effects models (REMs) were fitted to determine which model was most suited to the data. FEMs were based on the inverse variance method and REM on the DerSimonian and Laird methods. Because sperm parameters are continuous data, the mean differences (MD) and associated confidence intervals (CIs) between varicocele and control groups were calculated to determine the effect size. The heterogeneity of the studies was assessed using I2 statistic. When the heterogeneity was > 50% (I2 >50%), we applied the random effects model.8 Sensitivity analyses were conducted to assess the influence of individual studies on the results (Supplementary Material (2.1MB, pdf) ). Publication bias was assessed using the trim and fill method9 as provided in the supplementary material (2.1MB, pdf) . Statistical significance was set at P < 0.05.
RESULTS
Description of the included studies
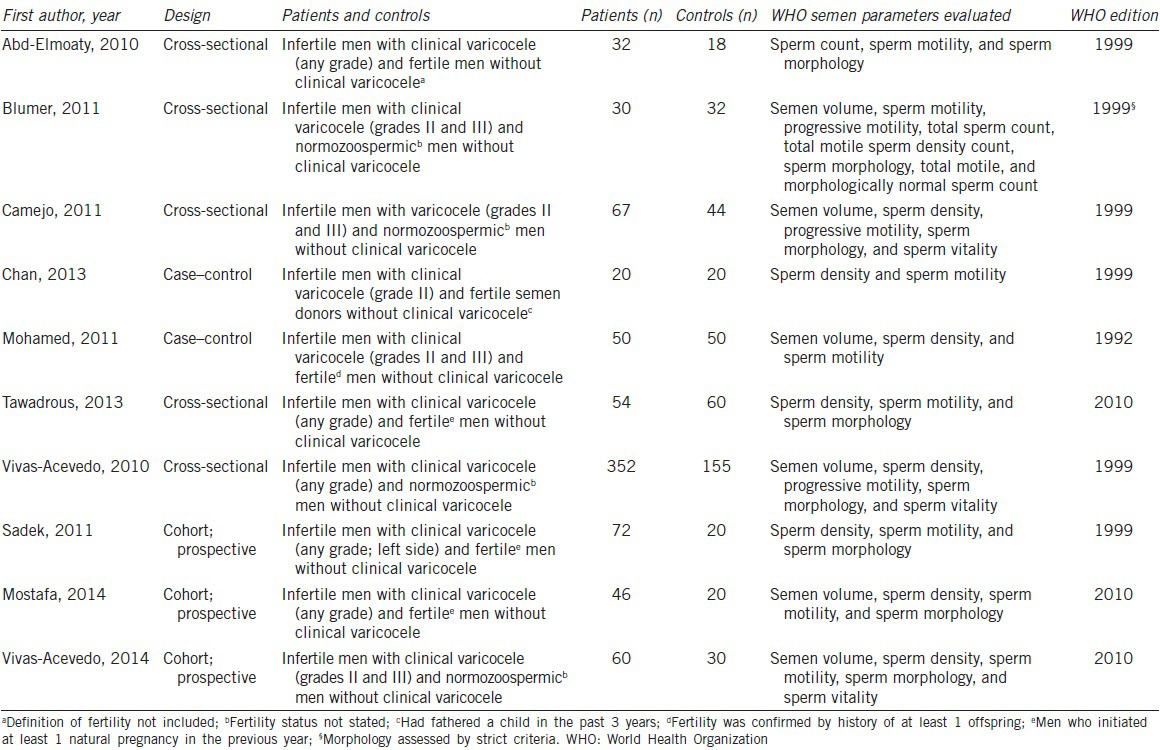
Overall, 10 suitable studies were qualified (five cross-sectional, three cohort, and two case-control studies), and these included 1232 men (783 with varicocele and 449 controls).10,11,12,13,14,15,16,17,18,19 The number of studies included in each meta-analysis varied according to the sperm parameter reported: six provided data on semen volume, 10 provided data on sperm count and motility, and eight provided data on morphology (Table 3). All semen analyses were carried out following the WHO laboratory manual for the examination of human semen. Despite including only studies published after the release of the 2010 WHO manual (fifth edition),5 only three of the studies specifically applied this new edition during semen analyses.15,18,19 Six studies10,11,12,13,16,17 utilized the previous version, namely the 1999 WHO manual (fourth edition),20 and one study14 applied the 1992 WHO manual (third edition) for the analyses.21 Of note, one of the studies that used the 1999 WHO manual utilized the strict criteria for sperm morphology assessment.11 Most of the included studies were designed to evaluate the effect of varicocele on sperm functional parameters; semen characteristics as per the WHO laboratory manual were mainly secondary outcome measures. Six studies10,13,14,15,17,18 included fertile controls without varicocele and four studies11,12,16,19 included healthy normozoospermic controls without varicocele (with all semen parameters within normal ranges according to the WHO criteria utilized) (Table 3).
Table 3.
Characteristics of included studies evaluating the effect of varicocele on semen parameters
Outcomes
Semen volume
Six studies reported data on semen volume including 936 men (605 with varicocele and 331 controls).11,12,14,16,18,19 Mean semen volume in patients and controls ranged from 2.6 to 3.3 ml and 2.6 to 3.7 ml, respectively. Five studies reported that semen volume was not statistically different between men with varicocele and controls.11,12,14,16,18 Heterogeneity was high and REM, therefore, provided the actual representation of the data. Overall, REM indicated that semen volume was not significantly affected by varicocele (mean difference: −0.23 ml; 95% CI: −0.64, 0.17; P = 0.26). To analyze the potential causes of the heterogeneity, a subgroup analysis was conducted to assess the effect of WHO manual editions with regards to semen volume. Performing separate analyses according to the WHO manual edition significantly reduced heterogeneity estimates, but the observed pooled effect size was not materially affected (Figure 2).
Figure 2.
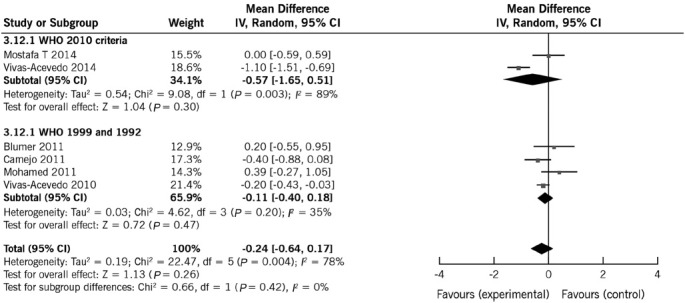
Forest plot showing the effect of varicocele on semen volume, including subgroup analyses according to different WHO criteria.
Sperm count
All included studies reported data on sperm count. Mean sperm count in patients and controls ranged from 9.62 to 96.6 × 106 ml−1 and 64.98 to 124.05 × 106 ml−1, respectively. Eight studies reported a significant negative effect of varicocele on sperm count.10,12,13,14,15,17,18,19 Overall, both FEM and REM indicated that sperm count significantly decreased in men with varicocele compared with controls. The pooled mean difference was FEM −62.28 × 106 ml−1 (95% CI: −64.15, −60.40 × 106 ml−1; P < 0.001) and REM −44.48 × 106 ml−1 (95% CI: −61.45, −27.51 × 106 ml−1; P < 0.001). Given the high heterogeneity (98%), REM provides the most appropriate representation of the data.
To analyze the potential causes of the heterogeneity, two subgroup analyses were conducted (Table 4). First, the effect of WHO manual editions on sperm count estimates was assessed. The heterogeneity estimates and the observed pooled effect size were not materially affected by the WHO manual edition (Figure 3). Then, the effect of control group type was analyzed. The heterogeneity estimates were slightly reduced by performing analyses separately by type of controls, which also affected the pooled mean differences (range of mean effect size with REM was −15.39 to −65.00), thus suggesting that some of the differences between studies might be explained by control groups (Table 4). The observed pooled effect size was larger for the studies using fertile controls compared with normozoospermic controls (P < 0.0001) (Supplementary Material (2.1MB, pdf) ).
Table 4.
Subgroup analyses
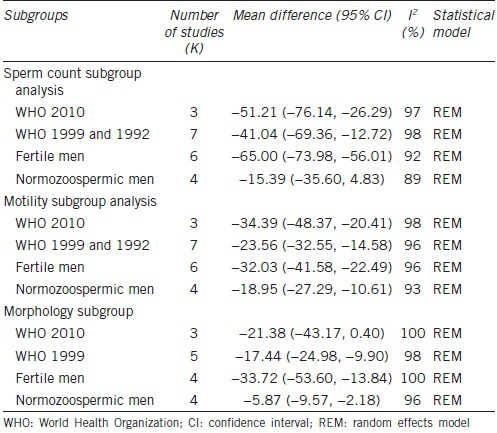
Figure 3.
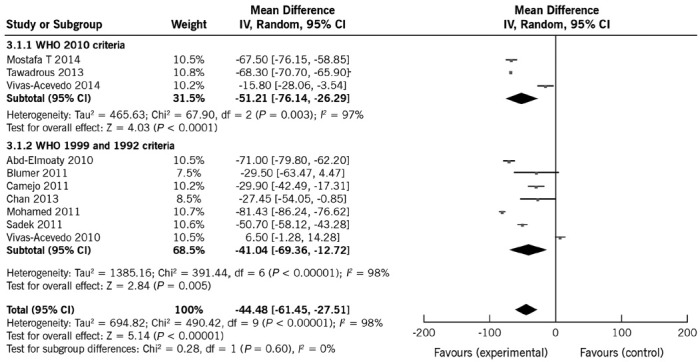
Forest plot showing the effect of varicocele on sperm count, including subgroup analyses according to different WHO criteria.
The consistency in the direction of the effect and overlap of the confidence intervals in most studies increase confidence in the results (Figure 3). Sensitivity analyses indicated that removing the study by Mohamed et al.,14 which was shown to have the largest influence on results, slightly reduced the mean difference to −40.34, but the observed effects were not affected by removing any of the other studies (range of mean effect size with REM was −53.12, −40.34; Supplementary Material (2.1MB, pdf) ).
Sperm motility
All included studies reported data on sperm motility. Mean motility in patients and controls ranged from 21.1% to 61.9% and 49.3% to 70.0%, respectively. Nine studies reported a significant negative effect of varicocele on sperm motility.10,12,13,14,15,16,17,18,19 Overall, varicocele was a risk factor for motility and affected it significantly. The pooled mean difference was −26.67% (95% CI: −34.27%, −19.08%; P < 0.0001). As heterogeneity was high (97%), REM is more appropriate for estimating mean differences. To analyze the potential causes of the heterogeneity, two subgroup analyses were conducted according to the WHO manual edition and type of control. The heterogeneity estimates and the observed pooled effect size were not materially affected by performing analyses separately by WHO manual editions (Figure 4). Like sperm count, the heterogeneity estimates were slightly reduced by performing analyses separately by type of controls (Table 4). The pooled mean differences were −32.03% (95% CI: −41.58%, −22.49%; P < 0.0001) for fertile controls and −18.95% (95% CI: −27.29%, −10.61%; P < 0.0001) for normozoospermic controls, thus suggesting that some of the differences are explained by control subgroups (Table 4). The observed pooled effect size was larger for the studies using fertile controls compared with normozoospermic controls (P = 0.04) (Supplementary Material (2.1MB, pdf) ).
Figure 4.
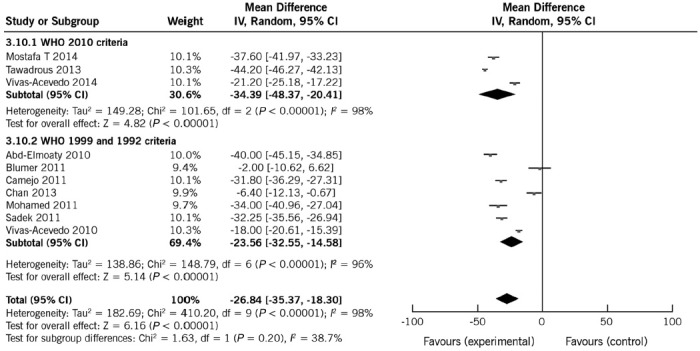
Forest plot showing the effect of varicocele on sperm motility, including subgroup analyses according to different WHO criteria.
Sensitivity analyses indicated that the observed pooled effect size was not affected by removal of any of the studies (Supplementary Material (2.1MB, pdf) ). These results are, therefore, consistent in suggesting a negative association between varicocele and sperm motility.
Sperm morphology
Eight studies, including 1092 subjects (713 with varicocele and 379 controls), were used in this analysis.10,11,12,15,16,17,18,19 In seven studies, varicocele was a risk factor for reduced sperm morphology.10,12,15,16,17,18,19 The pooled mean difference was −19.68% (95% CI: −29.28%, −10.07%; P < 0.0001). REM provided the most appropriate representation of the data since heterogeneity was high (100%) (Figure 5).
Figure 5.
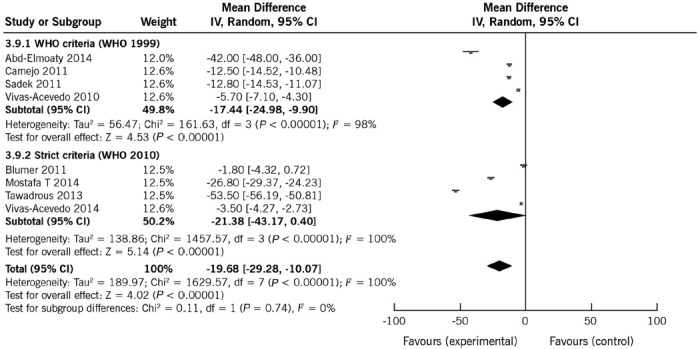
Forest plot showing the effect of varicocele on sperm morphology, including subgroup analyses according to different WHO criteria.
To analyze the potential causes of the heterogeneity, two subgroup analyses were conducted (Table 4 and Figure 5). First, the effect of method for sperm morphology assessments was examined. Mean sperm morphology as per the WHO criteria (1999 edition) in patients and controls ranged from 8.4% to 30.8% and 21.2% to 72.0%, respectively. The results according to the strict criteria (2010 edition) in patients and controls ranged from 6.2% to 8.6% and 10.0% to 61.8%, respectively. Heterogeneity estimates were not affected by performing analyses separately according to the sperm morphology method (Figure 5). Similarly, the observed pooled effect size was not significantly affected by the sperm morphology method (WHO criteria: mean effect size with REM: −17.44%; 95% CI: −24.98%, −9.90%; and Strict criteria: mean effect size with REM: −21.38%; 95% CI: −43.17%–0.40%).
The effect of varicocele on sperm morphology according to the control type was then assessed. The heterogeneity estimates were slightly reduced when analyses were conducted separately by type of controls, which also had a marked effect on the pooled mean differences (Table 4). The mean effect size was significantly reduced (P = 0.007) in studies where controls were normozoospermic men (MD: −5.87%; 95% CI: −9.57%, −2.18%) compared to studies including fertile controls (MD: −33.72%; 95% CI: −53.60, −13.84%), thus suggesting that some of the differences between studies might be explained by control subgroups (Supplementary Material (2.1MB, pdf) ). Sensitivity analyses showed that the removal of the study of Tawadrous et al.15 reduced the mean difference to −14.82%. In contrast, removal of the study of Blumer et al. increased the effect size to −22.3% (Supplementary Material (2.1MB, pdf) ). These results suggest that varicocele negatively affects sperm morphology, but they also indicate the need for further research to fully understand the strength of this association as well as the influence of control subgroups.
DISCUSSION
With changes in the reference ranges and laboratory evaluation methods for human semen,5,20,21,22 there is a need to clarify the relationships between varicocele and semen parameters. To our knowledge, this meta-analysis is the first to summarize the evidence currently available after the publication of the latest WHO laboratory manual for the examination of human semen.5 We included studies evaluating the effect of clinical varicoceles on semen parameters of infertile men. Our results strongly suggest that varicocele negatively affects individual sperm parameters. Varicocele was associated with reduced sperm count, sperm motility, and sperm morphology although it had no effect on semen volume. The consistency in the direction of overall effects estimated for all outcomes adds confidence to our findings.
Evidence from both animal and human studies show that varicocele affects sperm quality. Experimental varicocele has been associated with impairment of testicular and epididymis endocrine and exocrine function, which may contribute to the infertility seen in men with varicocele.23,24,25
The WHO periodically releases laboratory manuals for the examination of human semen. The first manual, published in 1980, summarized the clinical experience and research from the previous 80 years. In its subsequent updates in 1987, 1992, 1999, and 2010, the WHO manuals provided substantial improvements on how to assess the seminal parameters.5,20,21,26 In its latest (fifth) edition, the assessment of sperm volume is based on weighing the sample rather than using graduated pipettes, which is contrary to what had been previously recommended. Volume measurements made by aspirating a sample with a serological pipette have been shown to be approximately 17% lower than those done using an analytical scale, which may, therefore, influence total sperm count.27 Assessment of sperm motility also changed from grading sperm as “a,” “b,” “c,” and “d” to “progressively motile” and “nonprogressively motile,” thus decreasing inter-observer subjectivity. The strict (Tygerberg, also known as Kruger) criteria was incorporated as the recommended method for morphology assessment, which has been consistently associated with a lower percentage of spermatozoa being classified as normal.4,22,28
The reference values that were thought to be compatible with normal male fertility have also changed. In the 2010 WHO laboratory manual, semen analysis reference values were markedly lower than those of previous editions.5 Methodology issues related to data generation and semen analysis methods might explain the discrepancies in the reference thresholds among WHO manuals.4,22,28
Our results should be interpreted in view of the semen parameters of men classified as fertile, which served as the basis for the latest 2010 WHO reference values. The semen parameters of such men, whose female partners had a time-to-pregnancy of 1 year or less after stopping contraception, exhibit high heterogeneity as indicated by the median (95% CI) sperm count 73 × 106 ml−1 (15; 213), motility 61% (40; 78), and morphology 15% (4; 44).4 In our study, the average number of spermatozoa was estimated to be reduced by approximately 44 × 106 ml−1 in men with varicocele whereas the absolute reductions in motility and morphology were 26% and 19%, respectively. The clinical importance of an effect size may not be limited to men at the lower-end of the normal spectrum. It is therefore of no surprise that a significant proportion of men will fall below the fifth percentile, which has been proposed as the lower reference values by the WHO 2010 manual, when a risk factor, namely varicocele, inflicts such a marked change in sperm parameters as indicated by our study. As a result, many of the affected men might face infertility.
On the other hand, semen characteristics that discriminate fertile from infertile men are not well defined. Results are within reference range in up to 40% of those suffering from infertility.29,30 Moreover, sperm production varies widely in men, and conventional semen parameters do not evaluate putative sperm dysfunctions such as immature chromatin or fragmented DNA.31 So far, there is a wide variation in the methods used by different laboratories when performing semen analysis.32,33,34 Nevertheless, the sperm quality measures utilized in this study are still the most frequently used parameters in clinical settings to assess fertility.28
The largest study previous to our own that evaluated the effects of varicocele on individual semen parameters of infertile men was published in the early 1990s.3 The authors investigated the incidence of varicocele in a general population of men seeking fertility and its impact on semen parameters. Among 9038 men across 34 centers, clinical varicocele was identified in 25.4% and 11.7% of men with abnormal and normal semen analysis, respectively. Based on the 1989 WHO manual utilized at the time of publication, significant decreases in the mean total sperm count per ejaculate, percentage of sperm with motility and percentage of morphologically normal sperm were found in men with varicocele compared to controls without varicocele (P < 0.01).3 Nevertheless, this study neither included a control group of healthy men with proved or unproven fertility nor accounted for the magnitude of changes between patients and controls. Despite utilizing the WHO standardized method for semen evaluation and results reporting, the aforementioned study is not comparable to our own due to the inherent differences between the laboratory methods utilized for the examination of human semen.
There are some limitations to this study. Heterogeneity was high in all but one of our meta-analyses which may be associated with low number of included studies.35 The high heterogeneity and relatively low number of studies also precluded meaningful assessment of publication bias.36,37,38 Moreover, control groups of normozoospermic and fertile men might amplify the magnitude of pooled differences as they include a high proportion of men with sperm parameters within the WHO reference range. Along the same lines, a patient population taken from infertility centers may not be representative of the general population of men with varicocele. It is also possible that confounding factors have influenced the results; not all risk factors such as participant age, life-style habits, and obesity, which might have affected semen quality, were consistently reported. These variables are difficult to assess because semen parameters across these above mentioned subgroups have high heterogeneity. However, our meta-analysis included more than 1200 subjects, which increases confidence in the results. In addition, we conducted subgroup analyses to examine the impact of heterogeneity on the outcome data. The heterogeneity in the meta-analyses was partially due to the difference in controls subgroups. Nonetheless, pooled mean differences on sperm count, motility, and morphology indicated consistency between the subgroup types. Sensitivity analyses also demonstrated minimal differences when individual studies were excluded which suggest our results to be conservative.
Surprisingly enough, 6 of 10 studies published after 2010 still did not use the latest WHO manual for the laboratory examination of human semen. On the other hand, evidence has shown that it takes approximately 5 years for guidelines to be adopted into routine practice, and even the broadly accepted guidelines are often not fully followed.39 Subgroup analyses conducted on individual sperm parameters to assess the leverage of WHO manual editions on the results indicated that the observed pooled effect size was not materially affected by the different WHO editions used. Further research is required to confirm that the changes in the reference range and methods proposed by the 2010 WHO laboratory manual for the examination of human semen had no impact on the association between varicocele as a risk factor and semen parameter results compared to previous WHO manual editions. This would improve the precision of the estimated effect sizes and allow better judgment of the likely clinical importance of the findings.
CONCLUSIONS
Clinically detected varicocele was found to be a significant risk factor for decreased sperm count, motility, and morphology in adult infertile men. The observed pooled effect size does not seem to be affected by the WHO laboratory manual edition used for the examination of human semen. Given most of the studies published after 2010 still utilized the 1999 manual for semen analysis, further research is required to fully understand the impact of this change on the association between varicocele and semen parameters. A better understanding of the collective influence of varicocele on sperm quality and subsequently fertility will help improve counseling, treatment, and support for affected individuals.
AUTHOR CONTRIBUTIONS
AA proposed the conception of the idea and guided the study; RS performed literature search and review, meta-analysis, and interpretation of results; AH reviewed meta-analysis results and feedback on inclusion of studies and analysis; SCE analyzed included and excluded studies, helped summarize the collected evidence, interpretation of results, and drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declared no competing interests.
ACKNOWLEDGMENTS
Amy Moore, B.A., Medical Editing Services and Staff Editor, Cleveland Clinic Journal of Medicine, assisted with language editing.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Miyaoka R, Esteves SC. A critical appraisal on the role of varicocele in male infertility. Adv Urol 2012. 2012:597495. doi: 10.1155/2012/597495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 3.The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril. 1992;57:1289–93. [PubMed] [Google Scholar]
- 4.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 5.5th ed. Geneva: WHO Press; 2010. World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic review and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Sterne JA, Higgins JP, Reeves BC. on Behalf of the Development Group for ACROBAT-NRSI. A Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Interventions (ACROBAT-NRSI), Ver. 1.0.0; 24 September. 2014. [Last accessed on 2015 Oct 27]. Available from: http://www.riskofbias.info .
- 8.Higgins PT, Thompson SG, David J, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 10.Abd-Elmoaty MA, Saleh R, Sharma RK, Agarwal A. Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil Steril. 2010;94:1531–4. doi: 10.1016/j.fertnstert.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Blumer CG, Restelli AE, Del Giudice PT, Soler TB, Fraietta R, et al. Effect of varicocele on sperm function and semen oxidative stress. BJU Int. 2011;109:259–65. doi: 10.1111/j.1464-410X.2011.10240.x. [DOI] [PubMed] [Google Scholar]
- 12.Camejo MI, Abdala L, Vivas-Acevedo G, Lozano-Hernández R, Angeli-Greaves M, et al. Selenium, copper and zinc in seminal plasma of men with varicocele, relationship with seminal parameters. Biol Trace Elem Res. 2011;143:1247–54. doi: 10.1007/s12011-011-8957-5. [DOI] [PubMed] [Google Scholar]
- 13.Chan CC, Sun GH, Shui HA, Wu GJ. Differential spermatozoal protein expression profiles in men with varicocele compared to control subjects: upregulation of heat shock proteins 70 and 90 in varicocele. Urology. 2013;81:1379.e1–8. doi: 10.1016/j.urology.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed MA, ElShiekh MG, ElFayoumy HM, Fayad AS, Hussein IF, et al. Impact of inguinal varicocele ligation on testicular volume, sperm parameters, and pregnancy rates. Urotoday Int J. 2011;4:art2. [Google Scholar]
- 15.Tawadrous GA, Aziz AA, Mostafa T. Seminal soluble Fas relationship with oxidative stress in infertile men with varicocele. Urology. 2013;82:820–3. doi: 10.1016/j.urology.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Vivas-Acevedo G, Lozano JR, Camejo MI. Effect of varicocele grade and age on seminal parameters. Urol Int. 2010;85:194–9. doi: 10.1159/000314226. [DOI] [PubMed] [Google Scholar]
- 17.Sadek A, Almohamdy AS, Zaki A, Aref M, Ibrahim SM, et al. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril. 2011;95:1705–8. doi: 10.1016/j.fertnstert.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Mostafa T, Rashed L, Nabil N, Amin R. Seminal BAX and BCL2 gene and protein expressions in infertile men with varicocele. Urology. 2014;84:590–5. doi: 10.1016/j.urology.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Vivas-Acevedo G, Lozano-Hernández R, Camejo MI. Varicocele decreases epididymal neutral a-glucosidase and is associated with alteration of nuclear DNA and plasma membrane in spermatozoa. BJU Int. 2014;113:642–9. doi: 10.1111/bju.12523. [DOI] [PubMed] [Google Scholar]
- 20.4th ed. Cambridge: Cambridge University Press; 1999. World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. [Google Scholar]
- 21.3rd ed. Cambridge: Cambridge University Press; 1992. World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. [Google Scholar]
- 22.Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, Jr, et al. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Saypol DC, Howards SS, Turner TT, Miller ED., Jr Influence of surgically induced varicocele on testicular blood flow, temperature, and histology in adult rats and dogs. J Clin Invest. 1981;68:39–45. doi: 10.1172/JCI110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang QY, Qiu SD, Ma XN, Yu HM, Wu YW. Effect of experimental varicocele on structure and function of epididymis in adolescent rats. Asian J Androl. 2003;5:108–12. [PubMed] [Google Scholar]
- 25.Lehtihet M, Arver S, Kalin B, Kvist U, Pousette A. Left-sided grade 3 varicocele may affect the biological function of the epididymis. Scand J Urol. 2014;48:284–9. doi: 10.3109/21681805.2013.868513. [DOI] [PubMed] [Google Scholar]
- 26.2nd ed. Cambridge: Cambridge University Press; 1987. World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction; p. 80. [Google Scholar]
- 27.Pompeu C, Feijo C, Esteves S. Comparison between analytical scale and graduated serological pipette for semen volume analysis: a cross sectional study. Hum Reprod. 2015;30(Suppl 1):i331–2. [Google Scholar]
- 28.Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:443–53. doi: 10.1590/S1677-5538.IBJU.2014.04.02. [DOI] [PubMed] [Google Scholar]
- 29.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. National cooperative reproductive medicine network. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 30.van der Steeg JW, Steures P, Eijkemans MJ, F Habbema JD, Hompes PG, et al. Collaborative effort for clinical evaluation in reproductive medicine study group. Role of semen analysis in subfertile couples. Fertil Steril. 2011;95:1013–9. doi: 10.1016/j.fertnstert.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Esteves SC, Gosálvez J, López-Fernández C, Núñez-Calonge R, Caballero P, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol. 2015;47:1471–7. doi: 10.1007/s11255-015-1053-6. [DOI] [PubMed] [Google Scholar]
- 32.Berman NG, Wang C, Paulsen CA. Methodological issues in the analysis of human sperm concentration data. J Androl. 1996;17:68–73. [PubMed] [Google Scholar]
- 33.Keel BA, Stembridge TW, Pineda G, Serafy NT., Sr Lack of standardization in performance of the semen analysis among laboratories in the United States. Fertil Steril. 2002;78:603–8. doi: 10.1016/s0015-0282(02)03296-x. [DOI] [PubMed] [Google Scholar]
- 34.Riddell D, Pacey A, Whittington K. Lack of compliance by UK andrology laboratories with World Health Organization recommendations for sperm morphology assessment. Hum Reprod. 2005;20:3441–5. doi: 10.1093/humrep/dei230. [DOI] [PubMed] [Google Scholar]
- 35.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 36.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544–62. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 37.Ruzni N, Idris N. A comparison of methods to detect publication bias for meta-analysis of continuous data. J Appl Sci. 2012;12:1413–7. [Google Scholar]
- 38.Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113–26. doi: 10.1002/sim.1461. [DOI] [PubMed] [Google Scholar]
- 39.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342:1317–22. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


