Abstract
Varicoceles are the most common correctable etiology of male factor infertility. However, the detection and management of varicoceles have not been standardized. This has led to decades of debate regarding the effect of varicocele on male infertility and subsequently whether repair leads to an improved fertility status. The current body of evidence investigating the role of varicocele and varicocelectomy is weak and conflicting. The stance taken by the AUA and ASRM suggests that there is insufficient outcomes data to support evidenced-based guidelines, citing evidence used to provide current recommendations are generally of a low quality level. On the other hand, the EAU Guidelines give a level 1a of evidence for management of varicoceles that are clinically palpable, associated with subnormal semen analyses and having otherwise unexplained fertility. Besides aiding with clinical varicocele detection and management, clinical practice opinion statements and guidelines aim to direct and strengthen the infrastructure of future studies. We review the current status of opinion statements and guidelines in varicocele and management detection with focus on their application in practice.
Keywords: guidelines, varicocele, varicocele detection, varicocele management, varicocelectomy
INTRODUCTION
A varicocele is abnormal dilation of veins located in the pampiniform plexus of the spermatic cord. Varicoceles are a ubiquitous finding in men for any practitioner who performs genitourinary examinations regularly. The prevalence of varicocele varies widely within the literature which is likely attributable to differences in examination technique. Although variable, the prevalence of varicocele in the general population is 15% when not accounting for age.1 Most men who have a varicocele will be asymptomatic. Within the subfertile population, varicoceles are identified in 35%–50% of men with primary infertility and can be found in 69%–81% of men with secondary infertility.2,3
The clinical implication of the diagnosis and subsequent treatment of varicoceles remain controversial, as there is no level 1a evidence of a causal relationship between the presence of a varicocele and infertility. However, compelling evidence links the presence of a varicocele to male factor infertility, testicular hypotrophy, and abnormal semen parameters.4 Varicocelectomy is also the most commonly performed surgical procedure for the treatment male factor infertility.
Although several theories are proposed to explain the interaction between the presence of a varicocele and the effect on spermatogenesis and male infertility, it is likely that a multifactorial phenomenon comprised many etiologies. Venous hypertension transmitted to the testes, increased intratesticular temperature, reflux of adrenal metabolites, and increased concentration of reactive oxygen species (ROS) have all been theorized to lead to the pathogenicity of varicoceles.5,6,7,8
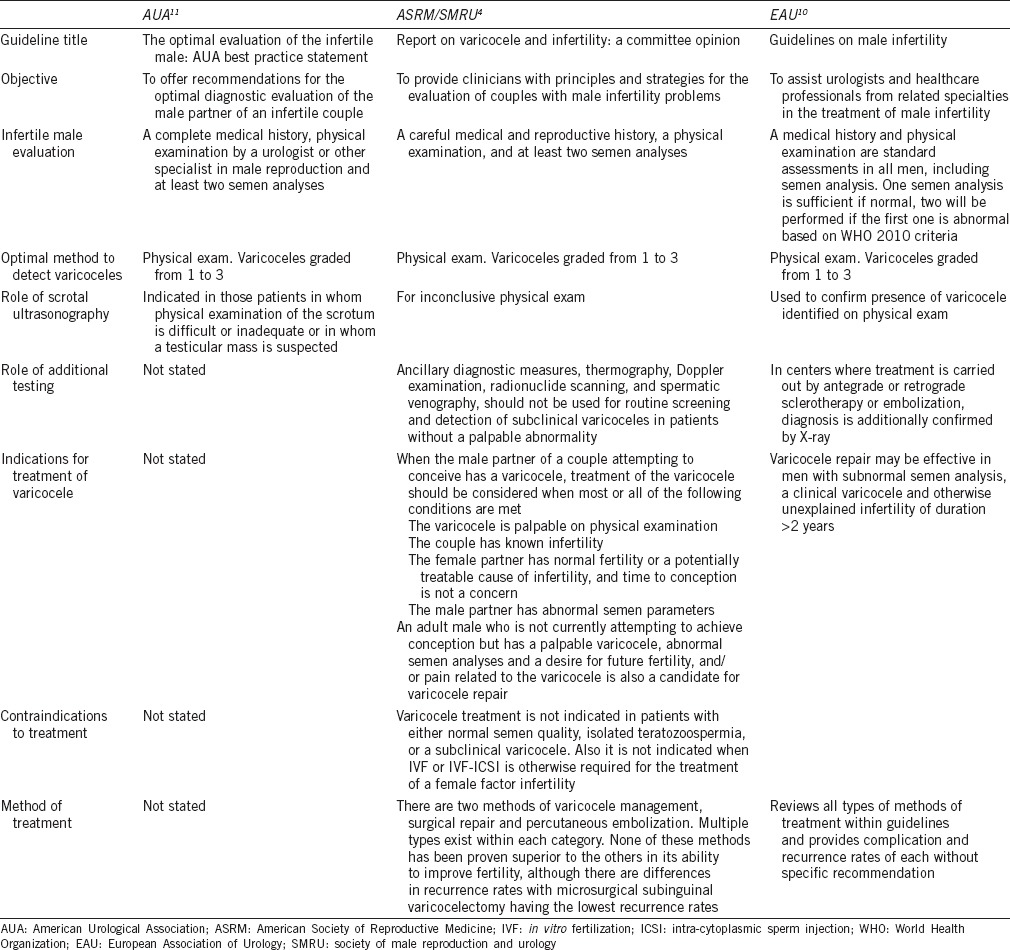
The diagnosis and treatment of varicoceles is embraced by the American Society for Reproductive Medicine, American Urological Association and European Urological Association (ASRM, AUA, EAU) yet guidelines regarding treatment are vague and inconsistent (Supplementary Table 1 (2.1MB, tif) ).4,9,10 The reason may be attributed to the paucity of well-designed studies and conflicting data on linking the impact of varicocele formation to infertility, abnormal semen parameters, decreased pregnancy rates and the results of varicocele treatment.
Scope and the methods used to formulate the AUA, ASRM and EAU guidelines for the evaluation of the infertile male [adapted13]
Historically, controversies have occurred after studies designed to answer clinical questions related to varicocele repair on improvement in semen parameters or pregnancy rates have lead to more confusion. Criticism of each analysis typically concludes with a statement addressing the lack of consistency among subjects. Some of the many purposes of guidelines are to define clinical conditions and recommend treatment actions that are consistent with evidence-based practice. This will aid in future study design, developing consistent, standardized protocols that will strengthen evidence further and improve patient care overall.
CURRENT PRACTICE REPORTS DISCUSSING VARICOCELE-RELATED INFERTILITY
The first report focusing on varicocele detection and management was a joint report formulated by the American Society of Reproductive Medicine (ASRM) and American Urological Society (AUA) in 2001. The Report on Varicocele and Infertility was created Male Infertility Best Practice Policy Committee of the American Urological Association11 and the Practice Committee of the American Society for Reproductive Medicine. The Male Infertility Best Practice Policy Committee was comprised nine urologists, one reproductive endocrinologist, one family physician, and one research andrologist. The mission of the Committee was to develop recommendations, based on expert opinion, for optimal clinical practices in the diagnosis and treatment of male infertility. In this Report on Varicoceles a review of expert opinions on detection and varicocele management was offered.11 The AUA has not updated their position in a dedicated statement on varicoceles since the report published in 2001. The AUA Optimal Evaluation of the Infertile Male: best Practice Statement (BPS) was written in 2007 by the Male Infertility BPS Panel comprised nine urologists, and one andrologist. This is the most updated statement from the AUA where varicocele detection is discussed, but it only does so briefly.
The ASRM has updated its position on varicoceles since the first joint report published in 2001 with the AUA. The ASRM and Society of Male Reproduction and Urology (SMRU) created the Report on Varicocele and Infertility: a Committee Opinion.12 This was first published in 2001 with the AUA, followed by full-text updates in 2008 and 2014 with the SMRU. The Practice Committee of the ASRM is a 21-person committee with male reproductive urologists, andrologists and reproductive endocrinology and infertility specialists. This report provides a comprehensive overview of varicocele detection and management recommendations based on literature review and expert opinion. They suggest that the recommendations should be regarded as “appropriate management” but not necessarily “the only standard of practice.”12
The only guideline that specifically addresses the detection and management of varicoceles is the EAU Guidelines on Male Infertility. The European Association of Urology (EAU) Guidelines Panel on Male Infertility10 has prepared these Guidelines to assist urologists and healthcare professionals from related specialties in the treatment of male infertility. The Male Infertility Guidelines Panel consists of seven members including urologists, endocrinologists, and gynecologists with special training in andrology and experience in the diagnosis and treatment of male infertility. The EAU Male Infertility Guidelines were first published in 2001, followed by full-text updates in 2004, 2007, 2010, 2013, and 2014. The Guidelines are unique in comparison to the other reports in their assignment of levels of evidence to references and their grading of recommendations based on the literature.
Supplementary Table 1 (2.1MB, tif) 13 offers a description of each guideline including the scope and methods involved to formulate recommendations regarding the diagnosis and treatment of varicoceles. Table 14,9,10 summarizes each respective guideline recommendations.
Table 1.
Summary of report recommendations
DETECTION OF VARICOCELE-RELATED INFERTILITY
The detection of varicocele-related infertility is preceded by evaluation of the infertile couple. The ASRM Practice Committee Report, and EAU Guidelines recommend an evaluation performed in couples that fail to achieve pregnancy after 12 months of unprotected intercourse. Exceptions for an earlier evaluation are granted for couples in which the female has known risk factors, for example, female 35 years or older, the male partner has a known risk factor, for example, history of cryptorchidism, or if the male partner has concerns about their future fertility. In the latter situation, the evaluation may be initiated at 6 months. The EAU Guidelines and AUA BPS suggest specialized evaluation by an andrologist/urologist if the male evaluation is suggestive of male factor or an abnormal semen analysis results based on WHO reference standards.
The complete evaluation of male infertility should be performed by a specialist in male reproduction or urology. A complete medical history, including a detailed reproductive assessment including the female partner is mandatory. A physical examination and at two semen analysis should be performed. The ASRM Report and AUA guidelines are more detailed in their recommendation about the evaluation whereas the EAU guidelines state the medical history and physical examination which are standard assessments in all men, including semen analysis. Furthermore, the EAU guidelines suggest only one semen analysis compared to two recommended by the AUA, ASRM/SMRU. Specialized male factor evaluation is only indicated in the setting of two abnormal semen analyses by the EAU guidelines. The EAU Guidelines when followed may exclude the evaluation of male or couple with unexplained male factor since the evaluation is dependent on abnormal semen analysis only. Unexplained fertility ranges from 6% to 39% and is dependent on the modalities used to diagnose male factor.14,15,16,17 Excluding this group from evaluation arguably would miss pathology that could be treated in some men.17
Physical examination is necessary for the diagnosis of varicocele. Only clinically palpable varicoceles are clearly associated with infertility. Varicocele is typically described as having a “bag of worms” appearance and texture. The distended veins are typically palpated within the scrotum above the testis. The male is examined in the upright and recumbent position, when reclined the veins should normally decompress. If the veins do not compress, either a retroperitoneal pathology or previous varicocele repair should be suspected.
Varicoceles are classified on a grading system first described by Dubin and Amelar.18 Grade 1: varicocele palpated during valsalva, Grade 2: varicocele palpable at rest, but not visible, Grade 3: varicocele visible. Subclinical varicoceles are those identified without palpation most often found on scrotal duplex ultrasonography.
Scrotal ultrasonography is not recommended in the screening of varicoceles and does not take the place of physical examination. The ASRM/SMRU Committee Opinion suggests scrotal ultrasonography in the setting of an “inconclusive examination.” The EAU guidelines take it a step further by stating that clinically palpable varicoceles should be confirmed by color Duplex ultrasonography (CDU). The WHO Manual for the Standardized Investigation, Diagnosis and Management of the Infertile Male is cited as the source for varicocele confirmation by CDU, however its dogmatic emphasis on further investigation of Grade 1 and subclinical varicoceles by CDU and thermography is not representative of the other aforementioned reports.19 The role of ultrasonography remains controversial since subclinical varicoceles have a poor concordance with those detected on physical examination.20 The presence of and repair of subclinical varicoceles are seldom clinically significant in the setting of male factor infertility.21,22,23 Nevertheless, in obese men, and men with high riding testis, inguinoscrotal surgery, or varicocele recurrence ultrasonography remains a useful tool.24
Similar to ultrasonography, venography for screening of varicoceles is also not recommended by the ASRM Committee Report. For the diagnosis of varicocele recurrence, however, venography is supported by the AUA, ASRM Report, and EAU Guidelines. Venography can be utilized with subsequent varicocele embolization for the treatment of varicocele recurrence after repair.
Semen analysis testing is the cornerstone of the male fertility evaluation. Semen analysis should be compared to the World Health Organization (WHO) 5th edition standards published in 2010. The AUA Best Practice Statement was published before the newest 2010 WHO guidelines were introduced and therefore may classify more men as having abnormal semen parameters. Specifically, the 2010 WHO standards lowered semen parameter reference values compared to the 1999 WHO standards.25 The clinical significance of the varicocele with regards to its effect on the man's fertility depends on the semen analysis being abnormal based on WHO reference values. Since the guidelines differ in the WHO reference standards utilized, varying results may be expected with regards to treatment outcomes when one is followed versus another.
McGarry et al. retrospectively investigated semen parameters of men undergoing varicocelectomy classified as having abnormal semen parameters based on 1999 and 1990 WHO standards, but having normozoospermia based on 2010 WHO standards.26 Fifty-six men out of 445 (13%) were classified as having a normal SA based on the 2010 standards and 24 underwent microsurgical varicocelectomy. The remainder were observed. Varicocelectomy led to a statistically significant increase in sperm concentration (50 ± 35 × 106 ml−1 [postsurgery] vs 32 ± 23 × 106 ml−1 [presurgery]; P = 0.003). Clinical pregnancy was 52% versus 38% in the observation group however did not reach statistical significance (P = 0.38). Lee et al. also retrospectively reviewed their microsurgical varicocelectomy population who were reclassified as having normal semen parameters based on 2010 WHO reference standards.27 Twenty-four of 70 men were reclassified as normozoospermia based on 2010 reference standards. Significant improvement was defined as >20% increase in semen parameters after varicocelectomy. Of the 2010 normal group 58.8% had semen parameter improvement whereas 85.9% of men who continued to have abnormal parameters based on 2010 reclassification significantly improved. The pregnancy data were not reported. Both studies highlight that, in isolation, semen parameters are not a classification of male infertility. Some men who would benefit from varicocelectomy with regards to pregnancy would be denied candidacy based on 2010 WHO reference standards. A strong argument can be made to offer varicocelectomy to men who were reclassified as normozoospermic by 2010 reference standards which are not explicitly stated in any guideline.
This data may reinforce the sentiment that guidelines should be used as a resource rather than law. The AUA's BPS states, “This best practice statement is intended to provide medical practitioners with a consensus of principles and strategies for the care of couples with male infertility problems. The document is based on current professional literature, clinical experience and expert opinion. It does not establish a fixed set of rules or define the legal standard of care and it does not preempt physician judgment in individual cases. Physician judgment must take into account variations in resources and in patient needs and preferences. Conformance with this best practice statement cannot ensure a successful result.”
INDICATIONS FOR VARICOCELE TREATMENT
Varicocele repair is the most commonly performed treatment for male factor infertility. However, the benefit of varicocele repair for male factor infertility has been an issue of much debate over the past two decades. Many retrospective studies investigating the benefit of repair have had conflicting results. The factors that have fueled the controversy include studies of small sample size, variation in varicocele definition and detection, the lack of a uniform standard of treatment and most notably the inclusion of subclinical varicoceles in the study groups. A recent meta-analysis of four RCTs of varicocelectomy in men with a clinically palpable varicocele, oligozoospermia and otherwise unexplained infertility a favorable trend toward surgical correction was identified.28 This sheds some light on the controversy and reinforces the current practice by most male reproductive urologists.
The ASRM Practice Committee Report Update12 reinforces the older AUA Best Practice Policy9 indications for varicocele repair. It states that in a male partner where male factor infertility is suspected treatment of the varicocele should be considered when the following conditions are met: (1) the varicocele is palpable on physical examination of the scrotum; (2) the couple has known infertility; (3) the female partner has normal fertility or a potentially treatable cause of infertility, and time to conception is not a concern; and (4) the male partner has abnormal semen parameters.
This contrasts with the EAU guidelines10 that are more stringent in their recommendation. The EAU Guidelines recommend consideration of repair in the case of clinically palpable varicocele, oligozoospermia, infertility duration of ≥2 years and otherwise unexplained infertility in the couple. The EAU guidelines increase the length of duration in their definition of infertility, and do not address management in the couple with potentially treatable female factor infertility. This recommendation is graded as “A” indicating randomized clinical trials support this evidence. The reasoning behind the difference in recommended duration of infertility prior to varicocele repair is not addressed in the Guidelines.
Contraindications to treatment highlighted by ASRM Report include normal semen quality, isolated teratozoospermia, or a subclinical varicocele.29 The EAU guideline excludes men with normal semen analysis, and subclinical varicocele. The EAU guidelines do not specifically address isolated teratozoospermia as a situation to consider repair or not. Nevertheless, the most contemporary data do not support male factor treatment for an isolated abnormal strict morphology.30,31,32,33,34,35 Varicocele repair does not lead to significant improvement in morphology, as well.36 The AUA Best Practice Statement on the Optimal Evaluation of the Infertile Male recommends that therapeutic decisions should not be based on abnormal strict morphology when not accompanied by other semen parameter abnormalities.
Another indication for repair that is addressed includes men who are not classified as infertile but have a desire for future fertility, clinically palpable varicocele and abnormal semen parameters. The ASRM Committee Opinion also includes further indications such as young men with increased risk of ipsilateral testicular dysfunction and normal semen parameters, men with varicocele-associated pain, and possibly men with large varicoceles and testosterone deficiency with symptoms. The EUA guidelines do not specifically address these relative indications where repair can be considered.
The repair of adolescent varicocele has been a controversial topic and was addressed by all the major society reports. A description and discussion of recommendations in the diagnosis and management of adolescent varicocele will be discussed elsewhere in this issue.
VARICOCELE TREATMENT OPTIONS
There are many different ways to treat varicoceles; but they can be all distilled to two different types of procedures, varicocele ligation and percutaneous embolization. Varicocele ligation may be performed open via a subinguinal, inguinal, or retroperitoneal approach as well as laparoscopically. Most types of varicocele repair have comparable outcomes in fertility; however, differ in recurrence rates.37 The guidelines acknowledge this and do not recommend one approach over another, but rather aim to inform the reader of the multiple different treatment options and the recurrence and complications associated with both. Percutaneous embolization involves cannulation of the venous system and access to the gonadal vein with subsequent embolization of the internal spermatic vein via coiling. Embolization can offer comparable rates of semen parameter improvement and pregnancy rates albeit at a higher risk of recurrence.38,39,40 Embolization is an excellent choice for treatment of recurrence of varicocele after surgical repair and vice versa.
Surgical repair
The aim of varicocele repair is to prevent spermatic vein pressure transmission to the testis as well as preventing venous pooling around the testis. As stated previously there are several ways to repair a varicocele. When comparing one approach to another, nuances exist that may either change or support the current practice of a reproductive urological surgeon. Most reproductive urologists would choose optical magnification via loupes or an operating microscope and access the varicocele via a subinguinal incision.37,41,42 The EAU guidelines suggest the microsurgical subinguinal approach has the lowest recurrence rate compared to other techniques and may have the least occurrence of complications.37,43 The ASRM Committee Report does not aim to direct the reader to one specific technique, however. The reasoning behind the lack of direction is likely intentional given the level of evidence to support an optimal technique is weak for fertility outcomes.
Percutaneous embolization
Embolization has solidified its role in the treatment of varicocele as the role of the interventional radiologist in medicine has expanded. The venous system is accessed percutaneous usually via the groin. Once the testicular venous system is accessed, either coils or sclerosing agents may be used to facilitate obstruction of the internal spermatic veins. The ASRM Report highlights two main factors that separate this technique from surgical repair. First, the testicular vein cannot be accessed about 20% of the time. Second, the external spermatic vein system cannot be easily accessed; therefore, the varicocele recurrence rate is high and similar to the rate of recurrence with retroperitoneal ligation. The EAU guidelines do not explicitly discuss embolization in their brief treatment section but include the recurrence rates and complications for different types of embolization in their table. Interestingly, the EUA guidelines do not cite any study newer than 20 years old. Most of the significant advances in the varicocele embolization experience have occurred in the past two decades.38,39,40 In experienced hands comparable fertility outcomes may be obtained in addition to being as least invasive as possible.
VARICOCELE OUTCOMES
The guidelines are clear that in the appropriately selected individual, such as a man classified as infertile, with abnormal semen parameters and a clinically palpable varicocele, that surgical repair will lead to improvement in the majority of cases. Semen parameter improvement and pregnancy rates are the usual endpoints used in varicocele outcome analysis. However, the definition of improvement varies greatly between studies siphoning strength from the societies to make clear recommendations regarding repair.
Several studies have grouped the mostly retrospective data into meta-analyses to increase the strength of evidence that varicocele repair leads to semen parameter improvement. Agarwal et al. examined 17 studies seeking a quantification of the impact of varicocelectomy on semen analysis. The inclusion criteria were men with clinically palpable varicoceles and multiple pre- and post-operative semen analyses (at least three). They found that sperm concentration, motility, and morphology increased by 9.7 × 106 ml−1, 9.9%, and 3.1%.36 Another meta-analysis attempted to stratify the semen parameter improvement by surgical approach. There were insignificant differences found between subinguinal, inguinal, and retroperitoneal approaches with regards to semen parameter improvement. Of note, a slight statistically significant advantage in pregnancy rates was seen in inguinal repair.
Abdel-Meguid et al. performed the most recent randomized clinical trial comparing varicocelectomy versus observation in 145 men.44 The study group included men with palpable varicoceles and confirmed abnormalities on semen analyses were included. Pregnancy rates improved with treatment, 32.9% versus 13.9% via natural conception in the control group. This resulted in an odds ratio of 3.04 (95% CI 1.3–6.9), with a number needed to treat of 5.3. A Cochrane Review was performed comparing surgery to embolization following this publication slightly favoring surgery over embolization. When the data were further tailored to exclude studies that included men with subclinical varicoceles and normal semen parameter the odds ratio improved to 2.39 (95% CI 1.56–3.66), with a number needed to treat of 7.45 This meta-analysis acknowledged the low quality of studies for varicocele management overall.
The limitations of the current studies are many. There are very few randomized control trials. This may be due to several factors including the difficulty recruiting men to an observation arm, loss to follow-up, financial considerations with repeated semen analysis, drop-out rates for couples seeking ART during the study period, and difficulty obtaining pregnancy data. Furthermore, some of the data have been diluted by inclusion of men with subclinical varicoceles and normal semen parameters. The lack of randomization, low power, exclusion of female factor data, and lack of control groups have further weakened the level of evidence supporting varicocele repair. The guidelines aim to improve the quality of studies by directing management toward appropriately selected individuals. Furthermore, the ASRM Report, AUA Best Practice Statement and EUA Guidelines acknowledge that better quality studies, in general, need to be performed. Nevertheless, both guidelines affirm “most studies” show semen parameter and fertility improvement by varicocele repair.
MANAGEMENT CONSIDERATIONS
In any discussion regarding management of the infertile male, all the treatment options should be reviewed with the couple. In the setting of varicocele, intrauterine insemination (IUI), and in vitro fertilization (IVF) with or without intra-cytoplasmic sperm injection (ICSI) can be offered. Addressing the varicocele may treat the underlying pathology leading to the male factor, but IUI, and IVF with or without ICSI can bypass the abnormal semen parameters to achieve pregnancy. One caveat is that IUI, and IVF require use of the assisted-reproductive technology (ART) for each attempt at pregnancy. This may lead to increased cost, and cost-effectiveness analyses comparing varicocele repair to other ARTs has been published previously.46 Potential considerations that may help a couple decide on which therapy to pursue include associated symptoms attributed to the varicocele, age, fertility potential of the female partner, and time available for conception as improvement in semen parameters after varicocele repair may take 3–6 months. Progressive decline of semen parameters may occur with observation of varicocele and this may argue to repair varicoceles in any event. However, semen parameters abnormalities in isolation do not predict future fertility.35
Usually, significant female factor warranting IVF may preclude the need for varicocele repair. However, in the setting of nonobstructive azoospermia and testis histology consistent with hypospermatogenesis or late maturation arrest, varicocelectomy may allow sperm to return to the ejaculate in 10%–55% of men.47,48 The ASRM Report statement suggests this consideration for repair in appropriately selected men because this may prevent a sperm extraction from being performed before or in-cycle with IVF. Again the controversial nature of this data is reaffirmed and the decision to repair in this setting is left up to the male reproductive specialist and couple.49
IMPACT OF GUIDELINES
The guidelines in general have an undefined impact on current practice. In theory, following practice guidelines leads to uniform, evidence-based patient care. In addition, epidemiologic data and outcomes analysis would also be improved due to guidelines-based patient selection. However, the actual impact of guidelines on practice has not been measured as of yet. In addition, guidelines are not often cited in recent studies. The EAU guidelines, and AUA/ASRM Committee Opinion Report on Varicoceles have been cited by publications 34 times in total. This includes previous EAU Guidelines, AUA/ASRM Joint Report since its publication in 2001, and subsequent ASRM Committee Opinion Statements. A PubMed search of “varicocele AND infertility” yields 958 publications since 2001. Five of the ten studies included in the most recent Cochrane Review comparing fertility outcomes between surgery and embolization had normal semen parameters and subclinical varicoceles. Although citations are not a direct measure of the impact of current guidelines it is thought provoking. Nevertheless, a lag time exists for the dissemination of information and acceptance of the recommendations into clinical practice which may explain this phenomenon.
The level of evidence existing for the detection and management on varicocele is generally poor. The AUA Best Practice Committee and ASRM Practice Committee acknowledged that currently there was insufficient outcome data to support a formal evidence-based guideline, and highlighted that the evidence used to provide recommendations was generally of a low quality level.9 The EAU Guidelines “Conclusions” section regarding the management of varicoceles give a 1a level of evidence for management of varicoceles that are clinical palpable, associated with subnormal semen analyses and have otherwise unexplained fertility.
The issues with delayed acceptance and low levels of evidence developed from previous poorly performed studies may actually argue in favor of generating evidence-based guidelines. Often guidelines are developed more to establish clinical standards aimed at directing future research efforts rather than changing everyday practice. The creation of measurable standardized variables may facilitate comparisons among studies despite the heterogeneity in patient populations.50 However, the final decisions regarding varicocele detection and management often rely on physician and patient preference. Preferences can be influenced by multiple factors including practice resources such as semen analysis testing, financial resources and considerations as well as cultural and religious values. It should be noted that guidelines aim to increase quality of care without consideration of cost.50 Guidelines offer a resource on which a standard of care can be established. However, it should be understood that they offer recommendations on ideal and index patients that may not translate into everyday practice. Those individualized decisions are up to the physician and patient.
FUTURE DIRECTIONS
The current Guidelines and Practice Committee reports will have more to address in the future as our knowledge base in the peer-reviewed literature evolves. First, by creating clinical standards and index parameters for varicocele diagnosis the strength of the data should increase. There are specific barriers to this such as resistance to randomization into a control arm described previously; however, randomized controlled studies are increasingly important as the field of male infertility and reproductive health gain mainstream acceptance. Second, the role of DNA fragmentation and reactive oxygen species (ROS) in varicocele diagnosis and outcomes assessment need to be addressed. There is a growing body of literature supporting the beneficial effect of varicocele repair on DNA fragmentation, and ROS damage.51,52 However, decreases in DNA fragmentation and ROS through repair have yet to be definitively linked to improved pregnancy outcomes. Finally, the role of varicocele repair in men with severe oligoasthenoospermia and azoospermia leading to return of sperm to the ejaculate has long been known.53 However, only small retrospective cohort studies exist and the role of varicocelectomy in preventing sperm retrieval prior to IVF/ICSI or improving the quality of sperm at the time of retrieval needs to be further delineated.48,49,54,55
CONCLUSIONS
Societal direction via guidelines and for the detection and management of varicocele is essential to establishing standards of care to assist with patient care and direct future studies. This in turn should promote improvement in healthcare delivery as well as discourage potentially harmful or ineffective interventions.15 Currently, the EAU Guidelines on Male Infertility is the only guideline that offer recommendations along with levels of evidence and include an implementation schema. The ASRM Practice Report gives a concise review of the current data supporting the opinion of the expert panel. The AUA had published a joint report with the ASRM in 2001, however have since elected not to update their position in another statement as of yet.
The levels of evidence of varicocele recommendations are low and generally derived from nonrandomized, retrospective studies. The EUA Guidelines and ASRM Practice Committee Report agree that infertile men with clinically palpable varicoceles and abnormal semen analysis are candidates for management but do not go much further in likeness. Without agreement between the standards set forth in the societal statements, clinicians must use sound judgment and individualize their care decisions based on the preferences and resources of the practice and couple.
AUTHOR CONTRIBUTIONS
AS and RCO contributed to drafting the manuscript. OOE and AS developed tables. AS and EDK coordinated review and guideline analysis.
COMPETING INTERESTS
All authors declare no competing interests.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Thomason A, Fariss B. The prevalence of varicoceles in a group of healthy young men. Mil Med. 1979;144:181–2. [PubMed] [Google Scholar]
- 2.Gorelick J, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–6. [PubMed] [Google Scholar]
- 3.Witt M, Lipshultz L. Varicocele: a progressive or static lesion? J Urol. 1993;42:541–3. doi: 10.1016/0090-4295(93)90268-f. [DOI] [PubMed] [Google Scholar]
- 4.American Society of Reproductive Medicine. Report on varicocele and infertility. Fertil Steril. 2008;90:S247–9. doi: 10.1016/j.fertnstert.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Nistal M, González-Peramato R, Serrano A, Pegadera J. Physiopathology of the infertile testicle. Etiopathogenesis of varicocele. Arch Esp Urol. 2004;57:883–904. [PubMed] [Google Scholar]
- 6.Marmar J. The pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Update. 2001;7:461–72. doi: 10.1093/humupd/7.5.461. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein M, Eid J. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol. 1989;142:743–45. doi: 10.1016/s0022-5347(17)38874-2. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Prabakara S, Allamaneni S. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006;12:630–3. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 9.Linthicum, MD: American Urological Association Education and Research, Inc; 2010. American Urological Association Education and Research, Inc. The optimal evaluation of the infertile male: AUA best practice statement. [Google Scholar]
- 10.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, et al. European association of urology guidelines on male infertility: the 2012 update. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Linthicum, MD: American Urological Association, Inc; 2001. American Urological Association Education and Research, Inc. Report on Varicocele and Infertility: An AUA Best Practice Policy and ASRM Practice Committee Report. Birmingham, AL: American Society for Reproductive Medicine. [Google Scholar]
- 12.American Society for Reproductive Medicine. Report on varicocele and infertility: a committee opinion. Fertil Steril. 2014;102:1556–60. doi: 10.1016/j.fertnstert.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Esteves SC, Chan P. A systematic review of recent clinical practice guidelines and best practice statements for the evaluation of the infertile male. Int Nephrol Urol. 2015;47:1441–56. doi: 10.1007/s11255-015-1059-0. [DOI] [PubMed] [Google Scholar]
- 14.Cates W, Farley T, Rowe P. Worldwide patterns of infertility: is Africa different? Lancet. 1985;2:596–8. doi: 10.1016/s0140-6736(85)90594-x. [DOI] [PubMed] [Google Scholar]
- 15.Sigman M, Baazeem A, Zini A. Semen analysis and sperm function assays: what do they mean? Semin Reprod Med. 2009;2:11–23. doi: 10.1055/s-0029-1202300. [DOI] [PubMed] [Google Scholar]
- 16.Miyaoka R, Esteves S. A critical appraisal on the role of varicocele in male infertility. Adv Urol 2012. 2012:597495. doi: 10.1155/2012/597495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamada A, Esteves S, Nizza M, Argawal A. Unexplained male infertility: diagnosis and management. Int Braz J Urol. 2012;38:576–94. doi: 10.1590/s1677-55382012000500002. [DOI] [PubMed] [Google Scholar]
- 18.Dubin L, Amelar R. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 19.Cambridge: Cambridge University Press; 2000. World Health Organization. WHO Manual for the Standardized Investigation, Diagnosis and Management of the Infertile Male. [Google Scholar]
- 20.Petros J, Andriole G, Middleton W, Picus D. Correlation of testicular color Doppler ultrasonography, physical examination and venography in the detection of left varicocele in men with infertility. J Urol. 1991;14:785–8. doi: 10.1016/s0022-5347(17)38451-3. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Gao X, Li Z, Yu Y, Zhang Z, et al. Efficacy of bilateral and left varicocelectomy in infertile men with left clinical and right subclinical varicoceles: a comparative study. J Urol. 2009;73:1236–40. doi: 10.1016/j.urology.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Hibi H, Hirata Y, Miyake K, Ishiaki T. Effect of varicocelectomy on sperm parameters and pregnancy rate in patients with subclinical varicocele: a randomized prospective controlled study. J Urol. 1996;155:1636–8. [PubMed] [Google Scholar]
- 23.Grasso M, Lania C, Castelli M, Galli L, Franzoso F, et al. Low-grade left varicocele in patients over 30 years old: the effect of spermatic vein ligation on fertility. BJU Int. 2000;85:305–7. doi: 10.1046/j.1464-410x.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- 24.Hayden R, Tanrikut C. Varicocele – Adult. In: Kim ED, Brannigan RE, editors. Case Studies in Male Infertility: Expert Analysis and Current Controversies. London: JP Medical; 2016. [In press] [Google Scholar]
- 25.5th edn. World Health Organization; 2010. World Health Organization, Dept. of Reproductive Health and Research. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 26.McGarry P, Alrabeeah K, Jarvi K, Zini A. Is varicocelectomy beneficial in men previously deemed subfertile but with normal semen parameters based on the new guidelines?. A retrospective study. Urology. 2015;85:357–62. doi: 10.1016/j.urology.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Lee YJ, Cho SY, Paick JS, Kim SW. Usefulness of 2010 World Health Organization reference values for determining indications for varicocelectomy. Urology. 2015;85:831–5. doi: 10.1016/j.urology.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Stahl P, Schlegel P. Standardization and documentation of varicocele evaluation. Curr Opin Urol. 2011;21:500–5. doi: 10.1097/MOU.0b013e32834b8698. [DOI] [PubMed] [Google Scholar]
- 30.Keegan B, Barton S, Sanchez S, Berkeley A, Krey L, et al. Isolated teratospermia does not affect in vitro fertilization outcome and is not an indication for intracytoplasmic sperm injection. Fertil Steril. 2007;88:1583–8. doi: 10.1016/j.fertnstert.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 31.Van Waart J, Kruger T, Lumbard C, Ombelet W. Predictive value of normal sperm morphology in intrauterine insemination (IUI): a structured literature review. Hum Reprod Update. 2001;7:495–500. doi: 10.1093/humupd/7.5.495. [DOI] [PubMed] [Google Scholar]
- 32.Spieccens C, Vanderschueren D, Meuleman C, D’Hooghe T. Isolated teratospermia and intrauterine insemination. Fertil Steril. 2003;80:1185–9. doi: 10.1016/s0015-0282(03)01172-5. [DOI] [PubMed] [Google Scholar]
- 33.Shibahara H, Obara H, Ayustawati, Hirano Y, Suzuki T, et al. Prediction of pregnancy by intrauterine insemination using CASA estimates and strict criteria in patients with male factor infertility. Int J Androl. 2004;27:63–8. doi: 10.1111/j.0105-6263.2004.00437.x. [DOI] [PubMed] [Google Scholar]
- 34.Gunalp S, Onculoglu C, Gurgan T, Kruger T, Lombard C. A study of semen parameters with emphasis on sperm morphology in a fertile population: an attempt to develop clinical thresholds. Hum Reprod. 2001;16:110–4. doi: 10.1093/humrep/16.1.110. [DOI] [PubMed] [Google Scholar]
- 35.Guzick D, Overstreet J, Factor-Litvac P, Brazil C, Nakajima S, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short R, et al. Efficacy of varicocelectomy in improving semen parameters: a new meta-analytical approach. Urology. 2007;70:532–8. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Ding H, Tian J, Du W, Zhang L, Wang H, et al. Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: a meta-analysis of randomized controlled trials. BJU Int. 2012;110:1536–42. doi: 10.1111/j.1464-410X.2012.11093.x. [DOI] [PubMed] [Google Scholar]
- 38.Schlansky-Goldberg R, VanArsdalen K, Rutter C, Soulen M, Haskal Z, et al. Percutaneous varicocele embolization versus surgical ligation for the treatment of infertility: changes in seminal parameters and pregnancy outcomes. J Vasc Interv Radiol. 1997;8:750–67. doi: 10.1016/s1051-0443(97)70657-2. [DOI] [PubMed] [Google Scholar]
- 39.Gandini R, Konda D, Reale C, Pampana E, Maresca L, et al. Male varicocele: transcatheter foam sclerotherapy with sodium tetradecyl sulfate – Outcome in 244 patients. Radiology. 2008;246:612–8. doi: 10.1148/radiol.2462061295. [DOI] [PubMed] [Google Scholar]
- 40.Bechara C, Weakley S, Kougias P, Athamneh H, Duffy P, et al. Percutaneous treatment of varicocele with microcoil emboliztion: comparison of treatment outcome with laparoscopic varicocelectomy. Vascular. 2009;3:S129–36. doi: 10.2310/6670.2009.00062. [DOI] [PubMed] [Google Scholar]
- 41.Al-Kandari A, Shabaan H, Ibrahim H, Elshebiny Y, Shokeir A. Comparison of outcomes of different varicocelectomy techniques: open inguinal, laparoscopic and subinguinal microscopic varicocelectomy: a randomized clinical trial. Urology. 2007;69:417–20. doi: 10.1016/j.urology.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 42.Kim S, Chung H, Park K. Modified microsurgical subinguinal varicocelectomy without testicular delivery. Andrologia. 2011;43:405–8. doi: 10.1111/j.1439-0272.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 43.Ghanem H, Anis T, El-Nashar A, Shamioul R. Subinguinal microvaricocelectomy versus retroperitoneal varicocelectomy comparative study of complications and surgical outcome. Urology. 2004;64:1005–9. doi: 10.1016/j.urology.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Meguid T, Al-Sayyad A, Tayib A, Farsi H. Does varicocele repair improve male infertility?. An evidenced-based perspective from a randomized, controlled trial. Eur Urol. 2011;59:455–61. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Kroese A, de Lange N, Collins J, Evers J. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 2012;10:CD000479. doi: 10.1002/14651858.CD000479.pub5. [DOI] [PubMed] [Google Scholar]
- 46.Schlegel P. Is assisted reproduction the optimal treatment for varicocele-associated male infertility?. A cost-effectiveness analysis. Urology. 1997;49:83–90. doi: 10.1016/S0090-4295(96)00379-2. [DOI] [PubMed] [Google Scholar]
- 47.Matthews G, Matthews E, Goldstein M. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe oligoasthenospermia. Fertil Steril. 1998;70:71–5. doi: 10.1016/s0015-0282(98)00108-3. [DOI] [PubMed] [Google Scholar]
- 48.Kim E, Leibman B, Grinblat D, Lipshultz L. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999;162:737–40. doi: 10.1097/00005392-199909010-00031. [DOI] [PubMed] [Google Scholar]
- 49.Schlegel P, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2004;81:1585–8. doi: 10.1016/j.fertnstert.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 50.Trost L, Nehra A. Guideline-based management of male infertility: why do we need it? Indian J Urol. 2011;27:49–57. doi: 10.4103/0970-1591.78426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mostafa T, Anis T, El-Nashar A, Imam H, Othman I. Varicocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicocele. Int J Androl. 2001;24:261–5. doi: 10.1046/j.1365-2605.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 52.Saleh R, Agarwal A, Sharma R, Said T, Sikka S, et al. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80:1431–6. doi: 10.1016/s0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 53.Tulloch W. A consideration of sterility factors in the light of subsequent pregnancies. II. Sub fertility in the male (Tr Edinburgh Obst Soc Session 104) Edinb Med J. 1952;59:29–34. [PMC free article] [PubMed] [Google Scholar]
- 54.Pasqualotto F, Sobreiro B, Hallak J, Pasqualotto E, Lucon A. Induction of spermatogenesis in azoospermic men after varicocelectomy repair: an update. Fertil Steril. 2006;85:635–9. doi: 10.1016/j.fertnstert.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 55.Zampieri N, Bosaro L, Costantini C, Zaffagnini S, Zampieri G. Relationship between testicular sperm extraction and varicocelectomy in patient with varicocele and nonobstructive azoospermia. Urology. 2013;82:74–7. doi: 10.1016/j.urology.2013.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scope and the methods used to formulate the AUA, ASRM and EAU guidelines for the evaluation of the infertile male [adapted13]


