Abstract
Varicocele repair is mainly indicated in young adult patients with clinical palpable varicocele and abnormal semen parameters. Varicocele treatment is associated with a significant improvement in sperm concentration, motility, morphology, and pregnancy rate. Antegrade scrotal sclerotherapy (ASS) represented one of the main alternatives to the traditional inguinal or suprainguinal surgical ligation. This article reviews the use of ASS for varicocele treatment. We provide a brief overview of the history of the procedure and present our methods used in ASS. In addition, we review complication and success of ASS, including our own retrospective data of treating 674 patients over the last 17 years. Herein, we analyzed step by step the ASS technique and described our results with an original modified technique with a long follow-up. Between December 1997 and December 2014, we performed 674 ASS. Mean operative time was 14 min (range 9 to 50 min). No significant intraoperative complications were reported. Within 90 days from the procedure, postoperative complications were recorded in overall 49 (7.2%) patients. No major complications were recorded. A persistent/recurrent varicocele was detected in 40 (5.9%) cases. In 32/40 (80%) cases, patients showed preoperative grade III varicoceles. In patients with a low sperm number before surgery, sperm count improved from 13 × 106 to 21 × 106 ml−1 (P < 0.001). The median value of the percentage of progressive motile forms at 1 h improved from 25% to 45% (P < 0.001). Percentage of normal forms increased from 17% before surgery to 35% 1 year after the procedure (P < 0.001). In the subgroup of the 168 infertile patients, 52 (31%) fathered offspring at a 12-month-minimum follow-up. Therefore, ASS is an effective minimal invasive treatment for varicocele with low recurrence/persistence rate.
Keywords: antegrade scrotal sclerotherapy, male infertility, outcomes, varicocele
INTRODUCTION
Varicocele repair is mainly indicated in young adult patients with clinical palpable varicocele and abnormal semen parameters. Indeed, evidences demonstrate that varicocele treatment is associated with a significant improvement in sperm concentration, motility, and normal morphology.1 Moreover, recent randomized controlled trials (RCTs) and not RCTs showed potential advantages also in terms of pregnancy rate in patients who received varicocelectomy in comparison with observation.2
Several surgical techniques were described for varicocele repair. In the last decades, laparoscopic ligation, microsurgical varicocelectomy, percutaneous retrograde sclero-embolization, and antegrade scrotal sclerotherapy (ASS) represented the main alternatives to the traditional inguinal or suprainguinal surgical ligation. None of the previous treatments demonstrated to be superior to the others in terms of success and complication rates. Only in the last years, microsurgical repair and sclero-embolization techniques seem to be more effective than inguinal and suprainguinal ligation in terms of recurrence rates. However, nowadays surgeon's preference is still the most relevant criteria to choose the technique for varicocele treatment.
In this article, we review the use of ASS for varicocele treatment. We also describe our step-by-step technique. Finally, we report the clinical outcomes observed in a large series of consecutive patients treated by the senior author.
HISTORY OF ANTEGRADE SCROTAL SCLEROTHERAPY
ASS of spermatic veins was described for the first time in 1993 by Tauber and Johnsen.3 The technique initially described in German language was popularized only after the publication in 1994 of the results of initial 285 patients treated in Hamburg.4 In our experience, in December 1997 ASS progressively replaced the open and laparoscopic retroperitoneal ligature of spermatic veins. However, after an initial experience using the original technique described by Tauber and Johnsen in 1994,4 the senior author of this article started to use an original modified technique reporting a high percentage of success and a low percentage of complications in an initial series of 201 consecutive cases.5 Initial positive results were further confirmed in a large series of patients treated by 21 different surgeons in the same academic institution.6 Obviously during the time, several tips and tricks were learned and used to further improve the safety and the effectiveness of this technique.
STEP BY STEP SURGICAL TECHNIQUE
Position
The patient is placed supine in a slight anti-Trendelenburg position. Usually, we invite the patient to close the thighs. This little trick allows us having the scrotum in a more superficial and accessible position above all in more robust patients.
Anesthesia
All cases were treated in our experience with local anesthesia. Before the local anesthetic administration, we suggest grasping the spermatic cord between the index finger and thumb at the scrotal root level. Then, the vas is palpated, separated from the other spermatic cord structures and abandoned within the scrotum (Figure 1). Indeed, a potential tip could be represented by the vagal reaction due to vas traction during the spermatic cord palpation and maneuvers to administer the local anesthetic. This trick does not impact on the efficacy of the procedure because the veins of pampiniform plexus are entirely included in the portion of spermatic cord grasped between the fingers of the surgeon. This maneuver is not performed only in cases with recurrent or persistent varicocele in which the failure could be related to the presence of reflux into the deferential vein. About 5 ml of 2% mepivacaine is infiltrated superficially at level of the site of the incision and then into the surrounding area of the spermatic cord. We suggest avoiding the infiltration of the spermatic cord because this condition could be responsible for bleeding and edema in the operative site. In general, after few minutes the scrotal skin is ready to be incised.
Figure 1.
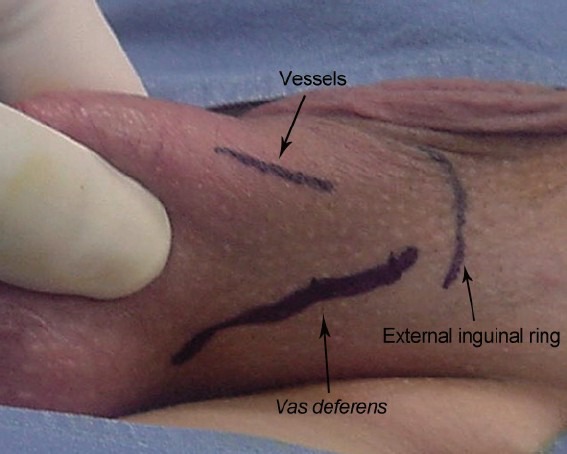
Spermatic cord is grasped, the vas is palpated, separated from the other spermatic cord structures and abandoned within the scrotum.
Incision
At the beginning of our surgeries, we performed a longitudinal, 2-cm incision at scrotal root level. However, the incision length can be further shortened until 1-cm. The level of the scrotal skin incision is very important, because a common mistake is represented by a lower incision exposing the spermatic cord in a segment in which the veins are smaller and more difficult to be incannulated. This condition will require a painful traction of the spermatic cord to expose bigger vein higher located. The spermatic cord is gently isolated and suspended using a Penrose drain (Figure 2). We recommend avoiding traction and/or compression of the spermatic cord to maintain an adequate flux within the veins of pampiniform plexus and facilitate their identification and isolation.
Figure 2.
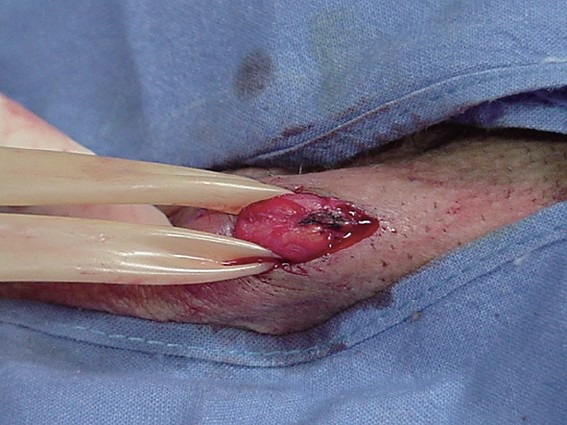
The vessels of spermatic cord are suspended using a Penrose drain.
Vein isolation and incannulation
After the incision of the vaginal fascia, dark yellow fat tissue covering the anterior portion of the pampiniform plexus is visible (Figure 3). The fat tissue is an important landmark to easily find the veins. Usually, more dilated veins are detectable. We suggest selecting the bigger and the more straight visible vein. In our experience, we clamp the selected vein and then we introduce into the selected vein a special 24-gauge venous catheter with a Y-adapter (Figure 4a).
Figure 3.
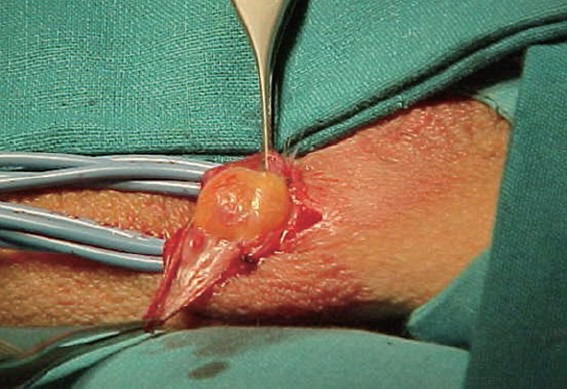
After the incision of vaginal fascia the dark yellow fat tissue is visible.
Figure 4.
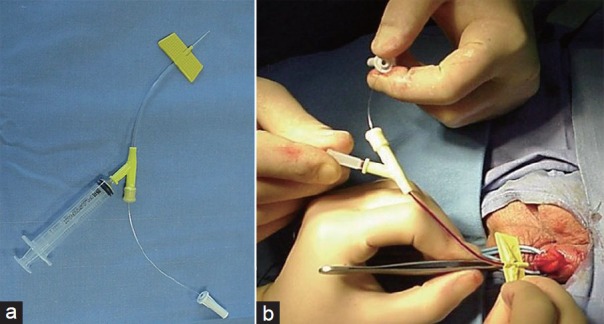
(a) 24-gauge venous catheter with a Y-adapter. (b) After the introduction of the needle into the vein, the mandrel is removed to avoid injuries of the vein wall and the soft segment of the catheter is pushed completely into the lumen of the vein.
The venous catheter comprises a 4-cm long distal portion with a butterfly thin-walled cannula, a 7-cm long flexible and transparent intermediate portion and a proximal portion with a two-way Y-adapter. One port is used to remove the fine mandrel and the second allows the infusion of contrast medium or sclerosant agent. The flexible part allows the second surgeon to move appropriately the catheter during the different steps of the procedure avoiding any conflict with the hands of the first surgeon. In details, the needle is inserted directly into the vein for few millimeters. Then, the mandrel is immediately removed to avoid injuries of the vein wall, and the soft segment of the catheter is pushed completely into the lumen of the vein (Figure 4a and 4b). Usually, a small clamp remains below the incannulation site and it is not necessary to fix the introduced needle. In our experience, this maneuver also allowed us incannulating small veins in patients with subclinical/grade I varicocele.
Antegrade phlebography
We still strongly support the use of antegrade phlebography before the administration of sclerosant agent. Antegrade phlebography is undertaken by infusing 4 ± 0.5 ml of contrast medium (Figure 5a and 5b). This step is fundamental to assess the correct positioning of the cannula into the pampiniform plexus. Moreover, the flow of contrast medium (iopamidolo) toward the renal vein on the left side and the vena cava on the right allows us to visualize the anatomy of the internal spermatic vein. Inviting patient to perform a feeble Valsalva maneuver during the injection of contrast medium, we can visualize small branches of main internal spermatic veins eventually present. We strongly recommended stopping the procedure when the contrasted veins do not reflect the normal anatomy or the most common variants of the internal spermatic veins. Indeed, the erroneous incannulation of superficial vein of spermatic cord or the presence of abnormal venous anastomosis could be responsible for relevant complications due to the occlusion of vein of other districts. This complication is rare but described in the literature and could happen in a necrosis of left colon.7 Moreover, in some cases, the flow of the well incannulated vein could be directed towards to the testicle and another puncture is necessary. Of course in this case, the antegrade phlebography is essential to avoid testicular damage.
Figure 5.
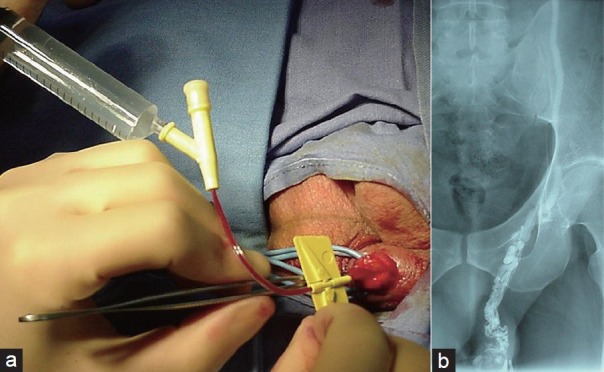
(a) Antegrade phlebography is undertaken by infusing 4 ± 0.5 ml of contrast medium. (b) Antegrade phlebography showing the correct position of the cannula into the pampiniform plexus.
Spermatic veins sclerotization
Before injecting the sclerosant agent into the vein, we recommend to clamp the spermatic cord using the Penrose drain. This maneuver prevents the potential and accidental testicular damage due to a reflux of sclerosant agent toward the testis. In our experience, the spermatic veins are sclerosed by introducing 1 ml of air followed by 4 ml of 3% ethoxysclerol (air-block technique). The air-block technique is commonly used to slow down the flow of sclerosant agent into the gonadal veins reducing the risk due to the passage into the renal vein and increasing the time of contact with the endothelium of the spermatic veins. Interestingly, the flexible portion of the catheter eases the administration of the sclerosant with the air-block technique, because the syringe tip can be directed upward, moving the air over the sclerosant solution. During the injection of sclerosant agent, which is performed in 4–5 s, the patient is asked to perform a weak Valsalva maneuver. The increased abdominal pressure opposes the downward flow of sclerosant into the spermatic vein system and hence there is a uniform and capillary diffusion into more collateral vessels. As previously described, to prevent reflux of the sclerosant towards the testis, the spermatic cord is clamped with a Penrose drain throughout the sclerosing phase and for the following 5 min. The injection of sclerosant does not need a radiological monitoring. Then, the cannula is immediately removed and the vein ligated below and above the point of injection. Finally, the spermatic cord fascia and the skin are closed with 4-0 Vicryl rapid.
Postoperative course
The procedure is usually performed in day surgery and patients can be discharged few hours after the end of the operation. Usually, we recommend the patients to avoid intensive physical activity increasing abdominal pressure for 7–10 days. Although no scientific evidence support this recommendation, we believe that avoiding relevant abdominal pressure during the early postoperative period can increase the chemical effect of the sclerosant agent within the spermatic vein. The follow-up schedule foresees the first visit 1 week after the procedure. Then, patients are invited to perform an eco-color Doppler of spermatic cord 3 months later and a seminal examination 6–9 months later.
CLINICAL DATA
Preoperative characteristics
In the framework between December 1997 and December 2014, senior author performed 674 ASS of spermatic vein in three different academic Institutions (Verona, Padova and Udine). Overall, 580 (86%) were left varicocele; 80 (12%) bilateral and 14 (2%) right. The treatment was performed in 67 (10%) adolescent patients and in 607 (90%) young adult men. All the adolescent patients showed a testicular hypotrophy and/or a high grade of reflux detected on color Doppler ultrasound. In adult patients, 380 (56%) cases presented a seminal impairment and 107 (16%) scrotal pain. One-hundred eighty-four (27%) cases were young adult not yet interested in fertility. Overall, the varicocele was subclinical in 7 (1%) cases, grade I in 101 (15%), grade II in 310 (46%) and grade III in 256 (38%) according to Dubin-Amelar classification.8
Perioperative data
Mean operative time was 14 min (range 9 to 50 min). No patient required intravenous sedation, or spinal or general anesthesia. Twenty-two (3.2%) patients had a vagal reaction during the procedure requiring intravenous atropine administration. In these cases, the procedure was normally completed without any further medical complication. The antegrade procedure failed in 6 (0.8%) cases because of difficulty in finding and/or catheterizing pampiniform veins. No significant intraoperative complications were reported. Patients were discharged home 4 h after surgery. Within 90 days from the procedure, postoperative complications were recorded in overall 49 (7.2%) patients. In details, we observed 12 (1.7%) slight hematoma spontaneously resolved, 9 (1.3%) infection treated with antibiotic therapy, 21 (3%) persistent scrotal pain requiring prolonged analgesic treatment. Moreover, one patient reported a persistent flank pain spontaneously resolved 2-month after the procedure and in two cases a wound infection was reported.
Follow-up data
All patients were evaluated with eco-color Doppler ultrasound. Only adult, preoperatively dyspermic patients performed a postoperative seminal examination. The first evaluation was performed at 3 months and the last evaluation was performed 1 year after the procedure.
In patients with a low sperm number before surgery, sperm count improved from a mean value of 13 × 106 (s.d. ± 3.5 × 106; 95% CI: 9.68–15.35) ml−1 to 21 × 106 (s.d. ± 4.2 × 106; 95% CI: 18.25–24.2) ml−1 (P < 0.001). The median value of the percentage of progressive motile forms at 1 h improved from 25% to 45% (P < 0.001). Percentage of normal forms increased from 17% before surgery to 35% 1 year after the procedure (P < 0.001). In the subgroup of the 168 infertile patients, 52 (31%) fathered offspring at a 12-month-minimum follow-up. The recurrence/persistence rate was 5%. We have not observed any hydrocele formation.
DISCUSSION
Despite for several years the benefit of varicocele treatment has not been clear, a recent meta-analysis of four randomized controlled trials of varicocelectomy in men with a clinical varicocele and semen parameters impairment showed a trend in favor of surgical correction.9 Varicocele treatment as demonstrated in a randomized controlled trial showed superiority over observation in infertile men with palpable varicoceles and impaired semen quality, with increasing the odds of natural pregnancy and improvements in semen characteristics.10 The benefit of varicocele treatment in infertile can produce a risk of overtreatment in adolescents, in fact the majority of adolescents patients with varicocele will have no problem achieving pregnancy later in life.11 Subinguinal approaches and percutaneous also have showed best functional results respect inguinal or high ligation approach in terms of recurrence rate. Microsurgical repair is probably the best option in terms of cost-effectiveness compared to percutaneous embolization.12
More than 15 years ago, the first procedure was performed by the senior author. Our ASS technique is a consolidate approach to repair idiopathic varicocele both in adolescent and in adult patients. Data reported confirmed a high percentage of success with a very low percentage of complication. In this single surgeon series, a persistent/recurrent varicocele was documented only in 5% of cases. Although this data could be considered slightly worse in comparison with the very low recurrent/persistence rates reported after microsurgical repair, we prefer to perform this approach avoiding the need to perform a specific learning curve with the operating microscope, the need to maintain during the time the acquired microsurgical skills, the longer operative time and the high costs of microdissection above all in a not dedicate center for the varicocele treatment. Moreover, similar to microsurgical approach, the ASS technique allows us to preserve the lymphatic vessels and testicular arteries offering a very low percentage of complications without risk of hydrocele and testicular hypotrophy.
A recent meta-analysis published by Wang et al.13 showed that ASS and microsurgical repair are superior to other techniques (open retroperitoneal surgery, laparoscopic approach) in terms of hydrocele formation. The lower risk of hydrocele formation for ASS in comparison with open surgical approach was previously highlighted by a randomized prospective study.14
In our experience, we did not observe any major complication after the procedure. Furthermore in the literature ASS demonstrated a lower complications rate than laparoscopic approach as published by May et al. In this series complications occurred in 13.1% of cases after laparoscopic approach versus 4.6% of cases for ASS.15 However, we highlighted the importance of an appropriate training before to start ASS. The described technique is quite easily performed but requires precise skills to isolate and catheterize the vein avoiding potential complications. In our opinion, surgeon approaching the ASS requiring a specific learning curve regardless their degree of experience with other surgical procedure. Usually, performing 10 cases with an expert tutor can be sufficient to have the necessary skill to reach good results.16
CONCLUSIONS
Our data confirmed that varicocele treatment is effective in terms of semen parameters improvement. ASS is an effective minimal invasive treatment for varicocele with low recurrence/persistence rate with relatively short learning curve. Complications rate is lower than laparoscopic approach above all in terms of hydrocele formation. We highlighted the importance of an appropriate training before to start ASS.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Ficarra V, Crestani A, Novara G, Mirone V. Varicocele repair for infertility: what is the evidence? Curr Opin Urol. 2012;22:489–94. doi: 10.1097/MOU.0b013e328358e115. [DOI] [PubMed] [Google Scholar]
- 2.Kroese AC, de Lange NM, Collins J, Evers JL. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 2012;10:CD000479. doi: 10.1002/14651858.CD000479.pub5. [DOI] [PubMed] [Google Scholar]
- 3.Tauber R, Johnsen N. [Antegrade scrotal sclerotherapy for treatment of testicular varicocele. Technique and late results] Urologe A. 1993;32:320–6. [PubMed] [Google Scholar]
- 4.Tauber R, Johnsen N. Antegrade scrotal sclerotherapy for the treatment of varicocele: technique and late results. J Urol. 1994;151:386–90. doi: 10.1016/s0022-5347(17)34956-x. [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V, Porcaro AB, Righetti R, Cerruto MA, Pilloni S, et al. Antegrade scrotal sclerotherapy in the treatment of varicocele: a prospective study. BJU Int. 2002;89:264–8. doi: 10.1046/j.1464-4096.2001.02418.x. [DOI] [PubMed] [Google Scholar]
- 6.Galfano A, Novara G, Iafrate M, Fracalanza S, Novella G, et al. Surgical outcomes after modified antegrade scrotal sclerotherapy: a prospective analysis of 700 consecutive patients with idiopathic varicocele. J Urol. 2008;179:1933–7. doi: 10.1016/j.juro.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 7.Fulcoli V, Costa G, Gigli F, Laurini L. Ischemic necrosis of the sigmoid colon after antegrade sclerotherapy of idiopathic varicocele: a case report. Urologia. 2013;80:162–4. doi: 10.5301/RU.2013.10722. [DOI] [PubMed] [Google Scholar]
- 8.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 9.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility?. An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59:455–61. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Ding H, Tian J, Du W, Zhang L, Wang H, et al. Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: a meta-analysis of randomized controlled trials. BJU Int. 2012;110:1536–42. doi: 10.1111/j.1464-410X.2012.11093.x. [DOI] [PubMed] [Google Scholar]
- 12.Kovac JR, Fantus J, Lipshultz LI, Fischer MA, Klinghoffer Z. Cost-effectiveness analysis reveals microsurgical varicocele repair is superior to percutaneous embolization in the treatment of male infertility. Can Urol Assoc J. 2014;8:E619–25. doi: 10.5489/cuaj.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Xia SJ, Liu ZH, Tao L, Ge JF, et al. Inguinal and subinguinal micro-varicocelectomy, the optimal surgical management of varicocele: a meta-analysis. Asian J Androl. 2015;17:74–80. doi: 10.4103/1008-682X.136443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayez A, El Shantaly KM, Abbas M, Hauser S, Müller SC, et al. Comparison of inguinal approach, scrotal sclerotherapy and subinguinal antegrade sclerotherapy in varicocele treatment: a randomized prospective study. Urol Int. 2010;85:200–3. doi: 10.1159/000316338. [DOI] [PubMed] [Google Scholar]
- 15.May M, Johannsen M, Beutner S, Helke C, Braun KP, et al. Laparoscopic surgery versus antegrade scrotal sclerotherapy: retrospective comparison of two different approaches for varicocele treatment. Eur Urol. 2006;49:384–7. doi: 10.1016/j.eururo.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Ficarra V, Sarti A, Novara G, Artibani W. Antegrade scrotal sclerotherapy and varicocele. Asian J Androl. 2002;4:221–4. [PubMed] [Google Scholar]


