Abstract
Varicocele has been associated with reduced male reproductive potential. With the advances in biomolecular techniques, it has been possible to better understand the mechanisms involved in testicular damage provoked by varicocele. Current evidence suggests the central role of reactive oxygen species (ROS) and the resultant oxidative stress (OS) in the pathogenesis of varicocele-associated male subfertility although the mechanisms have not yet been fully described and it is likely to be multifactorial. Excessive ROS is associated with sperm DNA fragmentation, which may mediate the clinical manifestation of poor sperm function and fertilization outcome related to varicocele. Testing of ROS/OS and DNA fragmentation has the potential to provide additional diagnostic and prognostic information compared to conventional semen analysis and may guide therapeutic management strategies in individual patient.
Keywords: antioxidants, infertility, male reproduction, oxidative stress, sperm DNA damage, sperm DNA fragmentation, varicocele, varicocelectomy
INTRODUCTION
Varicocele is a vascular abnormality of the testicular venous drainage system. It is manifested by a mass of abnormally dilated and tortuous veins of pampiniform and/or cremasteric venous plexus. It represents the most common cause of primary and secondary infertility in men.
Infertility is a major clinical problem and affects 13%–15% of couples worldwide.1 Male infertility attributes to approximately 50%–60% and varicocele alone accounts for 35% of the cases.2
The incidence of clinical varicocele was 25.4% in men with abnormal semen and 11.7% in men with normal semen.3 Varicoceles are considered the most common surgically correctable cause of male factor subfertility. Varicocelectomy results in improvement in semen parameters and natural pregnancy in 60%–80% and 20%–60% of couples, respectively.4
Despite the clear association between varicocele and male subfertility, skepticism persists. Some investigators also questioned the role of varicocelectomy in the era of assisted reproduction. A better understanding of the pathophysiology of varicocele-associated male subfertility is of paramount importance to elucidating the deleterious effects of varicocele on spermatogenesis and possibly formulating new treatment strategies. It also helps the selection of appropriate patients who will benefit from the treatment of varicocele.
In this review, we examine the role of oxidative stress in varicocele-associated male infertility, including its obvious association with sperm DNA fragmentation. First, we present a brief overview on the etiology of varicocele and the implications of ROS to male infertility. Then, we discuss the role of oxidative stress in the pathophysiology of varicocele-related infertility. Third, we present an overview of sperm DNA fragmentation and explain the association between oxidative stress-mediated sperm DNA fragmentation and varicocele. Finally, we summarize the clinical evidence of an association between varicocele treatment and alleviation of oxidative stress and improvement of DNA integrity of the male gamete.
Review criteria
An extensive search of studies was performed using PubMed, MEDLINE, and ScienceDirect. The start and end dates were October 2010 and September 2015, respectively. The search was limited to full articles in English. Study identification and data extraction were based on the following key words: “varicocele,” “oxidative stress”, “reactive oxygen species”, “free radicals”, “DNA fragmentation”, “varicocelectomy”, “male infertility”, and “semen parameters”. Cross references were referred to when necessary.
ETIOLOGY
Left varicocele is commonly observed and presents in 78%–93% of the cases.5 Left testicular vein is 8–10 cm longer than its counterpart. It runs vertically and inserts into the left renal vein at a right angle whereas the right testicular vein drains obliquely into the inferior vena cava. The left testicular vein acts as a long hydrostatic column in the upright position. The higher turbulent flow and back pressure related to the anatomy consequently lead to a higher incidence of venous dilation in the left spermatic cord. On the contrary, recent data indicate that bilateral palpable varicocele is found in >50% of the affected men.6 Such data are in agreement with venographic studies that show bilateral abnormal venous reflux in 84%–86% of men with varicocele.7,8 In contrast, isolated right-sided varicocele is found in only 2% of patients and may be associated with the presence of an obstructive lesion, such as a retroperitoneal or pelvic compressive mass.8 Vast majority of men do not develop a varicocele despite the universal anatomic relationships. There must be other active mechanism.
Absence of valves in the testicular veins has been documented and believed to be a significant contributing factor to the development of varicoceles.9 However, other investigators found that 26.2% of the patients with competent valve system still had a varicocele, being an evidence against the mechanism.10 Incompetent or absent valves probably contribute to the pathogenesis of varicoceles, but they are not the solely underlying cause.
Vascular contractions of the left testicular vein caused by reflux of catecholamines from left adrenal gland via left renal vein have been proposed as a contributing factor for the development of left varicocele.11 However, the contraction of left testicular vein has never been demonstrated on venography.
Another possible etiological factor is the “nut cracker phenomenon," which is the compression of the left renal vein between the aorta and the superior mesenteric artery. The incidence is found to be 0.7% on venographic study, making it an unlikely cause of most varicoceles.10 Rarely, development of left varicocele is secondary to renal or retroperitoneal tumors which exert pressure on the left renal and/or testicular veins. In summary, the etiology of varicocele is not fully understood and none of the above hypotheses can explain the occurrence of bilateral varicoceles in some patients.
OXIDATIVE STRESS (OS) AND REACTIVE OXYGEN SPECIES (ROS)
The presence of free radicals in spermatozoa and its possible implication in male infertility have been reported since 1943.12 Free radicals are normally formed during the intermediate steps of cellular metabolism. Superoxide anion radical (O2−) is formed when an extra electron is added to molecular oxygen (O2). The superoxide anion can be converted to other nonradicals, for example, hydrogen peroxide (H2O2), which are relatively stable powerful oxidants. Some ROS contains nitrogen atoms such as NO, NO3−, NO−, N2 O, and HNO3. These are the common free radicals in human semen, and they are collectively known as reactive oxygen species. Free radicals are very unstable due to the presence of one or more unpaired electrons. They are very reactive in the presence of amino acids, lipids, and nucleic acids.13
Multiple studies have demonstrated the deleterious effects of ROS on sperm function. Also, there are studies showing elevated ROS levels in 30%–80% of infertile men.14,15,16 However, a physiologic level of ROS is essential for successful fertilization by regulating sperm capacitation.13 A balanced cellular environment is maintained by the presence of scavenging system via enzymatic and nonenzymatic antioxidant pathways. OS is the result of imbalance of ROS and protective antioxidant system and is more likely due to a supraphysiological level of ROS rather than a low level of neutralizing antioxidants.17
ROS and OS probably act as the common pathway in the pathogenesis of testicular damage and male subfertility. Increase in OS has been demonstrated in clinical conditions related to male subfertility including varicocele,18 cryptorchidism,19 testicular torsion,20 genitourinary tract infection, and inflammation.21
Sources of ROS
Human spermatozoa generate ROS via aerobic metabolism. Impaired spermatogenesis and abnormal spermatozoa can produce excess ROS.22 OS may also affect normal spermatozoa when they come into proximity with ROS-producing abnormal sperm.23 Leukocytes are recognized as another major source of ROS in semen. Activated leukocytes produce 100-fold higher ROS than nonactivated leukocytes in situations like genitourinary tract infection and inflammation. Increased leukocytes in semen may also stimulate human spermatozoa to produce ROS.24
Effects of OS
OS exerts its negative effect by various mechanisms. ROS target cell membranes and increase peroxidation of membrane polyunsaturated fatty acids. The resultant reduced membrane fluidity has a detrimental effect on the structure of sperm head and midpiece membrane, ultimately leading to suboptimal motility and fertilization.25 Damage to axonemal proteins causes rapid depletion of adenosine triphosphate and decreased phosphorylation of axonemal proteins which may be another cause of impaired sperm motility.26
Spermatozoal nuclear and mitochondrial DNA is another important site of action of ROS.27 The damage to DNA can occur both on an amino acid or the backbone.28 Excessive ROS overwhelms the protective mechanisms and enzymes in sperm and oocyte that repair damaged DNA. The damage can be evident in the Yq chromosome where the AZF region is located.29 This can result in azoospermia in the offspring.
Excessive ROS can induce apoptosis in mature spermatozoa.30 Abnormal apoptosis may allow persistence of abnormal spermatozoa.31 The findings may explain the clinical presentation of oligo- and/or teratozoospermia in patients with male subfertility and elevated OS.
Elevated seminal OS in infertile men suggests that it plays a role in the impairment of sperm structural characteristics and functional capacities via several mechanisms. It is illustrated by the fact that elevated ROS levels are associated with impairment of sperm count, motility, morphology, and DNA integrity.32 The effect of sperm DNA damage on embryonic development, pregnancy, and offspring is of concern though the clinical evidence is still diverse.33
Measurement of OS
Seminal OS level is not measured directly. Elevated OS is reflected by excess ROS or lack of antioxidant capacity to buffer the ROS.
Measurement of ROS can be achieved by direct or indirect assays. Direct ROS assays include chemiluminescence, nitro blue tetrazolium test, ferricytochrome C reduction method, flow cytometry, electron spin resonance, and xylenol orange-based assay. The amount of oxidation within the sperm cell membrane reflects the net oxidative imbalance between ROS production and the antioxidant concentration in semen.34 Direct ROS assays are costly, which limit their clinical application, but they offer accurate results.35
Unlike the direct methods, indirect assays measure the stable downstream end products of the peroxidative process or DNA damage. In addition to the value as a surrogate marker of OS level, indirect assays provide information of ROS-related damage.36 Indirect assays include Endtz test, redox potential, and isoprostane method. Most of the ROS tests do not differentiate the source of ROS, i.e., leukocytes versus spermatozoa.
It is feasible to measure levels of individual antioxidants and the total antioxidant status of semen. Total antioxidant capacity (TAC) assesses the cumulative reducing ability of all antioxidants present in the semen. The reaction of seminal antioxidants against an oxidative reagent is measured by calorimeter or spectrophotometer.37 The lack of predictive value limits the application of TAC alone to routine infertility evaluation.38
ROS-TAC score has been proposed as a more predictive parameter than ROS levels or TAC alone.39 A lower score in infertile men is correlated with a higher risk of prolonged inability to conceive.40
VARICOCELE AND OXIDATIVE STRESS
Current evidence supports oxidative stress as a key element in the pathophysiology of varicocele-related infertility. The negative impact of OS on spermatozoa is evident although the mechanisms have not yet been fully described. The close relationship between varicocele and OS was demonstrated by the higher level of ROS, NO, and lipid peroxidation products in infertile men with varicocele than infertile men without varicocele.41 Several studies have also shown that fertile men with varicocele are more likely to have elevated OS in the reproductive tract compared to their counterpart without varicocele.42 A comprehensive review on oxidative stress in varicocele-associated infertility can be found elsewhere.29
Effects of left varicocele on the ipsilateral testicle
Scrotal hyperthermia
Scrotal hyperthermia is the most widely accepted theory supported by evidence. Varicoceles are thought to induce the elevation of scrotal temperature by reflux of warm abdominal blood through incompetent valves of the internal spermatic veins and cremasteric veins into the pampiniform plexus. The effect of varicocele is consistently demonstrated in varicocelized animal models. Significant elevation in testicular temperature resulting in decreased intratesticular testosterone levels and Sertoli cell secretory function was reported.43,44 The noxious impact of increased testicular temperature on Leydig cell secretory function has also been demonstrated.45 Varicocelectomy reduced testicular temperatures in the rat and rabbit models.44,46 Unlike the animal models, humans showed greater diversity. A significant difference in the mean left hemiscrotal temperature and sperm count has been demonstrated between men with varicoceles and controls.47 However, the overlap in temperatures between patients and controls was considerable.
Spermatogonia type B and developing spermatozoa are more vulnerable to heat stress compared to Sertoli and Leydig cells since the former have never been exposed to high temperature in utero. Spermatogenesis is optimal at temperature 2.5°C lower than core body temperature and heat stress can lead to impaired spermatogenesis.
The direct temperature-dependent relationship between heat exposure and ROS generation has been demonstrated in various studies. Varicocele grade has been correlated with seminal ROS level.48 ROS production from mitochondria, plasma membrane, cytoplasm, and peroxisome increases in the presence of heat stress.49,50 Enhanced production of mitochondrial ROS may be mediated by direct thermal inhibition of mitochondrial complexes which result in the transfer of electrons to molecular oxygen, and thus formation of ROS and inhibition of adenosine triphosphate synthesis.51 Enhanced NO production by heat-induced upregulation of inducible nitric oxide synthase (iNOS) may be contributed to the varicocele-associated testicular damage. It was shown that the levels of seminal NO and NOS are elevated in infertile men with varicocele. Excessive NO can result in sperm immobility and apoptosis of testicular sperm.52 Other possible mechanisms relating scrotal hyperthermia and ROS production include increased activity of xanthine oxidase,53 repression of cytoprotective heme oxygenase 1,54 and insufficient heat shock proteins.55
Testicular hypoxia
Study of fluid mechanics by venographic pressure and histopathology studies proposed that testicular tissue ischemia could occur if venous pressure of internal testicular vein exceeds the testicular arteriolar pressure. Histologic features of ischemia and arteriolar microthrombi were revealed on histologic examination of testicular tissue biopsies.56
Cellular response to hypoxia is mediated by hypoxia inducible factor 1 (HIF-1). The involvement of HIF pathway in pathogenesis of varicocele-induced testicular damage is evidenced by the high levels of HIF-1 alpha in the internal spermatic veins of infertile men with varicocele.57 Hypoxia may also lead to increased expression of cytokines in testicular tissue. Association between proinflammatory interleukin (IL) 6 has been shown in infertile men with varicocele.58 Higher incidence of leukocytospermia was noted in infertile men with varicocele.59 The presence of cytokines and inflammatory cells in seminal fluid may exert toxic effects on sperm since leukocytes are a major source of ROS.60
Reflux of adrenal or renal metabolites
It was hypothesized that retrograde flow of adrenal catecholamines to testicular vein results in damage to spermatogenesis.61,62 There was also a report of elevated prostaglandins in spermatic venous blood in varicocele patients as a result of reflux of renal venous blood.63 However, the literature does not unequivocally support the theory that retrograde blood flow through the left testicular vein is the major cause of varicocele-associated subfertility. Left adrenalectomy in varicocelized rats did not inhibit the development of physiologic changes in the testis related to varicocele.64 Although the role of reflux in the pathogenesis is uncertain, prostaglandins and renal and adrenal metabolites can induce cellular OS in various human cell cultures.63
Cadmium accumulation
Significantly elevated cadmium levels in testicular biopsy samples in infertile men with varicoceles have been reported. The level of cadmium is inversely related to the increase in sperm concentration after varicocelectomy.65 Cadmium may exert its negative effect on spermatogenesis by reducing zinc concentration and enhancing ROS production.66
Epididymal response
Epididymis is involved in sperm maturation and transportation. Various cell types lining the epididymal tubules are capable of generating ROS. The redox microenvironment is maintained by the counteracting antioxidants.67 Hypoxia and heat stress are the possible triggers for the imbalance between ROS and antioxidant in the epididymal tubules. Ultrastructural changes and apoptosis of principal epididymal cells in varicocelized animal model indicate both testicular and epididymal involvement in the pathogenesis of impaired spermatogenesis.68
Insufficiency of the hypothalamo–pituitary–gonadal axis is another theory that has been proposed as a cause of varicocele-associated male subfertility.69 This suggests the presence of other possible mechanisms in which ROS pathway is not involved.
Collectively, these data suggest that scrotal hyperthermia and ROS pathway have an important role in the pathophysiology, but the underlying mechanisms may be multifactorial. Figure 1 summarizes the etiologies of varicocele and the central role of oxidative stress in the pathogenesis of varicocele-associated male subfertility.
Figure 1.
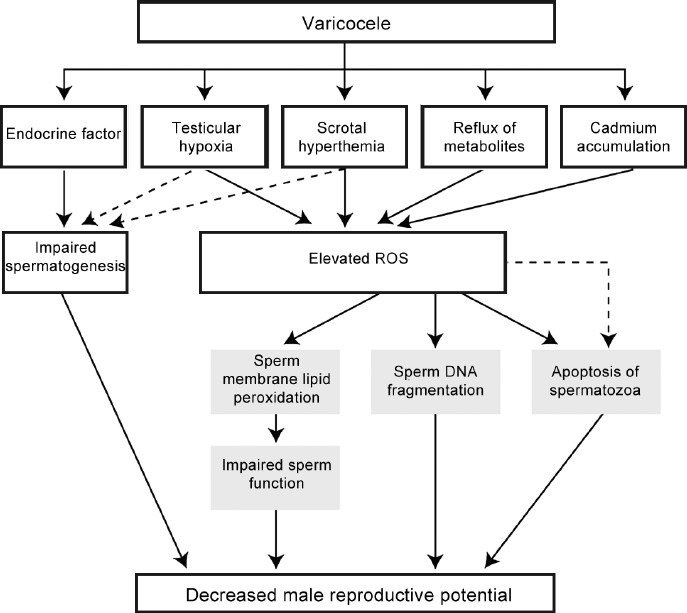
The detrimental effect of varicocele on male reproduction.
Effects of left varicocele on the contralateral testicle
The bilateral adverse effects were demonstrated in animal models and were not mediated through ipsilateral testis.70 The detrimental effects appear to be mediated by the sympathetic nervous system. Bilateral testicular degeneration and increase in tissue hypoxia were prevented by chemical sympathectomy.71
SPERM DNA FRAGMENTATION
DNA damage in male gamete is a major contributor to infertility, poor outcome in assisted reproductive technology (ART) treatment,72 miscarriage, and birth defects in the offspring.73 Among the different DNA anomalies, DNA fragmentation is the most frequent.74
Mechanisms
DNA fragmentation of germ cells may occur in the testis and excurrent duct system. Abortive apoptosis75 and defective maturation76 theories were proposed to explain the generation of DNA fragmentation in testicular sperm. There is evidence showing that there are more DNA fragmentation in epididymal and ejaculated sperm than in testicular sperm.77,78 And therefore, other mechanisms are involved in the generation of DNA fragmentation outside the testis.
ROS is considered the major cause of sperm DNA fragmentation (SDF). The positive relationship between ROS production and SDF in semen samples has been demonstrated.79 The source of oxidative stress (OS) responsible for creation of DNA damage has been discussed previously. Both mitochondrial and sperm nuclear DNA are potential targets of attack by ROS. While mitochondrial DNA is more vulnerable to ROS attack,80 a sperm nuclear DNA damage carries a higher clinical significance. Sperm nuclear DNA, unlike mitochondrial DNA, is tightly packed with protamines. The DNA damage is, therefore, a balance between oxidative stress and the inherent sperm susceptibility to DNA damage.73 This hypothesis is supported by the findings of a close relationship between efficiency of sperm chromatin protamination and degree of oxidative DNA damage.81 According to this theory, the first step in the DNA damage cascade constitutes of defects in chromatin remodeling leading to a reduction in the efficiency of protamination before chromatin is attacked by ROS in the second step.82 This includes ROS-triggered apoptotic events during sperm maturation within the epididymis that is later exacerbated by endogeneous redox reaction in the seminal plasma.29
Measurement
Sperm DNA fragmentation can be detected by flow cytometry and/or fluorescence microscopy. Among the commonly utilized techniques, sperm chromatin structure assay (SCSA) measures DNA denaturation by flow cytometry while terminal deoxynucleotidyl transferase-mediated fluorescein-dUTP nick-end labeling (TUNEL) assay uses either flow cytometry or microscopy in measuring DNA breaks following fluorescent enzymatic labeling. A variation of SCSA method that has been adapted to both bright and fluorescence microscopy is the sperm chromatin dispersion (SCD) assay.83 SCD assay is based on producing a controlled and species-specific DNA denaturation to produce single-stranded DNA stretches from any DNA break, coupled with controlled species-specific protein depletion.78
The lack of standardization of the various methods, except SCSA, made the comparison of results difficult. Different methods may reveal different types of DNA breaks. The SCSA is notably an expensive technique to set up, owing to the need for flow cytometry and specialized personnel. However, SCSA becomes more cost-effective if articulated with an external central laboratory. TUNEL test using flow cytometry is an advanced technique used by many investigators, but test results lack strict reference values.84 However, a modified TUNEL protocol using bench top flow cytometer allows accurate measurement of DNA damage in a large number of samples.85 While the COMET assay is very informative because it is possible to analyze the different types of DNA damage in a single cell, the method is not suited for rapid diagnosis and requires highly specialized personnel to analyze the results. On the contrary, SCD method is relatively inexpensive, rapid to use, and versatile since it can be conjugated with the simultaneous detection of DNA and protein damage. A comparison of the various techniques for sperm DNA fragmentation test, including advantages and shortcomings is provided elsewhere.72 It has been our experience that all the techniques can provide reliable results to discriminate men with different levels of SDF provided internal controls and proper standardization is carried out.74,84,86
Implications of DNA fragmentation
Sperm DNA fragmentation reflects to a certain extent, poor quality sperm.87 On the other hand, the fact that sperm with high DNA fragmentation can have normal motility and morphology suggests additional prognostic value of the assessment. The probability of in vivo pregnancy is reduced with higher DNA fragmentation in sperm.88 A DNA fragmentation index of more than 30% as measured by SCSA is associated with lower clinical pregnancy and delivery in intrauterine insemination cycles.89 However, the impact of sperm DNA integrity on the outcome of in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) is less clear. It appears that sperm with fragmented DNA retains the ability to fertilize oocytes.90 Although some degree of DNA repair by the oocyte is possible, the relationship between sperm DNA fragmentation and birth defect in offspring is of concern. Increased rates of aneuploidy were found to be associated with elevated sperm DNA fragmentation rates.91 It was also shown in mouse models that high levels of sperm DNA damage can result in premature aging, aberrant growth and behavior, and increased incidence of tumors in offsprings.92 On the contrary, sperm DNA fragmentation rates have been shown to be 5-fold lower in testicular than ejaculated sperm, and reproductive outcome of couples undergoing ICSI with testicular sperm was found to be superior as compared to ejaculated sperm.78 Figure 2 illustrates the possible etiologies of DNA fragmentation and its consequence.
Figure 2.
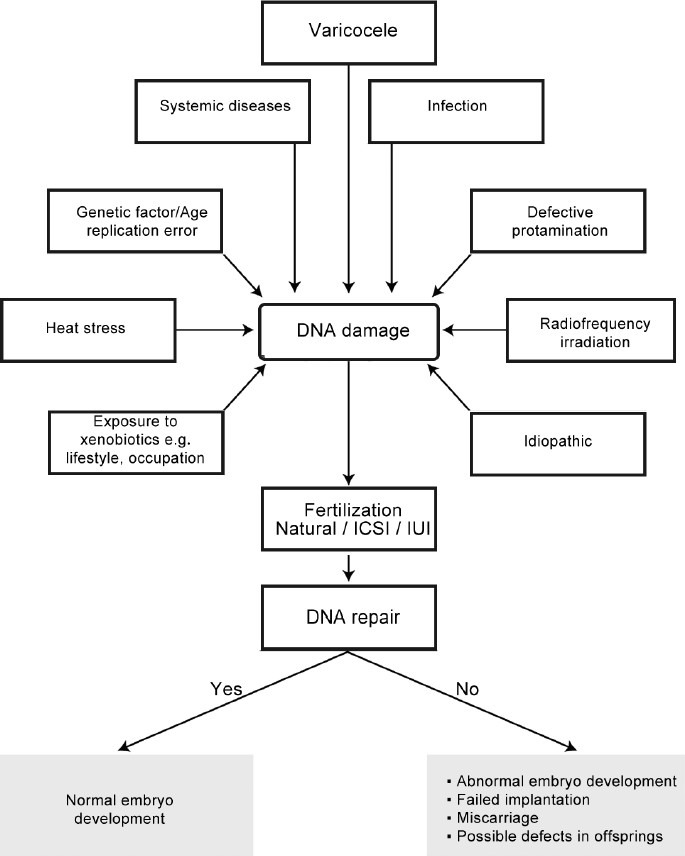
The possible etiologies and consequences of sperm DNA damage.
Sperm DNA fragmentation and varicocele
A number of studies have examined the association between varicocele and SDF. Fertile and infertile men with varicocele tend to have higher SDF than controls, thus suggesting that varicocele itself is associated with DNA damage even when fertility has not been compromised.93
Characterization of SDF in ejaculates of men with varicocele using the sperm chromatin dispersion test revealed two distinctive sperm subpopulations within the cluster of fragmented spermatozoa; namely, standard fragmented sperm and degraded sperm (DDS).94 Spermatozoa with degraded DNA exhibit a ghost-like morphology owed to extensive material loss after technical processing, resulting from massive single- and double-strand DNA breaks as well as nuclear protein damage. Although degraded DNA sperm is regularly seen in normal individuals at low frequencies ranging from 1% to 4%, as well as in infertile males, its frequency was shown to be 8 times higher in patients with varicocele compared to controls.95
Using receiver operating characteristics (ROC) analysis, DDSi, defined as the proportion of degraded sperm in the whole population of spermatozoa with fragmented DNA, was shown to be able to identify patients with varicocele with 94% accuracy, thus suggesting a possible role of the test to select candidates for early intervention.94
CLINICAL ASSOCIATIONS BETWEEN ROS AND SPERM DNA FRAGMENTATION PRE -AND POST-VARICOCELE TREATMENT
The benefit of varicocelectomy has been clearly demonstrated in meta-analyses. A significantly higher chances for pregnancy after varicocelectomy than either no treatment or medication in patients with clinical varicoceles and at least one abnormal semen parameter (OR: 2.87; 95% CI: 1.33–6.20; P < 0.001) was reported.96 However, given that not all patients with varicoceles are infertile, treatment of varicocele will not benefit all men. No beneficial effect of varicocele repair on fertility potential could be demonstrated in men with subclinical varicocele.97 Furthermore, varicocelectomy in men with varicocele and normal semen parameters did not show a clear benefit over observation.98 The surgical technique of choice is microsurgical subinguinal or inguinal approach since higher pregnancy rates and lower recurrence rates have been reported.99
Oxidative stress
Varicocelectomy has been shown to alleviate OS by reducing or normalizing OS markers in spermatozoa of infertile men with varicocele.41,100 Improvement in levels of seminal and peripheral blood plasma TAC and seminal antioxidants have been demonstrated.101 The beneficial effect of varicocele repair is time-dependent. It is generally believed that alleviation of OS and DNA fragmentation may take up to 6 months to occur after repair of varicocele.100
A comprehensive review into the effect of varicocele treatment and oxidative stress markers has been presented elsewhere.102
Sperm DNA fragmentation
Studies examining sperm DNA damage and pre- and post-varicocele treatment indicate that patients with varicoceles have significantly higher sperm DNA damage than controls, with a mean difference of 9.84% (95% CI: 9.19–10.49; P < 0.00001).103,104,105 It has been also shown that varicocelectomy decrease sperm DNA fragmentation with a mean difference of −3.37% (95% CI: −4.09–−2.65; P < 0.00001) compared to no treatment.105 It was also shown that varicocelectomy is associated with increased sperm DNA integrity postoperatively in adolescents.106
Due to the low magnitude of the effect size and heterogeneity of methods to evaluate SDF, further research is needed to elucidate the clinical significance of varicocelectomy on sperm DNA damage. At present, varicocele repair remains a viable option to decrease SDF, and, therefore, to restore or improve fertility. Sperm DNA fragmentation testing might be appropriate for controlling the postoperative outcomes after varicocele repair.
Antioxidant therapy
The understanding of OS as the central pathway in the pathogenesis of testicular damage in men with varicocele makes medical therapy, in the form of antioxidant or anti-inflammatory therapy, an attractive option. The enthusiasm was further escalated by the finding that Vitamin E effectively reduces seminal ROS levels in the varicocele rat model.107
Physiologic antioxidants are present in the seminal plasma. They can be classified as enzymatic and nonenzymatic antioxidants. Other sources of antioxidants are food or supplements in the form of either natural or synthetic antioxidants.
A statistically significant increase in live birth rates in unselected subfertile couples who underwent antioxidant therapy was suggested by a Cochrane Collaboration review, though the effect of antioxidants on semen parameters was not clear.108 Among the extensive list of substances studied, carnitines, Vitamins C and E have been shown to be beneficial in multiple studies.109,110 Glutathione, selenium, and coenzyme Q10 may be potentially helpful as suggested by a few studies.110 However, many of the studies are limited by methodologic flaws, including small sample size, short duration, lack of randomization, lack of double-blinded and placebo-controlled, nonstandardization of supplement regimen, and lack of control of baseline dietary consumption.110
As far as varicocele is concerned, few studies have explored the role of antioxidants as a therapeutic alternative or adjuvant therapy in varicocele. Collectively, limited data support the beneficial effect of improving the antioxidant defense system by exogenous antioxidant administration.111,112,113
CONCLUSIONS
The correlation between varicocele and OS and the negative impact of OS on spermatozoa suggest the central role of OS in the pathogenesis of varicocele-associated male subfertility. The exact mechanism of how varicoceles lead to an increase in ROS production awaits further clarification. It has been hypothesized that the detrimental effect OS on pregnancy outcomes is at least partly mediated by sperm DNA fragmentation. The understanding of OS as a central element in the pathophysiology of varicocele-associated infertility opens the possibility to rationalize and select treatments for individual patients. Better standardization of ROS/TAC and DNA fragmentation testing will allow the widespread utilization of such testing as surrogate markers of oxidative stress in varicocele.
AUTHOR CONTRIBUTIONS
CLC carried out the literature search and draft of the manuscript; AA and SCE supervised the conduction of literature review and finalize the manuscript. All authors have read and approved the final version of the manuscript.
COMPETING INTERESTS
None of the authors declared competing financial interests.
ACKNOWLEDGMENTS
The authors are grateful to the staff of American Center for Reproductive Medicine for support and advice.
REFERENCES
- 1.Jarow JP, Sharlip ID, Belker AM, Lipshultz LI, Sigman M, et al. Best practice policies for male infertility. J Urol. 2002;167:2138–44. [PubMed] [Google Scholar]
- 2.Witt MA, Lipshultz LI. Varicocele: a progressive or static lesion? Urology. 1993;42:541–3. doi: 10.1016/0090-4295(93)90268-f. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992;57:1289–93. [PubMed] [Google Scholar]
- 4.Schlesinger MH, Wilets IF, Nagler HM. Treatment outcome after varicocelectomy: a critical analysis. Urol Clin North Am. 1994;21:517–29. [PubMed] [Google Scholar]
- 5.Saypol DC, Lipshultz LI, Howards SS. Varicocele. In: Lipshultz LI, Howards SS, editors. Infertility in the Male. New York: Churchill Livingstone; 1983. [Google Scholar]
- 6.Akbay E, Cayan S, Doruk E, Duce MN, Bozlu M. The prevalence of varicocele and varicocele-related testicular atrophy in Turkish children and adolescents. BJU Int. 2000;86:490–3. doi: 10.1046/j.1464-410x.2000.00735.x. [DOI] [PubMed] [Google Scholar]
- 7.Graif M, Hauser R, Hirshebein A, Botchan A, Kessler A, et al. Varicocele and the testicular-renal venous route: hemodynamic Doppler sonographic investigation. J Ultrasound Med. 2000;19:627–31. doi: 10.7863/jum.2000.19.9.627. [DOI] [PubMed] [Google Scholar]
- 8.Gat Y, Gornish M, Navon U, Chakraborty J, Bachar GN, et al. Right varicocele and hypoxia, crucial factors in male infertility: fluid mechanics analysis of the impaired testicular drainage system. Reprod Biomed Online. 2006;13:510–5. doi: 10.1016/s1472-6483(10)60638-4. [DOI] [PubMed] [Google Scholar]
- 9.Sofikitis N, Dritsas K, Miyagawa I, Koutselinis A. Anatomical characteristics of the left testicular venous system in man. Arch Androl. 1993;30:79–85. doi: 10.3109/01485019308987738. [DOI] [PubMed] [Google Scholar]
- 10.Braedel HU, Steffens J, Ziegler M, Polsky MS, Platt ML. A possible ontogenic etiology for idiopathic left varicocele. J Urol. 1994;151:62–6. doi: 10.1016/s0022-5347(17)34872-3. [DOI] [PubMed] [Google Scholar]
- 11.Comhaire F, Vermeulen A. Varicocele sterility: cortisol and catecholamines. Fertil Steril. 1974;25:88–95. doi: 10.1016/s0015-0282(16)40159-7. [DOI] [PubMed] [Google Scholar]
- 12.MacLeod J. The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol. 1943;138:512–8. [Google Scholar]
- 13.Sharman RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–50. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 14.Tremellen K. Oxidative stress and male infertility: a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 15.Makker K, Agarwal A, Sharma R. Oxidative stress and male infertility. Indian J Med Res. 2009;129:357–66. [PubMed] [Google Scholar]
- 16.Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006;86:503–12. doi: 10.1016/j.fertnstert.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 17.Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in the semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma. Int J Androl. 1993;16:183–8. doi: 10.1111/j.1365-2605.1993.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 18.Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Agarwal A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999;161:1831–4. [PubMed] [Google Scholar]
- 19.Ahotupa M, Huhtaniemi I. Impaired detoxification of reactive oxygen and consequent oxidative stress in experimentally cryptorchid rate testis. Biol Reprod. 1992;46:1114–8. doi: 10.1095/biolreprod46.6.1114. [DOI] [PubMed] [Google Scholar]
- 20.Lysiak JJ, Turner SD, Nguyen QA, Singbarti K, Ley K, et al. Essential role of neutrophils in germ cell-specific apoptosis following ischemia/perfusion injury of the mouse testis. Biol Reprod. 2001;65:718–25. doi: 10.1095/biolreprod65.3.718. [DOI] [PubMed] [Google Scholar]
- 21.Reddy MM, Mahipal SV, Subhashini J, Reddy MC, Roy KR, et al. Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rates. Reprod Toxicol. 2006;22:493–500. doi: 10.1016/j.reprotox.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation and human sperm function. Biol Reprod Fertil. 1987;81:459–69. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 23.Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. 2001;16:1922–30. doi: 10.1093/humrep/16.9.1922. [DOI] [PubMed] [Google Scholar]
- 24.Ochsendorf FR. Infections in the male genital tract and reactive oxygen species. Hum Reprod Update. 1999;5:399–420. doi: 10.1093/humupd/5.5.399. [DOI] [PubMed] [Google Scholar]
- 25.Griveau JF, Dumont E, Renard B, Callegan JP, Lannou DL. Reactive oxygen species, lipid peroxidation and enzymatic defense systems in human spermatozoa. J Reprod Fertil. 1995;103:17–26. doi: 10.1530/jrf.0.1030017. [DOI] [PubMed] [Google Scholar]
- 26.de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J Androl. 1992;13:379–86. [PubMed] [Google Scholar]
- 27.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–90. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal A, Sekhon LH. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: is it justified? Indian J Urol. 2011;27:74–85. doi: 10.4103/0970-1591.78437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, et al. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol. 2014;12:33. doi: 10.1186/1477-7827-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis SE, Simon L. Clinical implications of sperm DNA damage. Hum Fertil. 2010;13:201–7. doi: 10.3109/14647273.2010.528823. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal A, Prabhakaran SA, Sikka SC. Clinical relevance of oxidative stress in patients with male factor infertility: evidence based analysis. AUA Update Ser. 2007;26:1–12. [Google Scholar]
- 35.Agarwal A, Ahmad G, Sharma R. Reference values of reactive oxygen species in seminal ejaculates using chemiluminescence assay. J Assist Reprod Genet. 2015 doi: 10.1007/s10815-015-0584-1. doi: 10.1007/s10815-015-0584-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79:1597–605. doi: 10.1016/s0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal A, Tvrda E, Sharma R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. 2014;12:45–52. doi: 10.1186/1477-7827-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasqualotto FF, Sharma RK, Pasqualotto EB, Agarwal A. Poor semen quality and ROS-TAC scores in patients with idiopathic infertility. Urol Int. 2008;81:263–70. doi: 10.1159/000151401. [DOI] [PubMed] [Google Scholar]
- 40.Saleh RA, Agarwal A. Oxidative stress and male infertility: from research to clinical practice. J Androl. 2002;23:737–62. [PubMed] [Google Scholar]
- 41.Sakamoto Y, Ishikawa T, Kondo Y, Yamaguchi K, Fujisawa M. The assessment of oxidative stress in infertile patients with varicocele. BJU Int. 2008;101:1547–52. doi: 10.1111/j.1464-410X.2008.07517.x. [DOI] [PubMed] [Google Scholar]
- 42.Mostafa T, Anis T, Imam H, El-Nashar AR, Osman IA. Seminal reactive oxygen species-antioxidant relationship in fertile males with and without varicocele. Andrologia. 2009;41:125–9. doi: 10.1111/j.1439-0272.2008.00900.x. [DOI] [PubMed] [Google Scholar]
- 43.Rajfer J, Turner TT, Rivera F, Howard SS, Sikka SC. Inhibition of testicular testosterone biosynthesis following experimental varicocele in rats. Biol Reprod. 1987;36:933–7. doi: 10.1095/biolreprod36.4.933. [DOI] [PubMed] [Google Scholar]
- 44.Sofikitis N, Takahashi I. Effects of surgical repair of experimental left varicocele on testicular temperature, spermatogenesis, sperm maturation, endocrine function, and fertility in rabbits. Arch Androl. 1992;29:163–75. doi: 10.3109/01485019208987721. [DOI] [PubMed] [Google Scholar]
- 45.Khera M, Lipshultz LI. Evolving approach to the varicocele. Urol Clin North Am. 2008;35:183–9. doi: 10.1016/j.ucl.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Hurt GS, Howards SS, Turner TT. Repair of experimental varicoceles in the rat. Long-term effects on testicular blood flow and temperature and cauda epididymal sperm concentration and motility. J Androl. 1986;7:271–6. doi: 10.1002/j.1939-4640.1986.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 47.Zorgniotti AW, MacLeod J. Studies in the temperature, human semen quality and varicocele. Fertil Steril. 1973;24:295–301. [PubMed] [Google Scholar]
- 48.Allamaneni SS, Naughton CK, Sharma RK, Thomas AJ, Jr, Agarwal A. Increased seminal reactive oxygen species levels in patients with varicoceles correlate with varicocele grade but not with testis size. Fertil Steril. 2004;82:1684–6. doi: 10.1016/j.fertnstert.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 49.Morgan D, Cherny VV, Murphy R, Xu W, Thomas LL, et al. Temperaturedependence of NADPH oxidase in human eosinophils. J Physiol. 2003;550:447–58. doi: 10.1113/jphysiol.2003.041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikeda M, Kodama H, Fukuda J, Shimizu Y, Murata M, et al. Role of radical oxygen species in rat testicular germ cell apoptosis induced by heat stress. Biol Reprod. 1999;61:393–9. doi: 10.1095/biolreprod61.2.393. [DOI] [PubMed] [Google Scholar]
- 51.Voglmayr JK, Setchell BP, White IG. The effects of heat on the metabolism and ultrastructure of ram testicular spermatozoa. J Reprod Fertil. 1971;24:71–80. doi: 10.1530/jrf.0.0240071. [DOI] [PubMed] [Google Scholar]
- 52.Rosselli M, Dubey RK, Imthurn B, Macas E, Keller PJ. Effects of nitric oxide on human spermatozoa: evidence that nitric oxide decreases sperm motility and induces sperm toxicity. Hum Reprod. 1995;10:1786–90. doi: 10.1093/oxfordjournals.humrep.a136174. [DOI] [PubMed] [Google Scholar]
- 53.Mitropoulos D, Deliconstantino G, Zervas A, Villiotou V, Dimopoulos C, et al. Nitric oxide synthase and xanthine oxidase activities in the spermatic vein of patients with varicocele: a potential role for nitric oxide and peroxynitrite in sperm dysfunction. J Urol. 1996;156:1952–8. [PubMed] [Google Scholar]
- 54.Abdel Aziz MT, Mostafa T, Atta H, Kamal O, Kamel M, et al. Heme oxygenase enzyme activity in seminal plasma of oligoasthenoteratozoospermic males with varicocele. Andrologia. 2008;42:236–41. doi: 10.1111/j.1439-0272.2009.00983.x. [DOI] [PubMed] [Google Scholar]
- 55.Yesilli C, Mungan G, Seckiner I, Akduman B, Acikgoz S, et al. Effect ofvaricocelectomy on sperm creatine kinase, HspA2 chaperone protein (creatine kinase-M type), LDH, LDH-X, and lipid peroxidation product levels in infertile men with varicocele. Urology. 2005;66:610–5. doi: 10.1016/j.urology.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 56.Gat Y, Zukerman Z, Chakraborty J, Gornish M. Varicocele, hypoxia and male infertility. Fluid mechanics analysis of the impaired testicular venous drainage system. Hum Reprod. 2005;20:2614–9. doi: 10.1093/humrep/dei089. [DOI] [PubMed] [Google Scholar]
- 57.Lee JD, Jeng SY, Lee TH. Increased expression of hypoxia-inducible factor-1alpha in the internal spermatic vein of patients with varicocele. J Urol. 2006;175:1045–8. doi: 10.1016/S0022-5347(05)00417-9. [DOI] [PubMed] [Google Scholar]
- 58.Nallella KP, Allamaneni SS, Pasqualotto FF, Sharma RK, Thomas AJ, Jr, et al. Relationship of interleukin-6 with semen characteristics and oxidative stress in patients with varicocele. Urology. 2004;64:1010–3. doi: 10.1016/j.urology.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 59.Tortolero I, Duarte Ojeda JM, Pamplona Casamayor M, Alvarez Gonzalez E, Arata-Bellabarba G, et al. The effect of seminal leukocytes on semen quality in subfertile males with and without varicocele. Arch Esp Urol. 2004;57:921–8. [PubMed] [Google Scholar]
- 60.Basu S, Aballa TC, Ferrell SM, Lynne CM, Brackett NL. Inflammatory cytokine concentrations are elevated in seminal plasma of men with spinal cord injuries. J Androl. 2004;25:250–4. doi: 10.1002/j.1939-4640.2004.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 61.Javert C, Clark R. Combined operation for varicocele and inguinal hernia: preliminary report. Surg Gynecol Obstet. 1944;79:644–50. [Google Scholar]
- 62.Cohen MS, Plaine L, Brown JS. The role of internal spermatic vein plasma catecholamine determinations in subfertile men with varicoceles. Fertil Steril. 1975;26:1243–9. doi: 10.1016/s0015-0282(16)41541-4. [DOI] [PubMed] [Google Scholar]
- 63.Ito H, Fuse H, Minagawa H, Kawamura K, Murakami M, et al. Internal spermatic vein prostaglandins in varicocele patients. Fertil Steril. 1982;37:218–22. doi: 10.1016/s0015-0282(16)46043-7. [DOI] [PubMed] [Google Scholar]
- 64.Sofikitis N, Miyagawa I. Left adrenalectomy in varicocelized rats does not inhibit the development of varicocele-related physiologic alterations. Int J Fertil Menopausal Stud. 1993;38:250–5. [PubMed] [Google Scholar]
- 65.Benoff SH, Millan C, Hurley HR, Napolitano B, Marmar JL. Bilateral increased apoptosis and bilateral accumulation of cadmium in infertile men with left varicocele. Hum Reprod. 2004;19:616–27. doi: 10.1093/humrep/deh139. [DOI] [PubMed] [Google Scholar]
- 66.Benoff S, Hurley IR, Barcia M, Mandel FS, Cooper GW, et al. A potential role for cadmium in the etiology of varicocele-associated infertility. Fertil Steril. 1997;67:336–47. doi: 10.1016/S0015-0282(97)81921-8. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki F. Microvasculature of the mouse testis and excurrent duct system. Am J Anat. 1982;163:309–25. doi: 10.1002/aja.1001630404. [DOI] [PubMed] [Google Scholar]
- 68.Ozturk U, Kefeli M, Asci R, Akpolat I, Buyukalpelli R, et al. The effects of experimental left varicocele on the epididymis. Syst Biol Reprod Med. 2008;54:177–84. doi: 10.1080/19396360802415752. [DOI] [PubMed] [Google Scholar]
- 69.Hudson RW, Perez-Marrero RA, Crawford VA, McKay DE. Hormonal parameters of men with varicoceles before and after varicocelectomy. Fertil Steril. 1985;43:905–10. [PubMed] [Google Scholar]
- 70.Hurt GS, Howards SS, Turner TT. The effects of unilateral, experimental varicocele are not mediated through the ipsilateral testis. J Androl. 1987;8:403–8. doi: 10.1002/j.1939-4640.1987.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 71.Ozturk H, Tander B, Aydin A, Okumus Z, Cetinkursun S. The effects of chemical sympathectomy on testicular injury in varicocele. BJU Int. 2001;87:232–4. doi: 10.1046/j.1464-410x.2001.01987.x. [DOI] [PubMed] [Google Scholar]
- 72.Esteves SC, Sharma RK, Gosálvez J, Agarwal A. A translational medicine ap-praisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46:1037–52. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 73.Aitken RJ, De Iuliis GN, McLachlan RI. Biological and clinical significance of DNA damage in the male germ line. Int J Androl. 2009;32:46–56. doi: 10.1111/j.1365-2605.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- 74.Gosálvez J, López-Fernández C, Fernández JL, Esteves SC, Johnston SD. Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotechnol Fertil. 2015;4:1–16. [Google Scholar]
- 75.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, et al. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–7. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 76.Sakkas D, Manicardi G, Bianchi PG, Bizzaro D, Bianchi U. Relationship between the presence of endogenous nicks and sperm chromatin packaging in maturing and fertilizing mouse spermatozoa. Biol Reprod. 1995;52:1149–55. doi: 10.1095/biolreprod52.5.1149. [DOI] [PubMed] [Google Scholar]
- 77.Moskovtsev SI, Jarvi K, Mullen JB, Cadesky KI, Hannam T, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril. 2010;93:1142–6. doi: 10.1016/j.fertnstert.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104:1376–77. doi: 10.1016/j.fertnstert.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 79.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–42. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 80.Sawyer DE, Roman SD, Aitken RJ. Relative susceptibilities of mitochondrial and nuclear DNA to damage induced by hydrogen peroxide in two mouse germ cell lines. Redox Rep. 2001;6:182–4. doi: 10.1179/135100001101536157. [DOI] [PubMed] [Google Scholar]
- 81.De Iuliis GN, Thomson LK, Mitchell LA, Finnie JM, Koppers AJ, et al. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2’- deoxyguanosine, a marker of oxidative stress. Biol Reprod. 2009;81:517–24. doi: 10.1095/biolreprod.109.076836. [DOI] [PubMed] [Google Scholar]
- 82.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 83.Fernández JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–42. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 84.Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril. 2014;101:58–63. doi: 10.1016/j.fertnstert.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 85.Sharma RK, Ahmad G, Esteves SC, Agarwal A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values and quality control. J Assist Reprod Genet. doi: 10.1007/s10815-015-0635-7. doi: 10.1007/s10815-015-0635-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharma RK, Sabanegh E, Mahfouz R, Gupta S, Thiyagarajan A, et al. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology. 2010;76:1380–6. doi: 10.1016/j.urology.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 87.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, et al. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:3–44. [PubMed] [Google Scholar]
- 88.Evenson DP, Wixon R. Data analysis of two in vivo fertility studies using sperm chromatin structure assay-derived DNA fragmentation index vs. pregnancy outcome. Fertil Steril. 2008;90:1229–31. doi: 10.1016/j.fertnstert.2007.10.066. [DOI] [PubMed] [Google Scholar]
- 89.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 90.Yamauchi Y, Riel JM, Ward MA. Paternal DNA damage resulting from various sperm treatments persists after fertilization and is similar prior and after DNA replication. J Androl. 2012;33:229–38. doi: 10.2164/jandrol.111.013532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Enciso M, Alfarawati S, Wells D. Increased numbers of DNA-damaged spermatozoa in samples presenting an elevated rate of numerical chromosome abnormalities. Hum Reprod. 2013;28:1707–15. doi: 10.1093/humrep/det077. [DOI] [PubMed] [Google Scholar]
- 92.Fernandez-Gonzalez R, Moreira PN, Perez-Crespo M, Sanchez-Martin M, Ramirez MA, et al. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–72. doi: 10.1095/biolreprod.107.065623. [DOI] [PubMed] [Google Scholar]
- 93.Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril. 2011;96:1283–7. doi: 10.1016/j.fertnstert.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Esteves SC, Gosálvez J, López-Fernández C, Núñez-Calonge R, Caballero P, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol. 2015;47:1471–7. doi: 10.1007/s11255-015-1053-6. [DOI] [PubMed] [Google Scholar]
- 95.Gosálvez J, Rodríguez-Predreira M, Mosquera A, López-Fernández C, Esteves SC, et al. Characterization of a subpopulation with massive nuclear damage, as recognized with the sperm chromatin dispersion (SCD) test. Andrologia. 2014;46:602–9. doi: 10.1111/and.12118. [DOI] [PubMed] [Google Scholar]
- 96.Marmar JL, Agarwal A, Prabakaran S, Agarwal R, Short RA, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88:639–48. doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 97.Grasso M, Lania C, Castelli M, Galli L, Franzoso F, et al. Low grade left varicocele in patients over 30 years old: the effect of spermatic vein ligation on fertility. BJU Int. 2000;85:305–7. doi: 10.1046/j.1464-410x.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- 98.Breznik R, Vlaisavljevic V, Borko E. Treatment of varicocele and male infertility. Arch Androl. 1993;30:157–60. doi: 10.3109/01485019308987750. [DOI] [PubMed] [Google Scholar]
- 99.Diegidio P, Jhaveri JK, Ghannam S, Pinkhasov R, Shabsigh R, et al. Review of current varicocelectomy techniques and their outcomes. BJU Int. 2011;108:1157–72. doi: 10.1111/j.1464-410X.2010.09959.x. [DOI] [PubMed] [Google Scholar]
- 100.Dada R, Shamsi MB, Venkatesh S, Gupta NP, Kumar R. Attenuation of oxidative stress and DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res. 2010;132:728–30. [PMC free article] [PubMed] [Google Scholar]
- 101.Chen SS, Huang WJ, Chang LS, Wei YH. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol. 2008;179:639–42. doi: 10.1016/j.juro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 102.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 103.Zini A, Azhar R, Baazeem A, Gabriel MS. Effect of microsurgical varicocelectomy on human sperm chromatin and DNA integrity: a prospective trial. Int. J Androl. 2011;34:14–9. doi: 10.1111/j.1365-2605.2009.01048.x. [DOI] [PubMed] [Google Scholar]
- 104.Li F, Yamaguchi K, Okada K, Matsushita K, Ando M, et al. Significant improvement of sperm DNA quality after microsurgical repair of varicocele. Syst Biol Reprod Med. 2012;58:274–7. doi: 10.3109/19396368.2012.692431. [DOI] [PubMed] [Google Scholar]
- 105.Wang YJ, Zhang RQ, Lin YJ, Zhang RG, Zhang WL. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–14. doi: 10.1016/j.rbmo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Lacerda JI, Del Giudice PT, da Silva BF, Nichi M, Fariello RM, et al. Adolescent varicocele: improved sperm function after varicocelectomy. Fertil Steril. 2011;95:994–9. doi: 10.1016/j.fertnstert.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 107.Cam K, Simsek F, Yuksel M, Turker L, Haklar G, et al. The role of reactive oxygen species and apoptosis in the pathogenesis of varicocele in a rat model and efficiency of Vitamin E treatment. Int J Androl. 2004;27:228–33. doi: 10.1111/j.1365-2605.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 108.Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011;1:CD007411. doi: 10.1002/14651858.CD007411.pub2. [DOI] [PubMed] [Google Scholar]
- 109.Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20:711–23. doi: 10.1016/j.rbmo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 110.Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil. 2010;13:217–25. doi: 10.3109/14647273.2010.532279. [DOI] [PubMed] [Google Scholar]
- 111.Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G. Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl. 2004;25:761–72. doi: 10.1002/j.1939-4640.2004.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 112.Cavallini G, Biagiotti G, Ferraretti AP, Gianaroli L, Vitali G. Medical therapy of oligoasthenospermia associated with left varicocele. BJU Int. 2003;91:513–8. doi: 10.1046/j.1464-410x.2003.04136.x. [DOI] [PubMed] [Google Scholar]
- 113.Paradiso Galatioto G, Sacchetti A, Innominato PF, Pace G, Ranieri G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol. 2008;26:97–102. doi: 10.1007/s00345-007-0218-z. [DOI] [PubMed] [Google Scholar]


