Abstract
To study the major differences in the distribution of spermatozoa proteins in infertile men with varicocele by comparative proteomics and validation of their level of expression. The study-specific estimates for each varicocele outcome were combined to identify the proteins involved in varicocele-associated infertility in men irrespective of stage and laterality of their clinical varicocele. Expression levels of 5 key proteins (PKAR1A, AK7, CCT6B, HSPA2, and ODF2) involved in stress response and sperm function including molecular chaperones were validated by Western blotting. Ninety-nine proteins were differentially expressed in the varicocele group. Over 87% of the DEP involved in major energy metabolism and key sperm functions were underexpressed in the varicocele group. Key protein functions affected in the varicocele group were spermatogenesis, sperm motility, and mitochondrial dysfunction, which were further validated by Western blotting, corroborating the proteomics analysis. Varicocele is essentially a state of energy deprivation, hypoxia, and hyperthermia due to impaired blood supply, which is corroborated by down-regulation of lipid metabolism, mitochondrial electron transport chain, and Krebs cycle enzymes. To corroborate the proteomic analysis, expression of the 5 identified proteins of interest was validated by Western blotting. This study contributes toward establishing a biomarker “fingerprint” to assess sperm quality on the basis of molecular parameters.
Keywords: bioinformatics, male infertility, proteomics, spermatozoa proteins, varicocele
INTRODUCTION
Varicocele is diagnosed in about 15%–20% of the adult male population and is implicated as a factor in about 40% of infertile men.1 The recommendation of the American Society of Reproductive Medicine is to treat a varicocele when it is palpable and present with at least one abnormal semen parameter in couples presenting with infertility or when the female partner is normal.2 Numerous factors have been proposed to explain the occurrence of this multifactorial disease, which include venous stasis, testicular hypothermia, testicular hypoxia, apoptosis, heavy metal toxicity, increased oxidative stress, and increased DNA damage.3,4 Although poor semen quality, increased oxidative stress and DNA fragmentation are frequently observed, not all men presenting with varicocele are infertile, and men with high-grade varicocele can still father children.5,6,7 However, the underlying molecular mechanism of varicocele-associated testicular dysfunction and infertility remains unclear.
Unilateral varicocele present on the left side is more common (35%–40%) compared to the presence of a bilateral varicocele (10%–15%).1 Varicocele repair has been shown to correct varicocele and improve semen parameters, DNA integrity and pregnancies in some, but not in all cases.6,7,8,9 In addition, significant improvement (49%) in natural pregnancy rates has been reported in bilateral varicocelectomy compared to 36% in unilateral varicocelectomy.10 While surgical repair eliminates varicocele in the majority of cases, its impact on infertility remains unclear.
Proteomics is a rapidly emerging technology that allows the simultaneous detection of thousands of proteins. Several comparative studies have been published on the sperm proteome, documenting alterations in the protein composition in the spermatozoa or seminal plasma in an effort to better understand the underlying etiology of male infertility.1,11,12,13 A number of studies have utilized high-throughput techniques such as LC-MS/MS, isobaric labeling strategies (such as isobaric tandem mass tags; TMTs), and isobaric tags for relative and absolute quantitation (iTRAQ) to study protein alterations in fertile versus infertile groups.14,15 Comparative studies on the sperm proteome have revealed the differential proteomic profile of varicocele patients in general with healthy fertile men or donors with normal seminal values. Hosseinifar et al. identified 15 distinct differences in the protein profile from oligozoospermic grade 3 varicocele group in comparison with normozoospermic men (n = 20), where 12 proteins showed low abundance, two were of high abundance, and one protein was not detected.16 Similarly, a study by Chan's group using a two-dimensional electrophoresis and MALDI-TOF-MS revealed a total of 15 differentially expressed proteins in men with grade 2 varicocele compared to fertile control subjects.17 Among the 15 proteins detected, Heat shock protein 70 and 90 were repeatedly upregulated in the varicocele group. Some of these differentially regulated proteins may be potential key proteins that could be helpful in predicting the success of varicocele repair and also shedding light on the crucial proteins involved in fertilization.18 A few studies have looked at altered protein profiles in varicocele patients before and after varicocele repair.19,20
We have recently examined the proteomic profile of patients with either unilateral or bilateral varicocele with fertile men21,22 and also compared infertile men with unilateral and bilateral varicocele only.23 The fundamental goal of our previous studies was to understand the specific pathophysiology of individual conditions, i.e., unilateral or bilateral varicocele. In all of the above-mentioned studies, comparisons were made using any two specific conditions. However, irrespective of the type (unilateral or bilateral) and/or grade of varicocele, a cohort of patients remained infertile. Therefore, in this study, we aim at comparing the protein profile of spermatozoa of infertile men with varicocele, with that of fertile men using the protein profiles obtained in our previous studies to identify novel protein markers that may be involved in the development of infertility along with validation of their expression levels.
MATERIALS AND METHODS
Patient enrollment and sample collection
Following approval of the study by the Institutional Review Board of Cleveland Clinic, semen samples were collected from 50 infertile varicocele patients seeking investigation for fertility. A separate set of 10 proven fertile men were included as a control group. All fertile men were confirmed for the absence of a clinical varicocele. All patients provided written consent to be enrolled in this prospective study. Varicocele was diagnosed by clinical analysis, including scrotal palpation and graded. Men with azoospermia and sperm concentration of <10 × 106 ml−1 were excluded. Samples with round cells >1 × 106 white blood cells were tested for leukocytospermia by Endtz or peroxidase test. Samples that were positive for leukocytospermia were not included in this study.
Semen analysis
Following liquefaction, manual semen analysis was performed according to the WHO guidelines to determine sperm concentration and motility.24 Sperm smears were air-dried, fixed, and stained with Diff-Quik for assessment of sperm morphology.
Measurement of reactive oxygen species, total antioxidant capacity, and DNA fragmentation
ROS formation was measured by chemiluminescence assay with luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) as the probe, using an AutoLumat LB 953 multi-tube luminometer (Berthold Technologies, Oak Ridge, TN, USA). Results were expressed as relative light units RLU/s/106 sperm.25 Total antioxidant capacity (TAC) was measured in the seminal plasma using the antioxidant assay kit (Cayman Chemical, Ann Arbor, MI, USA). Trolox standards and reagent were prepared as per the manufacturer's instructions at the time of the assay. Absorbance was monitored at 750 nm using ELx800 Absorbance Microplate Reader. Results were expressed as micromoles of Trolox.21
Sperm DNA fragmentation was evaluated using a terminal deoxynucleotidyl transferase–mediated fluorescein–dUTP nick end labeling (TUNEL) assay with an apoptosis-detection kit (Apo-Direct, BD Biosciences Pharmingen, San Diego, CA, USA) and flow cytometry. All fluorescence signals of labeled spermatozoa were analyzed by flow cytometer. Totally, 10 000 spermatozoa were examined, and the percentage of TUNEL-positive cells was calculated.26
Proteome profiling
Here, we used the list of proteins detected by proteomic analysis of spermatozoa proteins reported in our previous studies.21,22,23 The methodology is briefly described below.
Preparation of samples for proteomic analysis
Samples were thawed, and seminal plasma was removed after centrifugation for 7 min at 1000 ×g. Spermatozoa were washed 3 times in PBS by centrifugation. Sperm concentration was determined before adding the radioimmunoprecipitation assay (RIPA) lysis buffer (Sigma-Aldrich, St. Louis, MO, USA) containing proteinase inhibitor cocktail (Roche, Indianapolis, IN, USA). The spermatozoa samples were stored overnight at 4°C to allow for complete lysis of the spermatozoa. After centrifugation at 13 000 ×g for 20 min, the supernatant was aspirated, and the protein concentration was determined using a bicinchoninic acid (BCA) kit (Thermo, Rockford, IL, USA). Sperm concentration was normalized, so that equal number of spermatozoa is contributed to the protein pool. For proteomic analysis, samples were pooled. Both spermatozoa and protein concentrations were normalized in each group, i.e., equal quantity of protein was contributed by equal number of spermatozoa. Based on this normalization, we selected 5 of the 33 patients with unilateral varicocele, 3 of the 17 patients with bilateral varicocele, and 5 from the fertile group. Equal amounts of protein were diluted in the SDS-PAGE sample buffer and fractionated using 1D SDS-PAGE.
Global proteomic analysis
Global proteomic analysis was done in triplicate and quantified using the label-free spectral counting method. A 15 μg aliquot of each sample was boiled, and a standard SDS-PAGE was run on a 12.5% Tris-HCl 1-D gel with constant voltage of 150 V for 35 min. The gel was run for 1/3 of the total length to prepare for the downstream LC-MS/MS experiment. The gel was fixed for 30 min in 50% ethanol/10% acetic acid, washed with water thoroughly, and stained with Coomassie blue. For the protein digestion, the entire gel lane was cut and divided into 6 smaller pieces. The bands were reduced and alkylated before the in-gel digestion. The gel pieces were washed with water and dehydrated in acetonitrile. The bands were reduced with Dithiothreitol (DTT) and alkylated with iodoacetamide before the in-gel digestion. All bands were digested in-gel using trypsin by adding 5 μl of 10 ng μl−1 trypsin in 50 mmol l−1 ammonium bicarbonate and incubating overnight at room temperature to achieve complete digestion. The peptides were extracted from the polyacrylamide in two aliquots of 30 μl 50% acetonitrile with 5% formic acid. Gels from the fertile, unilateral, and bilateral varicoceles were run in triplicate for assessing technical and biological reproducibility.
Liquid chromatography mass spectrometer (LC-MS) analysis
The extracts were combined and evaporated to <10 μl in SpeedVac, and then resuspended in 1% acetic acid to make a final volume of ~30 μl for LC-MS analysis. The LC-MS system was a Finnigan LTQ-Orbitrap Elite™ Hybrid Ion Trap-Orbitrap Mass Spectrometer system. The HPLC column was a Dionex 15 cm × 75 μm internal diameter Acclaim Pepmap C18, 2 μm, 100 Ε reversed phase capillary chromatography column. Five microliters of the extract were injected, and the peptides eluted from the column by an acetonitrile/0.1% formic acid gradient at a flow rate of 0.25 μl min−1 were introduced into the source of the mass spectrometer on-line. The microelectrospray ion source was operated at 2.5 kV. The digest was analyzed using the data-dependent multitask capability of the instrument acquiring full scan mass spectra to determine the peptide molecular weights and product ion spectra (MS/MS) to determine the amino acid sequence in successive instrument scans.
Database searching and protein identification
Tandem mass spectra were extracted by Proteome Discoverer version 1.4.1.288 (Thermo Fisher Scientific, San Jose, CA, USA). All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version 2.3.02), Sequest (Thermo Fisher Scientific, San Jose, CA, USA; version 1.4.0.288) and X! Tandem (The GPM, thegpm.org; version CYCLONE [2010.12.01.1]). To validate MS/MS-based peptide and protein identifications, Scaffold (version Scaffold 4.0.6.1, Proteome Software Inc., Portland, OR, USA) was used. Protein identifications were accepted if they could be established at >99.0% probability to achieve a false detection rate (FDR) <1.0% and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm.27,28 Proteins were annotated with gene ontology (GO) terms from National Center for Biotechnology Information (NCBI).29
Quantitative proteomics for establishment of an expression database, data distribution, and quality
For proteomic analysis, the relative quantity of the proteins was determined by comparing the spectral counts used to identify each protein. The protein abundance in the complex mixture was measured by the spectral counts. Normalization of the spectral counts using the normalized spectral abundance factor (NSAF) approach was applied before quantification of the relative amount of protein present.30 This is important to account for the sample-to-sample variation seen during the replicate analysis of the samples.31 Accurate quantification and determination of real biological change is a function of the absolute number of spectral counts. Appropriate filters were used to identify the differentially expressed proteins (DEPs) that were dependent on the overall abundance of the proteins among the three replicate runs.
Based on the average spectral count of the proteins from the multiple runs, different constraints for fold change cutoffs were applied to obtain the differentially expressed proteins. Higher spectral counts have low variance and, therefore, require less stringent fold change cutoffs compared to low spectral count proteins. To minimize the bias and maintain a constant false positive rate, the abundance of the proteins was classified according to the average spectral count, different constraints for biological and statistical variance (P values), as well as the cutoff for the fold change (NSAF ratio) as:
Very low abundance: spectral count range 1.7–7; P ≤ 0.001 and NSAF ratio ≥2.5 for overexpressed, ≤0.4 for underexpressed proteins
Low abundance: spectral count range 8–19; P ≤ 0.01 and NSAF ratio ≥2.5 for overexpressed, ≤0.4 for underexpressed proteins
Medium abundance: spectral count range between 20 and 79; P ≤ 0.05 and NSAF ratio ≥2.0 for overexpressed, ≤0.5 for underexpressed proteins
High abundance: spectral counts >80; P ≤ 0.05 and NSAF ratio ≥1.5 for overexpressed, ≤0.67 for underexpressed proteins.
To assess the distribution and quality of the data for identification of any outliers present in the data set and to find the requirement of any normalization or standardization, various diagnostic plots were used. The intensity values were log2 transformed to reveal a rather homogenous data set with similar distributions of detected values for the different samples.
Filtering of missing values and establishment of an expression database
A missing value may result from the absence of the corresponding protein in the sample or its intensity was too low to be discriminated from the background level. Since the objective of this study is to compare spermatozoa proteomes in infertile varicocele patients with that of fertile donors to reveal proteins common to varicocele irrespective of laterality (unilateral or bilateral) and grades, the filter was based on the fraction of present values in both varicocele condition gels. Therefore, in this study, analysis was done using all proteins identified in each group taking all three replicates. Accordingly, an expression database of all proteins was established (Figure 1).
Figure 1.
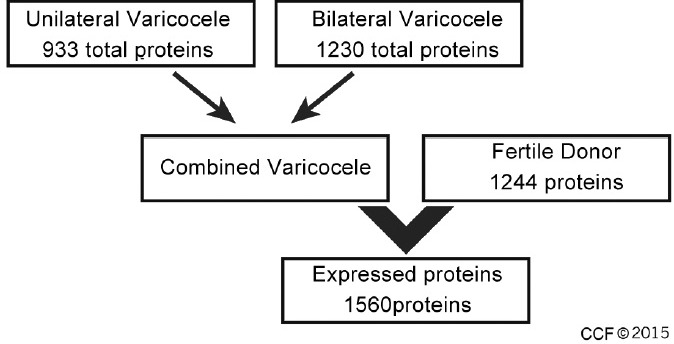
Flow diagram showing the expression database of all the three replicates.
Bioinformatics analysis
Functional annotation and enrichment analysis were performed using publicly available bioinformatics annotation tools and databases such as GO Term Finder,32 GO Term Mapper, UniProt, Software for Researching Annotations of Proteins (STRAP),33 Database for Annotation, Visualization, and Integrated Discovery (DAVID), and proprietary software packages such as IPA (Ingenuity Pathway Analysis) and Search Tool for the Retrieval of Interacting Genes/Proteins (STRING).
Protein confirmation by Western blotting
The differentially expressed proteins of interest were verified in each sample using Western blotting. Washed spermatozoa were lysed in RIPA lysis buffer containing 1 × PBS, 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 10 mg ml−1 phenylmethylsulfonyl fluoride (PMSF), aprotinin, 100 mmol l−1 sodium orthovanadate, and 4% protease inhibitor cocktails by microcentrifugation at 10 000 ×g for 10 min at 4°C. The supernatants were collected and treated with an equal volume of sample application buffer (125 mmol l−1 Tris-HCl, pH 6.8, 2% SDS, 5% glycerol, 0.003% bromophenol blue, and 1% β-mercaptoethanol). The mixture (approximately 20 μg of protein) was boiled for 5 min; 15 μl of each sample was applied to each well of a 4%–15% SDS–polyacrylamide gel and electrophoresed for 2 h at 90 V along with a set of molecular weight markers (Sigma Chemical Co., St. Louis, MO, USA). The resolved protein bands were then transferred onto PVDF membranes at 18 V for 30 min using a transfer buffer of 25 mmol l−1 Tris base, 192 mmol l−1 glycine, and 20% methanol. The blots were blocked overnight at 4°C with blocking buffer (5% nonfat milk in 10 mmol l−1 Tris pH 7.5, 100 mmol l−1 NaCl, and 0.1% Tween 20). The blocking buffer was decanted, and blots were incubated overnight at 4°C with constant rocking with primary antibody (rabbit IgG, Abcam, MA, USA) diluted 1:1000 in 5% milk PBS/Tween. As an internal control, blots were reprobed with an anti-β-actin antibody (Santa Cruz Biotechnology, Inc., TX, USA). Blots were then washed using Tris-Buffered Saline Triton (TBST) (10 mmol l−1 Tris pH 7.5, 100 mmol l−1 NaCl, 0.1% Tween 20) and incubated with horseradish peroxidase conjugated anti-rabbit IgG (1:15 000; Abcam, MA, USA) for 1 h at room temperature following washes in TBST. Peroxidase activity was revealed using 3,3-diaminobenzidine (Thermo Scientific, PI) as a substrate. The developed blots were subjected to densitometric analysis by ImageJ software (free software developed by NIH) and the ratio of intensity of the band to that of the internal control β-actin was taken for comparison among groups.
Statistical analysis
Statistical analysis for semen parameters was done using Wilcoxon Rank-Sum test and result was considered significant at P < 0.05. The densitometric data of expression levels of proteins were analyzed by one-way analysis of variance (ANOVA) followed by Duncan's New Multiple range test, with differences considered significant at P < 0.05.
RESULTS
Varicocele and semen parameters
Of the 50 patients diagnosed with varicocele, 66% (33/50) presented with unilateral varicocele and 34% with bilateral varicocele (17/50). The majority of the varicocele patients had left-sided varicocele (72.5%) compared to right side varicocele (27%). Grade 1 varicocele was seen in a higher percentage of patients with left side varicocele (44.4%) or right side varicocele (82.4%) and grade 2 was seen in 28.9% of left side varicocele and 17.6% with right side varicocele. Compared to the fertile group, sperm concentration (×106 ml−1) (69.90 ± 37.55 vs 29.49 ± 33.22; P < 0.002), motility (%) (57.1 ± 16.0 vs 41.3 ± 18.4; P < 0.023), and strict morphology (%) (8.4 ± 3.4 vs 2.4 ± 1.9; P < 0.001) were significantly poor in the varicocele group. Significantly higher levels of ROS (RLU/s/106 sperm; median [25th, 75th percentile values; 142.7 (36.2, 337.7) vs 896.1 (165.6, 2990.5), (P < 0.008)]) and DNA fragmentation (%) (range: 8.5–41.7 vs 3.3–48.3; P < 0.009) were seen in the varicocele group.
Global proteomic profiling and abundance of proteins
For the global protein profiling, each pooled sample from control, unilateral, and bilateral groups was run in triplicate to minimize biological and technical variation. For the fertile control group, 1055, 1010, and 1042 proteins were identified in the 3 LC-MS runs, respectively. On the other hand, 795, 713, and 763 proteins were detected for unilateral varicocele group and while a total of 1024, 999, and 1017 proteins were identified in bilateral varicocele group. Thus, after filtering the missing values, a total of 1244, 933, and 1230 proteins were reported for fertile donor and infertile unilateral and bilateral varicocele patients, respectively (Figure 2a).
Figure 2.
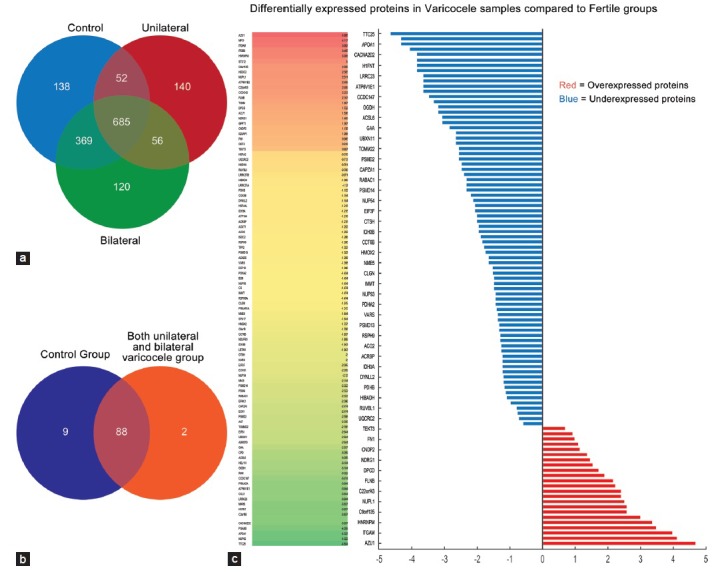
Venn diagram. (a) Distribution of global proteins in fertile, unilateral and bilateral varicocele group and (b) differentially expressed proteins in fertile and unilateral and bilateral varicocele group. (c) Heat map showing the DEP proteins that were overexpressed or underexpressed in varicocele group.
Differentially expressed proteins in fertile and varicocele group
In this study, the goal was to identify proteins that were differentially expressed in infertile varicocele men to those in fertile men. We also examined proteins involved in key functions related to spermatogenesis, sperm motility, and mitochondrial dysfunction.
Of the 1250 proteins identified by global proteomics, 99 were differentially expressed proteins (DEPs). The majority of the DEP (87.5%, 77/88) were underexpressed in patients with varicocele. Only 12.5% of the DEP were overexpressed. Of these, 88 were common to both fertile and the varicocele group as shown in the Venn diagram (Figure 2b). The heat map generated for the DEP that were overexpressed or underexpressed is shown in Figure 2c.
Classification of the differentially expressed proteins
Functional annotations and enrichment were examined using GO Term Finder, GO Term Mapper, UniProt, DAVID, STRAP and Reactome for the DEP in the fertile and the varicocele group is shown in Supplementary Table 1 (68.4KB, pdf) . DAVID functional annotation analysis revealed that both underexpressed and overexpressed proteins were located in the mitochondria, cytosol, organelle membranes, nuclear pore complex, and the cAMP-dependent protein complex. Metabolism including energy metabolism, metabolism of the amino acids, pyruvate and TCA cycle, lipid and lipoprotein, fatty acid metabolism, and oxidative phosphorylation was significantly affected in varicocele group as a result of the underexpressed proteins. Overexpressed proteins affected the integrin-mediated signaling pathway in addition to integrin-cell surface interaction and adhesions. Underexpressed proteins also influenced biological processes such as generation of precursor metabolites, oxidation-reduction associated processes, spermatogenesis, sperm development/differentiation, and germ cell development. Overexpressed proteins affected cell motion, chemotaxis, cell activation, and inflammatory response. Key molecular functions affected by underexpressed proteins were nucleotide and purine nucleotide binding, nucleoside binding as well as ATP binding, peptidase activity, and to a smaller extent kinase regulatory activity and unfolded protein binding. Overexpressed proteins were involved in carbohydrate metabolism, polysaccharide binding, and glycoprotein binding.
Summary data of all DEP in varicocele group compared to the fertile group highlighting protein abundance, expression (overexpressed, underexpressed, and unique) and enriched pathways with respect to related proteins as revealed by GO, STRAP, DAVID, and reactome analysis
Underexpressed proteins were shown to be involved in spermatogenesis, sperm motility, mitochondrial dysfunction, metabolism of nucleotides, and fatty acid metabolism (Supplementary Table 2 (32.3KB, pdf) ). Overexpressed proteins were involved in the formation of cellular protrusions, cellular compromise, and loss of phosphatidyl-ionositol-4-5 bisphosphate in varicocele group.
Proteins involved in key functions that are underexpressed and may contribute to sperm dysfunction or in the development of varicocele as revealed by GO, STRAP, DAVID and reactome analysis
Functional categories identified by IPA for overexpressed and underexpressed DEP are shown in Supplementary Table 1 (68.4KB, pdf) . In varicocele group, the majority of the DEP (87.5%) were underexpressed. Nine proteins were unique to the fertile group, which included aspartate-rich protein 1 (DRICH1), nucleoporin p58/p45 (NUPL1), uncharacterized protein C9orf135 (C9orf135), coiled-coil domain-containing protein 42A (CCDC42), HD domain-containing protein 2 (HDDC2), protein DPCD (DPCD), V-proton ATPase subunit B brain isoform (ATP6V1B2), Heterogeneous nuclear ribonucleoprotein M (HNRNPM), and syntaxin-12 (STX12). The two proteins that were unique to the varicocele group were integrin alpha-M (ITGAM) and integrin beta-2 (ITGB2).
Proteins involved in major networks
Interaction network was generated using IPA for all key proteins in fertile and varicocele group. We identified two networks that may affect key sperm functions such as spermatogenesis, sperm motility, mitochondrial dysfunction, as well as nucleic acid, and fatty acid metabolism, which in turn might potentially affect the overall sperm quality in infertile men with varicocele. The first network involved 17 focus molecules that participated in the nucleic acid metabolism, small molecule biochemistry, and molecular transport and were underexpressed in varicocele group compared to fertile group (Figure 3a). Of these, only two proteins were overexpressed (NDRG1 and ODF2). Eleven proteins were identified in the second network that was involved in energy production, metabolism, lipid metabolism, and small molecule biochemistry. Of these, eight were underexpressed and three were overexpressed (Figure 3b).
Figure 3.
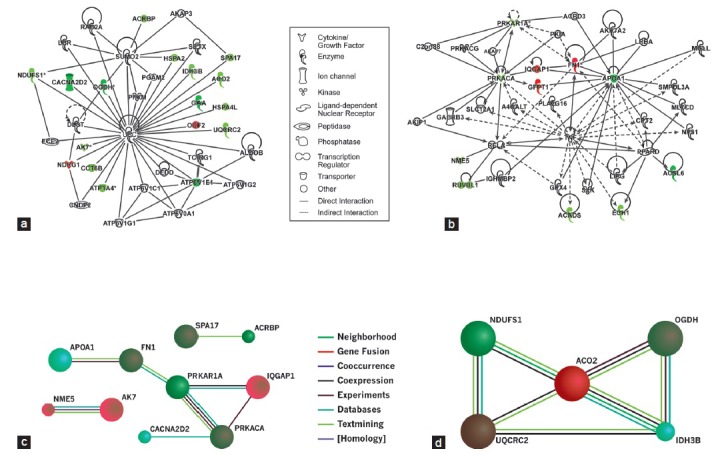
Interaction network generated using IPA for all key proteins in varicocele group compared to fertile group. (a) Nucleic acid metabolism, small molecule biochemistry and molecular transport and (b) energy production, lipid metabolism and small molecule biochemistry and STRING database showing evidence of functional link between protein-protein interactions among (c) spermatogenesis, sperm motility and (d) mitochondrial dysfunction.
Further, we identified protein-protein interaction network using STRING database to identify the key functions that were underexpressed in varicocele group that may contribute to the onset of varicocele and ultimately to sperm dysfunction. These interactions are derived through high-throughput experiments, co-array experiments, and PubMed or other public data sources. The interaction network for all key proteins shows their involvement in reproductive functions such as spermatogenesis, sperm motility, and mitochondrial dysfunction (Figure 3c and 3d).
Validation of expression profile of proteins by Western blotting
From the list of proteins, 5 proteins were selected for validation studies based on their role in stress response and regulation of structural and functional integrity of the spermatozoa. Of the 5 proteins, 4 (PKAR1A, AK7, CCT6B, and HSPA2) were shown to be downregulated while 1 (ODF2) was upregulated by NASF ratio. Expression of these proteins of interest was validated by Western blot densitometric analysis using β-actin as an internal control (Figure 4).
Figure 4.
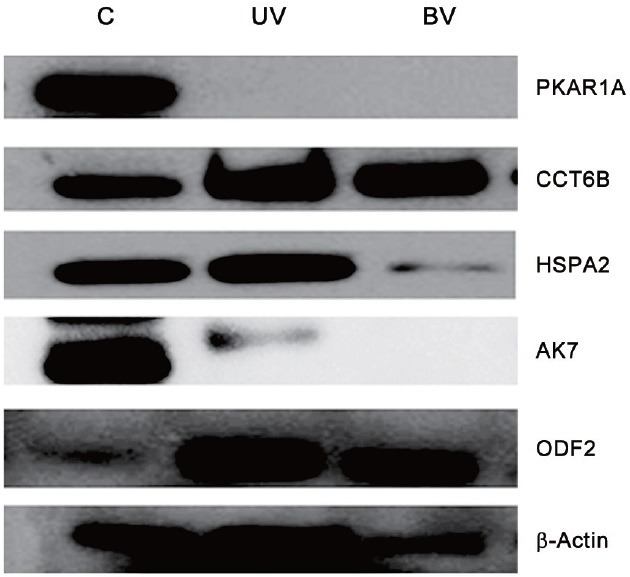
Representative immunoblot of expression profile of selected proteins (n = 5 per group run in duplicate).
DISCUSSION
Although many differentially expressed proteins in the spermatozoa involved in sperm motility, sperm capacitation, and fertilization have been identified,34,35,36 studies on varicocele-related male infertility are far from conclusive in narrowing down the potential candidate biomarkers of varicocele-associated infertility.16,17,18,37,38 This challenge is mainly attributed to the selection criteria of the study subjects, instrumentation, and techniques, as well as the available software and search engines utilized in conducting these studies. While some of these shortcomings can be overcome using the LC-MS/MS technique,36 some of the integral membrane proteins have been identified using other techniques.39
In our recent studies, we have utilized a robust LC-MS Orbitrap system and identified differentially expressed proteins in men with unilateral varicocele compared to fertile men,21 bilateral varicocele compared to fertile men,22 and differentially expressed proteins in infertile men with unilateral and bilateral varicocele.23 Comparative analyses of the unilateral varicocele and the fertile group identified 29 proteins that were involved in key reproductive functions.21 Some of the key proteins associated with fertility related functions that were underexpressed were AKAP3, APOPA1, SEMG1, ACR, SPA17, and DNAH17. Proteins such as GSTM3, TGM4, ODF2, HIST1H2BA, and PARK7 were overexpressed. CRISP-2 and ARG2 were uniquely expressed in the unilateral varicocele group only.21
In bilateral varicocele and fertile group comparison, majority of the proteins were involved in metabolic processes, stress response, and oxidoreductase activity. Key proteins involved in sperm function that were overexpressed were TEKT3, TCP11, and TGM4, whereas CLMGN, TOM22 were underexpressed and may attribute to infertility in these patients.22 We next compared infertile men with unilateral and bilateral varicocele to identify the DEP among the two varicocele groups. This was important to help understand how the protein expression is altered in a specific diagnosis of varicocele, either unilateral, or bilateral.23 We identified 21 DEP that were involved in key reproductive functions. GSTM3, SPANXB1, PARK7, PSM8, DLD, SEMG1, and SEMG2 were the candidates of interest and likely to be involved in varicocele development and infertility.23
In the present study, we identified two proteins (ITGAM and ITGB2) that were unique to varicocele group (Supplementary Table 1 (68.4KB, pdf) ). ITGAM (integrin alpha-M) is implicated in various adhesive interactions of various types of cells and molecules. Adhesion molecules such as integrin, fibronectin, and ADAMS are involved in spermatogenesis and gamete interactions. Varying expression of integrin is associated with the release of spermatids into the tubule lumen.40 Most of the known steps in leukocyte trafficking across the vascular endothelium bear resemblance with the intracellular reactions in spermatogenesis and sperm-oocyte apposition involving protein families such as integrin.40
Integrin β (CD11b/CD18) is the primary adhesive glycoprotein complex involved in neutrophil-mediated immune injury to the spermatozoa.41 These have been shown to be associated with ROS-mediated destruction of motile sperm by neutrophils that adhere to the sperm. The presence of these two integrin proteins only in varicocele patients also suggests the potential role of these two adhesion molecules and presence of activated neutrophil-associated with production of ROS in varicocele men. They can serve as a biomarker for neutrophil-mediated immune injury to spermatozoa and subsequent onset of infertility in these men.
In fertile group, nine unique proteins were identified as mentioned above. Studies suggest that these proteins may have novel reproductive functions, not previously established in fertile men. Among them, nucleoporin is a component of the nuclear pore complex and constitutes a large supramolecular assembly that mediates macromolecular trafficking across the nuclear envelope involving interactions between cytosolic transport factors and nuclear pore complex proteins.42 V-ATPase is involved in luminal acidification in the epididymis where an acidic environment is essential to keep the sperm in a dormant immotile state and create an optimal acidic environment critical for male fertility.43 Secretory vesicles are used during spermatogenesis to deliver proteins to the cell surface. The acrosome is considered an acidic secretory vesicle containing hydrolytic enzymes that are involved in the passage of the sperm across the zona pellucida. The assembly of the proton pump is an important step for the biogenesis of the acrosome.44
HNRNP M is an abundant heterogeneous nuclear ribonucleoprotein responsible for spliceosome mediated constitutive and alternative splicing.45 Its ability not only includes exon skipping but also promoting exon inclusion.46 It plays a role in the generation of varied proteins and small regulatory RNAs from heterogeneous RNA. The unique presence of HNRNP M in fertile men might be responsible for the production of new proteins and small nuclear RNAs required for successful fertilization and embryo development. Syntaxin-12 is a member of syntaxin SNARE (Soluble NSF Attachment Protein Receptor) family of proteins that acts to regulate protein transport between late endosomes and the trans-Golgi network. Sperm use the SNARE fusion machinery and regulatory components as characterized for other secretory events.47
Increasing evidence has shown that a-SNAP/NSF, Rab3A, toxin-sensitive members of SNARE family, and interaction of complexin/synaptotagmin are required in Ca2+ -triggered acrosome exocytosis before intra-acrosomal Ca2+ efflux of the acrosome exocytosis.48,49,50 Therefore, their absence in varicocele patients might be the causative factor for impaired acrosomal reaction leading to fertilization failure. To corroborate this fact, we found acrosin binding protein (ACRBP) and voltage-dependent calcium channel subunit alpha-2/delta-2 (CACNA2D) to be underexpressed in varicocele patients. CACNA2D is the alpha-2/delta subunit of voltage-dependent calcium channels regulating calcium current density and is highly expressed in the testis. ACRBP can bind to proacrosin zymogen and delay its maturation.51 During fertilization, ACRBP regulates autoactivation of proacrosin to the mature isoforms of acrosin, and thereby accelerating the release of acrosomal commitments during acrosome reaction.52 The underexpression of this protein reflects that some of the otherwise normal appearing spermatozoa in varicocele men may have compromised the ability of undergoing acrosome reaction, which is a prerequisite for natural fertilization.
We generated two interaction networks using ingenuity pathway analysis for all key proteins identified to be differentially expressed in varicocele group (Figure 3a and 3b). In the first network, these molecules were associated with the nucleic acid metabolism, small molecule biochemistry, and molecular transport. Of the 17 molecules identified participating in this network, 15 were underexpressed and 2 were overexpressed. The underexpressed molecules participated in energy metabolism, molecular chaperone, ion channel transport, kinase and peptidase activities, or functioned as transport molecules.
Varicocele is characterized by low blood flow into the testis resulting in a hypoxic and hypothermic state. The hypoxic state is evidenced in the present study by the underexpression of key mitochondrial electron transport complex proteins, namely, Complex-I enzyme NADH-ubiquinone oxidoreductase (NDUFS1, 75 kDa subunit) and Complex-III Cytochrome b-c1 complex subunit 2 (UQCRC2). This condition is further substantiated by detection of underexpressed enzymes of the Krebs cycle such as Aconitate hydratase (ACO2), Isocitrate dehydrogenase (NAD) subunit beta (IDH3B), and 2-oxoglutarate dehydrogenase (OGDH). Thus, the spermatozoa from infertile varicocele men are basically in an energy-deprived state which may be responsible for the decreased motility and DNA damage as reported previously.3,9,34,53 In fact, mitochondrial Complex-III is suggested to be the oxygen sensor during hypoxic condition and generation of ROS54 and a single, mild, transient scrotal heat stress is reported to cause hypoxia and oxidative stress in mouse testes leading to germ cell death.55 The lysosomal enzyme alpha-glucosidase (GAA) is found to be underexpressed in infertile patients with varicocele. It is essential for degradation of glycogen to glucose in the lysosomes. Varicocele has been reported to cause a decrease in epididymal neutral alpha-glucosidase suggesting its epididymal dysfunction and possible association with sperm membrane damage, impaired sperm quality, and increased DNA fragmentation.56,57
Reduced levels of alpha-glucosidase are also associated with oxidative stress and infection resulting in increased DNA fragmentation. Varicocele-induced reduced levels of alpha 1, 4 glucosidase have also been reported.58 Similarly, sodium/potassium-transporting ATPase subunit alpha-4 (ATP1A4), the catalytic component of the active enzyme which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane is underexpressed in varicocele group. It provides energy for active transport of various nutrients and plays a role in sperm motility. It is specifically expressed in the testis and mature sperm at the protein level and essential for germ cell gene expression (sperm with deficiency show bent tail), abnormal ion regulation, reduced motility, and hyperactivation, which is necessary for capacitation.59 We have also demonstrated this protein in unilateral varicocele patients.21 Therefore, it is within reason to speculate that spermatozoa from varicocele patients, which are essentially in a hypoxic-hyperthermic state, have impaired motility and harbored DNA damage, in conformity with our previous findings.21,22,23 In this study, we also report dysregulation of proteins involved in sperm motility and sperm chromatin compaction.
Molecular chaperons are the best sensors of intrinsic and extrinsic environmental stress in a cell. HSPA4L encodes a heat shock protein that belongs to the HSP 110 family. In somatic cells, HSPA4L is inducible by heat shock at temperatures of 32–39°C. They can also be induced by chemical or physical stress, viral infection, drugs, and transforming agents where they confer cytoprotective effects by maintaining protein homeostasis and blocking the caspase-dependent apoptosis.60,61 They act by refolding of denatured proteins and prevent the adverse metabolic effects occurring as a result of the accumulation of misfolded proteins. Under normal conditions, they are constitutively present and function in the folding, trafficking, and translocation of proteins across the membranes.62
We recently have demonstrated low expression of this protein in spermatozoa of men with ROS higher than the physiological levels (low, medium, and high ROS levels) suggesting disruption of the spermatogenesis process, resulting in ROS-induced infertility.63 Differential regulation of HSP has also been reported in infertile men with varicocele. Hosseinifar et al. also reported underexpression of HSPA5, but overexpression of HSP70, HSP90 in infertile men with varicocele.16 Increased expression of HSP79, HSP90A4, and heat shock factors HSF1, HSF2 was reported in both men with varicocele and oligozoospermia.18 HSPA2 was downregulated in ejaculated sperm of men with oligozoospermia37 and re-expressed at a higher level after varicocelectomy.38 The presence of HSP chaperones on the sperm surface is suggestive of their multifunctional roles, one of which is critical for sperm-zona pellucida binding.61 Besides HSPs, T-complex protein 1 subunit zeta-2 (CCT6B) is another molecular chaperone involved in protein (actin and tubulin) folding mediated by cytoplasmic chaperon in containing TCP-1, which is highly expressed in the testis. It plays an important role in the cytoskeletal organization during spermatogenesis. It is also confined to nuclear heterochromatin and associated with nuclear compaction of the spermatozoa.64
In fact, a defective organization of the flagellum is evidenced by the overexpression of the outer dense fiber protein 2 (ODF2). ODF2 is the major cytoskeleton protein of the sperm tail. It may contribute to assorted ciliopathies. Developmental defects of ODF will cause severe tail abnormalities resulting in abnormal sperm motility, morphology, and infertility. This protein was also identified in unilateral and bilateral varicocele patients.22 Studies have reported increased presence of ODF and failed fertilization in IVF65 and asthenozoospermic men.66 Another protein adenylate kinase 7 (AK7) involved in maintaining ciliary structure and function67 is also underexpressed in varicocele group. SPA17 is localized in the fibrous sheath of the sperm flagellum. Its presence from ejaculation to oocyte fertilization indicates its involvement in the regulation of postmaturation processes such as capacitation, acrosomal reaction, and sperm-oocyte binding during fertilization.68 Underexpression of this protein and its presence in low abundance may explain why men with varicocele have poor sperm concentration and motility. We also reported this protein in the unilateral varicocele patients.21
N-myc downstream-regulated protein 1 (NDRG1) is a stress-responsive protein involved in hormone responses, cell growth, and differentiation induced by hypoxia and DNA damage. NDRG1 is overexpressed in varicocele group. Apart from its role in stress signaling, it also plays a role in vesicular trafficking.69 V-type proton ATPase subunit E 1 (ATP6V1EJ) is a subunit of the peripheral V1 complex of vacuolar ATPase essential for assembly or catalytic function. A decreased level of a2 vacuolar ATPase, a member of the V-type ATPase could be used as a marker for infertility.
The second network identified was involved in lipid metabolism and small molecule biochemistry. In this network, we identified 11 proteins, of which 8 were underexpressed and 3 were overexpressed. Three enzymes related to fatty acid oxidation, namely, long chain fatty acid-CoA ligase 6 (ACSL6), short-chain specific acyl-CoA dehydrogenase (ACADS), and Delta(3,5)-Delta(2,4)-dienoyl-CoA isomerase (ECHI) are underexpressed in varicocele group pointing toward energy deprivation for the spermatozoa in varicocele patients. Similarly, apolipoprotein A-I (APOA1) involved in lipid transport is underexpressed. It activates sperm motility.70,71 We also identified this protein in the unilateral varicocele patients.21 The low expression of this enzyme also reflects poor motility as seen in varicocele patients. Increased nitrotyrosine is reported in the blood plasma collected from spermatic vein of adolescent varicocele patients.72
Nucleoside diphosphate kinase homolog 5 (NME5) confers protection from cell death by Bax and alters the cellular levels of several antioxidant enzymes including GPX5. It may play a role in spermiogenesis by increasing the ability of late-stage spermatids to eliminate ROS.73 NME5 is also involved in purine metabolism and is uniquely expressed in the fertile group only. Lack of NEM5 in varicocele patients shows that the varicocele patients are at a disadvantage, as they are likely to be more susceptible to apoptosis and have inadequate antioxidant protection. We also demonstrated the presence of NEM5 in infertile men with unilateral varicocele.21
cAMP-dependent protein kinase catalytic subunit alpha (PRKAC) and cAMP-dependent protein kinase type I-alpha regulatory subunit (PRKAR1A) are regulatory subunits of the cAMP-dependent protein kinases involved in cAMP signaling in cells. Heterozygotes for PRKAR1A were reported to be infertile and had lower sperm count with morphologically aberrant spermatozoa.74
RuvB-like 1 (RUVBL1) is expressed essentially in germ cells.75 Reduced expression in spermatozoa of varicocele patients indicates defective chromatin packaging during their development and maturation.
Ras GTPase-activating-like protein IQGAP1 (IQGAP1) is a novel vascular endothelial growth factor receptor type 2 (VGEFR2) binding protein.76 It is a critical regulator of VEGF-induced ROS production linked to endothelial cell migration by interacting with gp 91 phox (NOX2). Thus, its role in ROS-related injury/sperm damage in varicocele patients due to ischemia/hypoxia cannot be ruled out.
Glutamine: fructose 6 phosphate amidotransferase (isomerizing) 1 (GFPT1) controls the flux of glucose into the hexosamine pathway. It is a protein phosphorylated in glucose deprived cells.77 Since glycoproteins in sperm is involved in sperm function in the female reproductive tract and mediates interaction with zona pellucida,78 a hypo-glycosylated protein in varicocele patient may lead to decreased fertility power.
Fibronectins (FN1) could bind cell surfaces and various compounds including collagen, fibrin, heparin, DNA, and actin. Both semenogelin and fibronectin play an important role in coagulation. It is highly expressed on the surface of ejaculated spermatozoa and is a marker of human sperm maturation and plays an important role in sperm capacitation.79 Both Eppin and fibronectin bind together and are located in the postacrosomal and midpiece region of the sperm head. During ejaculation, it helps inhibit sperm capacitation, making initial ejaculated spermatozoa to become immotile. The Eppin-FN complex found on ejaculated spermatozoa also provides a protective shield before capacitation in the female reproductive tract. It also plays a role in sperm-oolemmal adhesions.79,80
The key interactions identified using STRING analysis were direct (physical) or indirect (functional) associations. ACRBP, SPA17, AK7, and NME5 were identified by STRING database and may play a critical role in spermatogenesis. Three of them (ACRBP, SPA17, and AK7) were involved in nucleic acid metabolism; small molecule biochemistry and molecule transport (Network 1). NME5 is involved in energy production, lipid metabolism, and small molecule biochemistry (Network 2). All of them were underexpressed in the varicocele group.
Oxoglutarate (alpha-ketoglutarate) dehydrogenase (OGDH), aconitase 2, mitochondrial (ACO2), ubiquinol-cytochrome c reductase core protein II (UQCRC2), and NADH dehydrogenase (ubiquinone) Fe-S protein 1 (NDUFS1) are associated with mitochondrial dysfunction. Three of these were involved in network 1 and were underexpressed. All these co-expressed proteins are associated with altered mitochondrial activity in the spermatozoa of infertile men with varicocele. This is a strong reason to associate spermatozoa mitochondrial dysfunction in these men with the onset, development, and progression of varicocele and eventual infertility in these men. Figure 3c and 3d show the evidence for interaction network for all key proteins associated with varicocele when compared with the fertile men. In addition to the proteins mentioned in the above interactions, four other proteins that participate in the overall interaction are APOA1, CACNA2D2, FN1, and IDH3B. Of these, all except FN1and IQGAP1 are underexpressed and involved in the two networks.
Many of the proteins identified in our previous study have not been reported in earlier studies on varicocele patients. At the same time, there are new proteins that have not been reported earlier in our studies. This is attributed to the study groups that are being compared, as these proteins may still be present but they may not be differentially expressed and, therefore, are not reported. The majority of the men in the unilateral as well as bilateral group had significantly poor semen parameters, especially sperm concentration and, therefore, could not be included for proteomic analysis. Because of the limited samples size for proteomic analysis, we did not group the varicocele patients in the proteomic analysis based on the grade of the varicocele. It will be important to examine the impact of varicocele grade on the differential expression of the proteins.
CONCLUSIONS
We have for the first time identified key proteins that are altered or modified in the presence of varicocele and result in male infertility. Infertile men with varicocele have a large number of spermatozoa proteins that are underexpressed compared to fertile men. The majority of the underexpressed proteins are involved in major energy metabolism pathways, transport, protein folding, and proton pumps. Furthermore, fertile men exhibit unique proteins that are completely missing in infertile men with varicocele. The underexpression of these proteins in varicocele men sheds important light on the development of varicocele and sperm dysfunction that ultimately results in male infertility. The results of the present study suggest that irrespective of the varicocele disease status, the condition essentially leads to a state of energy deprivation, hypoxia, and hyperthermia due to impaired blood supply as evidenced by the down-regulation of lipid metabolism, mitochondrial electron transport chain, and Krebs cycle enzymes. The hypoxia sensor Complex-III of ETC (Cytochrome b-cI complex subunit) is downregulated, suggesting hypoxia-induced ROS release. Key protein functions affected in varicocele group are spermatogenesis, sperm motility, and mitochondrial dysfunction. Validation of the DEP identified in this study further strengthens the hypothesis. This will also help the clinicians identify patients who are more likely to benefit from varicocelectomy and have improvement in sperm quality and, therefore, increase likelihood of a successful pregnancy.
AUTHOR CONTRIBUTIONS
DD and LS conducted the study and helped with the data collection and management of this study. RS helped with the writing, reviewing, and editing of the manuscript. AA conceived the idea, supervised the study, and edited the article for submission. ES helped with the enrollment of patients and review of the article. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declared that they have no competing interests.
ACKNOWLEDGMENTS
The authors are grateful to the Andrology Center technologists for scheduling the study subjects. Belinda Willard, Director, Proteomic Core Lab, Lerner Research Institute for providing assistance with proteomic analysis and Banu Gopalan, Lerner Research Institute with Bioinformatics data analysis. The Orbitrap Elite mass spectrometer used in this study was purchased with funds from an NIH shared instrument grant (No. 1S10RR031537-01) to Belinda Willard. Financial support was provided by the American Center for Reproductive Medicine, Cleveland Clinic. Luna Samanta thanks the University Grants Commission, India for the award of Raman Postdoctoral Fellowship.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committee of American Society for Reproductive Medicine. Report on varicocele and infertility. Fertil Steril. 2008;90:S247–9. doi: 10.1016/j.fertnstert.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006;12:630–3. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 4.Sheehan MM, Ramasamy R, Lamb DJ. Molecular mechanisms involved in varicocele-associated infertility. J Assist Reprod Genet. 2014;31:521–6. doi: 10.1007/s10815-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocuzza M, Cocuzza MA, Bragais FM, Agarwal A. The role of varicocele repair in the new era of assisted reproductive technology. Clinics. 2008;63:395–404. doi: 10.1590/S1807-59322008000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marmar JL. The pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Update. 2001;7:461–72. doi: 10.1093/humupd/7.5.461. [DOI] [PubMed] [Google Scholar]
- 7.Bertolla RP, Cedenho AP, Hassun Filho PA, Lima SB, Ortiz V, et al. Sperm nuclear DNA fragmentation in adolescents with varicocele. Fertil Steril. 2006;85:625–8. doi: 10.1016/j.fertnstert.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Blumer CG, Fariello RM, Restelli AE, Spaine DM, Bertolla RP, et al. Sperm nuclear DNA fragmentation and mitochondrial activity in men with varicocele. Fertil Steril. 2008;90:1716–22. doi: 10.1016/j.fertnstert.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril. 2011;96:1283–7. doi: 10.1016/j.fertnstert.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Libman J, Jarvi K, Lo K, Zini A. Beneficial effect of microsurgical varicocelectomy is superior for men with bilateral versus unilateral repair. J Urol. 2006;176:2602–5. doi: 10.1016/j.juro.2006.07.161. [DOI] [PubMed] [Google Scholar]
- 11.Hamada A, Sharma R, du Plessis SS, Willard B, Yadav SP, et al. Two-dimensional differential in-gel electrophoresis-based proteomics of male gametes in relation to oxidative stress. Fertil Steril. 2013;99:1216–26. doi: 10.1016/j.fertnstert.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 12.Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013a;11:85. doi: 10.1186/1477-7827-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma R, Agarwal A, Mohanty G, Hamada AJ, Gopalan B, et al. Proteomic analysis of human spermatozoa proteins with oxidative stress. Reprod Biol Endocrinol. 2013;11:8. doi: 10.1186/1477-7827-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amaral A, Paiva C, Attardo Parrinello C, Estanyol JM, Ballescà JL, et al. Identification of proteins involved in human sperm motility using high-throughput differential proteomics. J Proteome Res. 2014;13:5670–84. doi: 10.1021/pr500652y. [DOI] [PubMed] [Google Scholar]
- 15.Légaré C, Droit A, Fournier F, Bourassa S, Force A, et al. Investigation of male infertility using quantitative comparative proteomics. J Proteome Res. 2014;13:5403–14. doi: 10.1021/pr501031x. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinifar H, Gourabi H, Salekdeh GH, Alikhani M, Mirshahvaladi S, et al. Study of sperm protein profile in men with and without varicocele using two-dimensional gel electrophoresis. Urology. 2013;81:293–300. doi: 10.1016/j.urology.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Chan CC, Sun GH, Shui HA, Wu GJ. Differential spermatozoal protein expression profiles in men with varicocele compared to control subjects: upregulation of heat shock proteins 70 and 90 in varicocele. Urology. 2013;81:1379.e1–8. doi: 10.1016/j.urology.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Ferlin A, Speltra E, Patassini C, Pati MA, Garolla A, et al. Heat shock protein and heat shock factor expression in sperm: relation to oligozoospermia and varicocele. J Urol. 2010;183:1248–52. doi: 10.1016/j.juro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Camargo M, Intasqui Lopes P, Del Giudice PT, Carvalho VM, Cardozo KH, et al. Unbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomy. Hum Reprod. 2013;28:33–46. doi: 10.1093/humrep/des357. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinifar H, Sabbaghian M, Nasrabadi D, Modarresi T, Dizaj AV, et al. Study of the effect of varicocelectomy on sperm proteins expression in patients with varicocele and poor sperm quality by using two-dimensional gel electrophoresis. J Assist Reprod Genet. 2014;31:725–9. doi: 10.1007/s10815-014-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A, Sharma R, Durairajanayagam D, Ayaz A, Cui Z, et al. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod Biol Endocrinol. 2015;13:7. doi: 10.1186/s12958-015-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal A, Sharma R, Durairajanayagam D, Cui Z, Ayaz A, et al. Spermatozoa protein alterations in infertile men with bilateral varicocele. Asian J Androl. 2015 May 22; doi: 10.4103/1008-682X.153848. doi: 10.4103/1008-682X.153848. Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal A, Sharma R, Durairajanayagam D, Cui Z, Ayaz A, et al. Differential proteomic profiling of spermatozoal proteins of infertile men with unilateral or bilateral varicocele. Urology. 2015;85:580–3. doi: 10.1016/j.urology.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 24.5th ed. Switzerland, Geneva: World Health Organization Press; 2010. WHO. World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 25.Kashou AH, Sharma R, Agarwal A. Assessment of oxidative stress in sperm and semen. Methods Mol Biol. 2013;927:351–61. doi: 10.1007/978-1-62703-038-0_30. [DOI] [PubMed] [Google Scholar]
- 26.Sharma RK, Sabanegh E, Mahfouz R, Gupta S, Thiyagarajan A, et al. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology. 2010;76:1380–6. doi: 10.1016/j.urology.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 28.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner M. A biologist's view of the Drosophila genome annotation assessment project. Genome Res. 2000;10:391–3. doi: 10.1101/gr.10.4.391. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wen Z, Washburn MP, Florens L. Refinements to label free proteome quantitation. How to deal with peptides shared by multiple proteins. Anal Chem. 2010;82:2272–81. doi: 10.1021/ac9023999. [DOI] [PubMed] [Google Scholar]
- 31.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–24. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 32.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, et al. GO: TermFinder-open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–5. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatia VN, Perlman DH, Costello CE, McComb ME. Software tool for researching annotations of proteins: open-source protein annotation software with data visualization. Anal Chem. 2009;81:9819–23. doi: 10.1021/ac901335x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naaby-Hansen S, Flickinger CJ, Herr JC. Two-dimensional gel electrophoretic analysis of vectorially labelled surface proteins of human spermatozoa. Biol Reprod. 1997;56:771–87. doi: 10.1095/biolreprod56.3.771. [DOI] [PubMed] [Google Scholar]
- 35.Baker MA, Reeves G, Hetherington L, Muller J, Baur I, et al. Identification of gene products present in Triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis. Proteomics Clin Appl. 2007;1:524–32. doi: 10.1002/prca.200601013. [DOI] [PubMed] [Google Scholar]
- 36.Brewis IA, Gadella BM. Sperm surface proteomics: from protein lists to biological function. Mol Hum Reprod. 2010;16:68–79. doi: 10.1093/molehr/gap077. [DOI] [PubMed] [Google Scholar]
- 37.Lima SB, Cenedeze MA, Bertolla RP, Filho PA, Oehninger S, et al. Expression of the HSPA2 gene in ejaculated spermatozoa from adolescents with and without varicocele. Fertil Steril. 2006;86:1659–63. doi: 10.1016/j.fertnstert.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 38.Yeºilli C, Mungan G, Seçkiner I, Akduman B, Açikgöz S, et al. Effect of varicocelectomy on sperm creatine kinase, HspA2 chaperone protein (creatine kinase-M type), LDH, LDH-X, and lipid peroxidation product levels in infertile men with varicocele. Urology. 2005;66:610–5. doi: 10.1016/j.urology.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 39.Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, et al. Proteomics-based study on asthenozoospermia: differential expression of proteasome alpha complex. Mol Hum Reprod. 2010;16:452–62. doi: 10.1093/molehr/gaq009. [DOI] [PubMed] [Google Scholar]
- 40.Preissner KT, Bronson RA. The role of multifunctional adhesion molecules in spermatogenesis and sperm function: lessons from hemostasis and defense? Semin Thromb Hemost. 2007;33:100–10. doi: 10.1055/s-2006-958468. [DOI] [PubMed] [Google Scholar]
- 41.D’Cruz OJ, Haas GG., Jr Beta 2-integrin (CD11b/CD18) is the primary adhesive glycoprotein complex involved in neutrophil-mediated immune injury to human sperm. Biol Reprod. 1995;53:1118–30. doi: 10.1095/biolreprod53.5.1118. [DOI] [PubMed] [Google Scholar]
- 42.Hu T, Guan T, Gerace L. Molecular and functional characterization of the p62 complex, an assembly of nuclearporecomplex glycoproteins. J Cell Biol. 1996;134:589–601. doi: 10.1083/jcb.134.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shum WW, Da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol. 2009;212:1753–61. doi: 10.1242/jeb.027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun-Wada GH, Imai-Senga Y, Yamamoto A, Murata Y, Hirata T, et al. A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification. J Biol Chem. 2002;277:18098–105. doi: 10.1074/jbc.M111567200. [DOI] [PubMed] [Google Scholar]
- 45.Llères D, Denegri M, Biggiogera M, Ajuh P, Lamond AI. Direct interaction between hnRNP-M and CDC5L/PLRG1 proteins affects alternative splice site choice. EMBO Rep. 2010;11:445–51. doi: 10.1038/embor.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hovhannisyan RH, Carstens RP. Heterogeneous ribonucleoprotein m is a splicing regulatory protein that can enhance or silence splicing of alternatively spliced exons. J Biol Chem. 2007;282:36265–74. doi: 10.1074/jbc.M704188200. [DOI] [PubMed] [Google Scholar]
- 47.Tomes CN, De Blas GA, Michaut MA, Farré EV, Cherhitin O, et al. alpha-SNAP and NSF are required in a priming step during the human sperm acrosome reaction. Mol Hum Reprod. 2005;11:43–51. doi: 10.1093/molehr/gah126. [DOI] [PubMed] [Google Scholar]
- 48.Michaut M, Tomes CN, De Blas G, Yunes R, Mayorga LS. Calcium-triggered acrosomal exocytosis in human spermatozoa requires the coordinated activation of Rab3A and N-ethylmaleimide-sensitive factor. Proc Natl Acad Sci USA. 2000;97:9996–10001. doi: 10.1073/pnas.180206197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roggero CM, De Blas GA, Da H, Tomes CN, Rizo J, et al. Complexin/synaptotagmin interplay controls acrosomal exocytosis. J Biol Chem. 2007;282:26335–43. doi: 10.1074/jbc.M700854200. [DOI] [PubMed] [Google Scholar]
- 50.Yunes R, Michaut M, Tomes C, Mayorga LS. Rab3A triggers the acrosome reaction in permeabilized human spermatozoa. Biol Reprod. 2000;62:1084–9. doi: 10.1095/biolreprod62.4.1084. [DOI] [PubMed] [Google Scholar]
- 51.Baba T, Niida Y, Michikawa Y, Kashiwabara S, Kodaira K, et al. An acrosomal protein, sp32, in mammalian sperm is a binding protein specific for two proacrosins and an acrosin intermediate. J Biol Chem. 1994;269:10133–40. [PubMed] [Google Scholar]
- 52.Tardif S, Guyonnet B, Cormier N, Cornwall GA. Alteration in the processing of the ACRBP/sp32 protein and sperm head/acrosome malformations in proprotein convertase 4 (PCSK4) null mice. Mol Hum Reprod. 2012;18:298–307. doi: 10.1093/molehr/gas009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001;7:473–81. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]
- 54.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–19. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 55.Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80:913–9. doi: 10.1095/biolreprod.108.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vivas-Acevedo G, Lozano-Hernandez R, Camejo MI. Markers of accessory sex glands function in men with varicocele, relationship with seminal parameters. Can J Urol. 2011;18:5884–9. [PubMed] [Google Scholar]
- 57.Vivas-Acevedo G, Lozano-Hernández R, Camejo MI. Varicocele decreases epididymal neutral a-glucosidase and is associated with alteration of nuclear DNA and plasma membrane in spermatozoa. BJU Int. 2014;113:642–9. doi: 10.1111/bju.12523. [DOI] [PubMed] [Google Scholar]
- 58.Roaiah MM, Mostafa T, Salem D, El-Nashar AR, Kamel II, et al. Alpha-1,4-Glucosidase activity in infertile oligoasthenozoospermic men with and without varicocele. Andrologia. 2007;39:28–32. doi: 10.1111/j.1439-0272.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 59.Rodova M, Nguyen AN, Blanco G. The transcription factor CREMtau and cAMP regulate promoter activity of the Na, K-ATPase alpha4 isoform. Mol Reprod Dev. 2006;73:1435–47. doi: 10.1002/mrd.20518. [DOI] [PubMed] [Google Scholar]
- 60.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 61.Naaby-Hansen S, Herr JC. Heat shock proteins on the human sperm surface. J Rep Immunol. 2010;84:32–40. doi: 10.1016/j.jri.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–51. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Ayaz A, Agarwal A, Sharma R, Arafa M, Elbardisi H, et al. Impact of precise modulation of reactive oxygen species levels on spermatozoa proteins in infertile men. Clin Proteomics. 2015;12:4. doi: 10.1186/1559-0275-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kubota H, Hynes GM, Kerr SM, Willison KR. Tissue-specific subunit of the mouse cytosolic chaperonin-containing TCP-1. FEBS Lett. 1997;402:53–6. doi: 10.1016/s0014-5793(96)01501-3. [DOI] [PubMed] [Google Scholar]
- 65.Pixton KL, Deeks ED, Flesch FM, Moseley FL, Bjorndahl L, et al. Sperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case report. Hum Reprod. 2004;19:1438–47. doi: 10.1093/humrep/deh224. [DOI] [PubMed] [Google Scholar]
- 66.Zhao C, Huo R, Wang FQ, Lin M, Zhou ZM, et al. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril. 2007;87:436–8. doi: 10.1016/j.fertnstert.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 67.Panayiotou C, Solaroli N, Xu Y, Johansson M, Karlsson A. The characterization of human adenylate kinases 7 and 8 demonstrates differences in kinetic parameters and structural organization among the family of adenylate kinase isoenzymes. Biochem J. 2011;433:527–34. doi: 10.1042/BJ20101443. [DOI] [PubMed] [Google Scholar]
- 68.Chiriva-Internati M, Gagliano N, Donetti E, Costa F, Grizzi F, et al. Sperm protein 17 is expressed in the sperm fibrous sheath. J Transl Med. 2009;7:61. doi: 10.1186/1479-5876-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Askautrud HA, Gjernes E, Gunnes G, Sletten M, Ross DT, et al. Global gene expression analysis reveals a link between NDRG1 and vesicle transport. PLoS One. 2014;9:e87268. doi: 10.1371/journal.pone.0087268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brosens JJ, Hodgetts A, Feroze-Zaidi F, Sherwin JR, Fusi L, et al. Proteomic analysis of endometrium from fertile and infertile patients suggests a role for apolipoprotein A-I in embryo implantation failure and endometriosis. Mol Hum Reprod. 2010;16:273–85. doi: 10.1093/molehr/gap108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee YH, Choi SH, Lee KW, Kim DJ. Apolipoprotein B/A1 ratio is associated with free androgen index and visceral adiposity and may be an indicator of metabolic syndrome in male children and adolescents. Clin Endocrinol (Oxf) 2011;74:579–86. doi: 10.1111/j.1365-2265.2010.03953.x. [DOI] [PubMed] [Google Scholar]
- 72.Romeo C, Ientile R, Impellizzeri P, Turiaco N, Teletta M, et al. Preliminary report on nitric oxide-mediated oxidative damage in adolescent varicocele. Hum Reprod. 2003;18:26–9. doi: 10.1093/humrep/deg004. [DOI] [PubMed] [Google Scholar]
- 73.Munier A, Feral C, Milon L, Pinon VP, Gyapay G, et al. A new human nm23 homologue (nm23-H5) specifically expressed in testis germinal cells. FEBS lett. 1998;434:289–94. doi: 10.1016/s0014-5793(98)00996-x. [DOI] [PubMed] [Google Scholar]
- 74.Burton KA, McDermott DA, Wilkes D, Poulsen MN, Nolan MA, et al. Haploinsufficiency at the protein kinase A RI alpha gene locus leads to fertility defects in male mice and men. Mol Endocrinol. 2006;20:2504–13. doi: 10.1210/me.2006-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makino Y, Kanemaki M, Kurokawa Y, Koji T, Tamura TA. A rat RuvB-like protein, TIP49a, is a germ cell-enriched novel DNA helicase. J Biol Chem. 1999;274:15329–35. doi: 10.1074/jbc.274.22.15329. [DOI] [PubMed] [Google Scholar]
- 76.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, et al. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 77.Diekman AB, Norton EJ, Klotz KL, Westbrook VA, Shibahara H, et al. N-linked glycan of a sperm CD52 glycoform associated with human infertility. FASEB J. 1999;13:1303–13. doi: 10.1096/fasebj.13.11.1303. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, Roux C, Lazereg S, LeCaer JP, Laprévote O, et al. Identification of a novel serine phosphorylation site in human glutamine: fructose-6-phosphateamidotransferase isoform 1. Biochemistry. 2007;46:13163–9. doi: 10.1021/bi700694c. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X, Fang J, Xu B, Zhang S, Su S, et al. correlation of epididymal protease inhibitor and fibronectin in human semen. PLoS One. 2013;8:e82600. doi: 10.1371/journal.pone.0082600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diaz ES, Kong M, Morales P. Effect of fibronectin on proteasome activity, acrosome reaction, tyrosine phosphorylation and intracellular calcium concentrations of human sperm. Hum Reprod. 2007;22:1420–30. doi: 10.1093/humrep/dem023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary data of all DEP in varicocele group compared to the fertile group highlighting protein abundance, expression (overexpressed, underexpressed, and unique) and enriched pathways with respect to related proteins as revealed by GO, STRAP, DAVID, and reactome analysis
Proteins involved in key functions that are underexpressed and may contribute to sperm dysfunction or in the development of varicocele as revealed by GO, STRAP, DAVID and reactome analysis


