Abstract
β-defensins are components of host defense, with antimicrobial and pleiotropic immuno-modulatory properties. Research over the last 15 years has demonstrated abundant expression of a variety of β-defensins in the postnatal epididymis of different species. A gradient of region- and cell-specific expression of these proteins is observed in the epithelium of the postnatal epididymis. Their secretion into the luminal fluid and binding to spermatozoa as they travel along the epididymis has suggested their involvement in reproduction-specific tasks. Therefore, continuous attention has been given to various β-defensins for their role in sperm function and fertility. Although β-defensins are largely dependent on androgens, the underlying mechanisms regulating their expression and function in the epididymis are not well understood. Recent investigation has pointed out to a new and interesting scenario where β-defensins emerge with a different expression pattern in the Wolffian duct, the embryonic precursor of the epididymis, as opposed to the adult epididymis, thereby redefining the concept concerning the multifunctional roles of β-defensins in the developing epididymis. In this review, we summarize some current views of β-defensins in the epididymis highlighting our most recent data and speculations on their role in the developing epididymis during the prenatal-to-postnatal transition, bringing attention to the many unanswered questions in this research area that may contribute to a better understanding of epididymal biology and male fertility.
Keywords: androgens, embryogenesis, epididymis, organogenesis, β-defensins
INTRODUCTION
The mammalian epididymis, a highly convoluted duct that links the efferent ductules to the vas deferens, plays a critical role in sperm maturation, transportation, concentration, protection against pathogenic and metabolic injuries, and storage before ejaculation. It can be subdivided into the initial segment, caput, corpus, and cauda regions on the basis of the histological and functional differences. Further subdivisions of these regions into intra-regional segments, limited by connective tissue septa, have been identified in rodents.1,2 These discrete segments present specific patterns of gene expression and protein localization, representing distinct regulatory subunits of the epididymis that tightly regulate the composition of the epididymal luminal fluid, which dynamically interacts with the spermatozoa during their journey along the epididymis.1,2 Androgens and other steroid hormones, paracrine and lumicrine factors and, more recently, microRNAs are among important factors that regulate the segment-dependent gene expression in the epididymis.3,4 The maintenance of the regional normal fluid microenvironment along the epididymal duct is essential for sperm maturation and, therefore, contributes to male fertility.3,4,5
The advances in the last 15 years in genome and gene and protein expression profiling have greatly expanded our view of the complexity of the function of the epididymal epithelium and its contribution to the formation of the highly specialized luminal fluid milieu. Nowadays one of the more interesting scenarios in this tissue is the high number of epididymal genes encoding a variety of innate immunity secretory proteins.6 Among these genes, several encode β-defensins, which are members of a large family of cationic cysteine-rich proteins exhibiting a broad-spectrum antimicrobial activity.7,8,9,10 β-defensins are normally made up of fewer than 80 amino acid residues with 5–12 positively charged residues, usually with a very stable structure composed of one alpha-helix and three beta-sheets, and generally encoded by a gene with two exons. These characteristics are found in β-defensins such as β-defensin 1 (DEFB1) and DEFB2, among others. Some protein isoforms encoded by the SPAG11B (sperm-associated antigen 11B; variants SPAG11C, SPAG11D and SPAG11E, also known as BIN1B) and human DEFB126 (the orthologue of the rat and mouse Defb22) genes are exceptions, being longer owing to a more complex gene structure, with more than two exons that encode additional amino acids after the N-terminal secretion signal leader sequence or at the C-terminus adjacent to the C5 and C6 residues.7,11,12 Herein, we review the current understanding on the contributions of β-defensins to the epididymis, with a special focus on the recent discoveries indicating that these proteins may play different roles in the epididymis during prenatal and postnatal life. We shed light on evidence and perspectives regarding new potential roles for β-defensins in the developing epididymis.
β-DEFENSINS IN THE ADULT EPIDIDYMIS – ROLE IN SPERM PROTECTION AND FUNCTION
Since their first identification as antimicrobials in cattle airway epithelial cells, more than 30 β-defensins genes have been described in human and other species.10,13,14 Several are constitutively expressed in adult tissues from the male reproductive tract and are found to be particularly abundant in the testis and epididymis.11,13,15 In some cases, their expression is almost restricted to or very abundant in the adult epididymal epithelium, where most of them display unique region- and cell-specific expression patterns depending on the β-defensin evaluated.15,16,17,18,19
One question in this scenario is why so many β-defensins are expressed in the epididymal epithelia and secreted into the epididymal lumen, where they are usually found on the surface of maturing spermatozoa15,17,20 Would they all be required for sperm protection and maturation while the spermatozoon travels through the epididymis? Since the β-defensin genes are found in clusters that have arisen from gene duplication,13,21,22 many family members are believed to be functionally redundant. Thus, one point of view is that redundancy of their functions would allow them to back each other up, perhaps explaining why β-defensin knockout mice have not been reported as being developmentally lethal or with a severely impaired phenotype.23,24,25,26 On the basis of the region- and epithelial cell-specific β-defensin expression pattern in the adult epididymis, Zhang et al.18 have speculated that multiple β-defensins act in a synergistic and sequential manner in the epididymal luminal fluid, contributing collaboratively in this way to the achievement of sperm protection, maturation and fertilization ability along the epididymal duct.
In fact, there is increasing evidence that β-defensins display multifunctional roles in host defense, both as effectors and regulators, as well as in the modulation of the immune system during an infection or inflammatory response.14,15,17,27 On one hand, they are effectors of host defense by presenting in vitro and in vivo antimicrobial activities against bacteria and fungi.7,15 In addition, several recombinant β-defensins have been shown to regulate host defense by their ability to bind to or neutralize the activity of the lipopolysaccharide (LPS) endotoxin of Gram-negative bacteria,27,28 suggesting ways by which β-defensins can protect epididymal spermatozoa against the inflammatory effects of LPS or infection by Gram-negative bacteria.
On the other hand, β-defensins can inhibit in vitro and in vivo LPS-mediated inflammatory responses,28 which are events not necessarily correlated with their capacity to bind LPS, and that may involve their interference with signaling cascades triggered by the activation of Toll-like receptor 4 (TLR4) by LPS and other inflammatory factors.28,29 In this context, there are data supporting both anti- and pro-inflammatory roles for β-defensins,10,28 indicating the need for further studies to uncover their specific roles in the epididymis as modulators of the immune system. How β-defensins communicate and participate with other key innate immunity effectors such as TLR4, humoral mediators (e.g., cytokines, chemokines) and immune cells (e.g., phagocytes and dendritic cells)30,31,32 in the maintenance of the epididymal function and in the ability of this organ to nurture the spermatozoa in normal and in infectious and inflammatory conditions, are still poorly understood.
Aside from antimicrobial and immune system modulation properties, different epididymal β-defensins have been implicated in performing reproduction-specific tasks, such as modulation of sperm function in their physiological repertoires. Examples are rat SPAG11E33,34 and rat DEFB1535 (the orthologue of human DEFB106B), which are differentially expressed in the caput epididymidis and found to affect sperm motility acquisition33,34 and sperm motility maintenance.35 Primate DEFB126, on the other hand, is predominantly expressed in the corpus epididymidis and plays a role in the transport of spermatozoa in the female reproductive tract.36,37 More recently, the deletion of a subset of nine adjacent β-defensin genes (Defb1, Defb2, Defb9, Defb10, Defb11, Defb13, Defb15, Defb35, and Defb50) located in a cluster in the mouse chromosome 8 was shown to significantly impair sperm function in vivo, by affecting the control of intracellular calcium and regulation of the acrosome reaction, which resulted in sterility.26 In addition, a common mutation in the human DEFB126 gene was reported to reduce sperm penetration ability into cervical mucus and was associated with a male subfertility condition.12 A detailed overview on β-defensin and their impact on sperm function can be found in Dorin and Barratt.20 In spite of the above data, there is still the need for more studies focusing on the underlying mechanisms by which the sophisticated β-defensin expression patterns, their functional repertoire and cross-talk contribute to sperm maturation and protection in the epididymis.
REGULATION OF β-DEFENSIN EXPRESSION IN THE POSTNATAL AND ADULT EPIDIDYMIS
Is an expression of β-defensins under the regulation of infectious or inflammatory conditions in the epididymis? The data on this matter are still scarce and controversial. There are reports indicating no change38 or an increase39 in different β-defensin mRNA levels in tissues from the rat male reproductive tract following in vivo treatment with LPS from Escherichia coli. In a rat epididymitis model induced by Gram-negative bacteria, however, Spag11e (Bin1b) mRNA levels decreased 3 days after infection.32 In other experimental models of epididymitis induced by LPS, Defb2, Defb21 and Defb27 mRNA levels decreased in the caput epididymidis,39,40 while other β-defensins such as Defb29, Defb41 and Defb42 were unaffected by the treatment.40 Although the abundance of Spag11b (Bin1b) mRNA decreased following bacterial infection in the mouse epididymis,41 its overexpression gave mice added resistance to E. coli-induced epididymitis.42 Although differences in the experimental models of epididymitis (whole bacteria vs bacterial products; time of infection or inflammatory induction; epididymal region evaluated, etc.) may be responsible for these various results, the scenario indicates the complex regulation of β-defensin expression in the epididymis during inflammation or infection. This may have a significant impact on the clinical outcomes of epididymitis, a condition that often results in impairment of sperm function and fertility.32
Considering the host defense properties of β-defensins, and the still limited information on the modulation of their gene expression and function in the epididymis, a closer investigation of the segment-specific expression pattern of β-defensins in response to infectious, or even noninfectious, epididymitis is interesting and should be pursued. Animal models of bacterial epididymitis established in the last few years31,32 are important tools to show experimentally the exact roles played by the various epididymal β-defensins in epididymal physiology and pathology, either by themselves or in association with one another. It is known that LPS secreted by a Gram-negative bacterium, such as E. coli, and the subsequent LPS-induced inflammatory host response, together play a significant role as contributing factors to male infertility following cases of epididymitis and orchitis.31,32 The fact that human DEFB102 expression is induced in the epididymis in response to LPS from E. coli and other pro-inflammatory agents raises the idea of β-defensins as potential targets for the development of therapeutics in the prevention or treatment of infection- or inflammation-related diseases.14,43
Androgens, the primary modulators of epididymal structure, gene expression and function,4 are acknowledged as important regulators of epididymal β-defensin expression in multiple species. In fact, a gradual upregulation of the mRNA levels of several rat and mouse epididymal β-defensins is observed from early postnatal ages to adulthood, when timely increases in gonadal and plasma testosterone concentrations occur.22,35,44,45,46,47 In different species, the epididymis from castrated animals presents down-regulation of different β-defensin transcripts that is reversed in tissues from testosterone-treated castrated animals, suggesting their positive androgen regulation.16,22,35,38,45,46,47,48,49,50,51 More recently, we discovered a down-regulation in the β-defensin Spag11c mRNA levels in the caput, but not in the cauda epididymidis from castrated adult rats, demonstrating that influence of androgens on β-defensin expression is dependent on the epididymal region analyzed.44 In addition, mouse Spag11a (Bin1b)50 and Spag11c mRNA levels44 were not fully maintained at normal levels in the caput epididymidis from testosterone-treated castrated rats, suggesting that their expression is not only dependent on androgens, but also on luminal testicular factors. Furthermore, the SPAG11C immuno-distribution is differentially affected by androgen deprivation and testosterone treatment, not only in a region-dependent, but also a cell-specific, fashion in the adult rat caput and cauda epididymidis.44 The identification of androgen receptor (AR) response elements in the 5’-flanking region of β-defensin genes of different species has provided further proof of the potential androgen modulation of these genes.52,53 By using ChIP-PCR/qPCR assays, Hu et al.53 studied the binding of AR to AREs identified in mouse caput β-defensin genes. They observed twelve genes presenting AR binding sites in their promoter or intronic regions (indicating direct regulation of these genes by AR), another six exhibiting an androgen-independent expression pattern and yet one gene showing high dependence on testicular factors rather other than androgens, thus confirming defensins’ differential androgenic regulation depends on the epididymal cell type and region analyzed.
Furthermore, by contrasting the differential mRNA expression profile of six different splicing variants originating from the SPAG11B gene in fetal and adult reproductive and nonreproductive tissues from bulls,19 our group raised the hypothesis of differential mechanisms contributing to the modulation of β-defensin expression in the developing epididymis. Likewise, our most recent work focusing on the expression of a β-defensin in the prenatal and postnatal rat epididymis44 has shed light on a novel understanding of the putative roles of β-defensins in epididymal ontogenesis, which we discuss below.
β-DEFENSINS IN THE PRENATAL AND POSTNATAL EPIDIDYMIS – EVIDENCE OF NEW PHYSIOLOGICAL ROLES?
During epididymal development, the formation of the straight Wolffian duct (WD), the anlage of the epididymis, and its progression to the three-dimensionally coiled and highly regionalized postnatal epididymis depends on a highly coordinated succession of molecular and morphogenic events involving a complex and essential interplay of different modulatory factors, primarily androgens.54 Briefly, the rat fetal testis begins to secrete testosterone at the embryonic age of 15.5 days (E15.5),55 1 day after the detection of AR in the mesenchyme of the WD. Peak prenatal plasma concentrations peak between E17.5 and E18.5,56,57 which precedes the coiling of the future epididymis.58,59,60 During this period, androgens act indirectly on WD epithelium by androgen-dependent mesenchymal-derived regulators and, shortly after E18.5, directly through the AR that is now expressed by the WD epithelium.61,62 Disorders of androgens and AR signaling in this critical period of WD development, due to congenital causes or exposure to endocrine disruptors, impair reproductive tract masculinization and normal epididymal development, resulting in later infertility during adulthood.58,59,60 After another plasma testosterone peak that occurs shortly after birth,63 a low plasma level of this steroid hormone is maintained in immature animals, increasing steadily throughout puberty, plateauing in adulthood.64 Postnatal growth and differentiation of the rat epididymis is composed of an undifferentiated period between 1 and 15 days of age, a differentiation phase between 15 and 44 days, followed by an expansion period from 44 days of age to adulthood.4
Recently, we reported that significant temporal, cell-type and region-specific changes occur in the expression of the β-defensin Spag11c gene as the rat epididymis develops from prenatal to postnatal life.44 Spag11c mRNA, detected as early as E12.5 in rat WD, increased in expression at E17.5 and decreased at E20.5, a period when the androgen-induced differentiation of WD into epididymis occurs (Figure 1a and 1C). Unexpectedly, SPAG11C immuno-localization in WD mesenchymal cells gradually switched after birth (after postnatal 20 days of age) to a more predominant localization in the epididymal epithelia that persisted into adulthood (Figure 1b and 1C). The hypothesized effects of SPAG11C on cellular growth and differentiation during tissue morphogenesis were further supported by the ubiquitous distribution of Spag11c mRNA in fetal rat reproductive and nonreproductive organs versus the preferential and abundant expression of Spag11c in the postnatal rat epididymis.44
Figure 1.
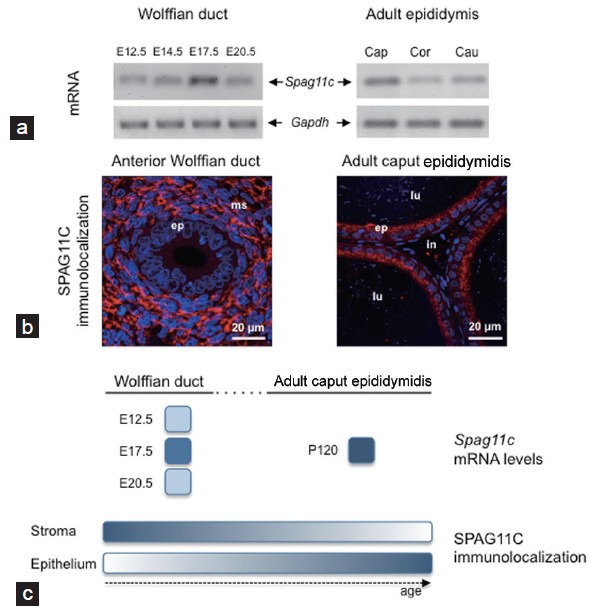
Spatiotemporal expression of the β-defensin SPAG11C in the developing rat epididymis. (a) Spag11c mRNA was detected by end-point RT-PCR in the male Wistar rat urogenital rudiment as early as embryonic day 12.5 (E12.5), before the onset of androgen receptor (AR) expression and testosterone synthesis in the embryo. Increased Spag11c mRNA levels were observed in the Wolffian duct (WD) at E17.5, in contrast to decreasing levels detected at E20.5, when the WD morphologically differentiates into the epididymis under androgen influence. In the adult rat epididymis, Spag11c mRNA was more abundant in the caput (Cap) than in corpus (Cor) or cauda (Cau) epididymidis. The housekeeping gene Gapdh was used as an endogenous control. (b) Immunofluorescence studies performed in paraffin-embedded sections from whole fetuses (E18.5) and adult caput epididymidis (120 days old, P120) revealed that SPAG11C was prenatally mainly located in mesenchymal cells of the anterior WD (the future epididymis) (left panel), shifting gradually to epithelial cells after birth and became mainly distributed in the epithelium of adult caput epididymidis (right panel). Nuclei were stained with DAPI (blue). lu: lumen; ms: mesenchyme; ep: epithelium; in: interstitium. (c) Schematic representation of SPAG11C (mRNA levels and immunolocalization) developmental changes in the rat WD and adult epididymis. Relative expression levels were represented by a gradient of blue shades ranging from white (minimal intensity) to dark blue (maximal intensity) based on RT-qPCR data.44 P: postnatal day. Results from panels (a) and (b) are representative of previously published data by our group.44
Does the differential expression of β-defensins in the epididymis between prenatal and postnatal life reflect distinct biological roles or specific regulatory mechanisms throughout its lifespan? What ultimately drives the dynamic changes of the spatio-temporal expression of SPAG11C in the developing epididymis? We do not have answers for these questions. Since expression of Spag11c mRNA in the rat urogenital ridge is detected as early as E12.5, before the onset of testosterone (Figure 1a), other collaborative developmental factors besides androgens may be important for the regulation of the expression levels of this particular β-defensin.
Preliminary RT-PCR studies from our group with WDs collected at E17.5 and E20.5 indicated, however, that the mRNA expression of five other β-defensins were either not readily detected at these two embryonic time points (Spag11e and Defb12) or only observed in WDs at the latter E20.5 time point (Defb1, Def2 and Defb22; Figure 2). The discovery of this differential expression pattern between Spag11c and these other β-defensins in the WD contrasts with constitutive expression in the adult epididymis (Figure 2). Thus, this additional layer of complexity of β-defensins in the prenatal versus postnatal epididymis may provide clues to additional physiological roles for these multifaceted proteins.
Figure 2.
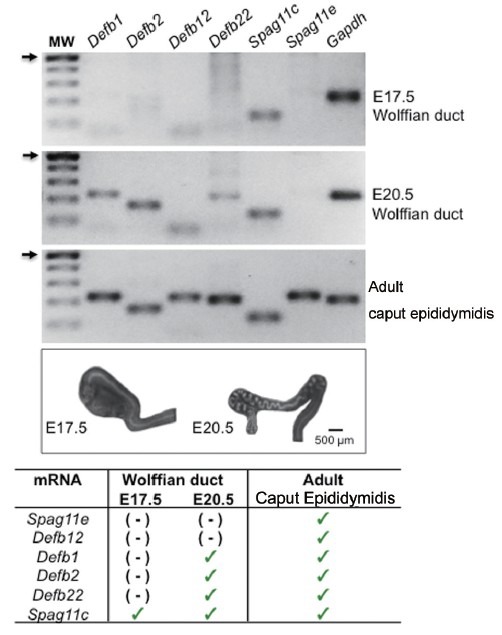
mRNA expression profile of different β-defensins in the rat Wolffian duct and adult caput epididymidis. Inverted image of an agarose gel of end-point RT-PCR showing the expression of Defb1, Def2, Def12, Defb22, Spag11c and Spag11e in the Wolffian duct (WD) isolated at embryonic ages (E) E17.5 and E20.5 and in the adult caput epididymidis (120 days old, P120). Amplicon expected sizes (in base pairs): Defb1 (222), Def2 (165), Def12 (219), Defb22 (208), Spag11c (123) and Spag11e (224). Housekeeping gene Gapdh (207 bp) was used as an endogenous control. MW indicates a 100 base pair (bp) standard DNA ladder. Left arrows indicate 500 bp. Whole-mount inverted photographs show elongation and coiling of rat WD. Data are representative from analyses of pool of tissues from 3 to 5 rats in each time point. Table summarizes the amplicons detected either in their expected sizes (✓) or not readily detected in the experimental conditions used (-). Results relative to Spag11c transcript in time points E17.5 and E20.5 in panel (a) are representative of previously published data by our group.44
The mechanisms of action of β-defensins and how they respond in expression and function to insults, or changes in steroid hormone concentration during embryonic life, are not well understood and are now the subject of investigation in our laboratory. Together, these findings constitute a baseline for future studies addressing how β-defensins contribute to the achievement and maintenance of male fertility. In addition, the understanding of β-defensin roles is of clinical and therapeutic relevance, since it can be instructive not only in identifying their involvement in phenotype and disease susceptibility, including male infertility but also in helping the identification of novel druggable targets.
CONCLUSIONS AND PERSPECTIVES
There has been a great deal of scientific interest in recent years in studying the function of β-defensins in the “normal” epididymis since it may provide clues on how they contribute to physiological conditions. Several questions still remain, however, concerning the in vivo role of these proteins and their effects on epididymal function and sperm maturation. What are the relevant conditions for the control of β-defensin expression and function in the epididymis? Developmental? Physiological? Pathological? Acute, chronic or both? Could β-defensins have relevance as key biomarkers of developmental events or as targets for the treatment of diseases in the developing epididymis after injuries or anti-infectious/inflammatory responses? These are interesting and fascinating hypotheses to be addressed experimentally in further studies and then translated into the clinical setting. Aside from their known action in host defense, the understanding of the full physiological range of the β-defensin functions in the epididymis may expand their therapeutic potential in sperm protection and male fertility optimization, and beyond.
AUTHOR CONTRIBUTIONS
MCWA and CMR carried out the conception, writing and revision of the manuscript. BH and EJRS were responsible for the suggestions to the body of the manuscript, revision and helpful comments.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We thank Daniel S. Thimoteo and Lucas GA Ferreira and all other members from Avellar's laboratory for their contribution and assistance with experiments, figures, and helpful comments. Financial support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, #480452/2008-3, #455450/2014-5 and #308349/2010-5), CNPq/Science Without Borders (#PVE401932/2013-3, PDJ #167292-2013-7 and BJT#401718/2012-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, #02921/09-2), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, #2010/52711-0 and #2009/14649-3) and National Institutes of Health Eunice Kennedy Shriver NICHD (#HD069654 and #HD068365, BTH).
REFERENCES
- 1.Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, et al. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–70. doi: 10.1095/biolreprod.106.057323. [DOI] [PubMed] [Google Scholar]
- 2.Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, et al. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- 3.Belleannée C, Légaré C, Calvo É, Thimon V, Sullivan R. microRNA signature is altered in both human epididymis and seminal microvesicles following vasectomy. Hum Reprod. 2013;28:1455–67. doi: 10.1093/humrep/det088. [DOI] [PubMed] [Google Scholar]
- 4.Robaire B, Hinton BT. The epididymis. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. 4th ed. San Diego: Academic Press; 2015. pp. 691–771. [Google Scholar]
- 5.Corwall GA, Lareyre JJ, Matusiki RJ, Hinton BT, Orgebin-Crist MC. Gene expression and epididymal function. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Springer; 2002. pp. 169–200. [Google Scholar]
- 6.Dean MD, Good JM, Nachman MW. Adaptive evolution of proteins secreted during sperm maturation: an analysis of the mouse epididymal transcriptome. Mol Biol Evol. 2008;25:383–92. doi: 10.1093/molbev/msm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 8.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–56. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 9.Klüver E, Adermann K, Schulz A. Synthesis and structure-activity relationship of β-defensins, multi-functional peptides of the immune system. J Pept Sci. 2006;12:243–57. doi: 10.1002/psc.749. [DOI] [PubMed] [Google Scholar]
- 10.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human beta-defensins. Cell Mol Life Sci. 2006;63:1294–313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro CM, Romano RM, Avellar MC. Beta-defensins in the epididymis: clues to multifunctional roles. Anim Reprod. 2012;9:751–9. [Google Scholar]
- 12.Tollner TL, Venners SA, Hollox EJ, Yudin AI, Liu x, et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci Transl Med. 2011;3:92ra65. doi: 10.1126/scitranslmed.3002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patil AA, Cai Y, Sang Y, Blecha F, Zhang G. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics. 2005;23:5–17. doi: 10.1152/physiolgenomics.00104.2005. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Ouchi Y. Antimicrobial peptide defensin: identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:152–66. doi: 10.2183/pjab.88.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall SH, Yenugu S, Radhakrishnan Y, Avellar MC, Petrusz P, et al. Characterization and functions of beta defensins in the epididymis. Asian J Androl. 2007;9:453–62. doi: 10.1111/j.1745-7262.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 16.Avellar MC, Honda L, Hamil KG, Yenugu S, Grossman G, et al. Differential expression and antibacterial activity of epididymis protein 2 isoforms in the male reproductive tract of human and rhesus monkey (Macaca mulatta) Biol Reprod. 2004;71:1453–60. doi: 10.1095/biolreprod.104.031740. [DOI] [PubMed] [Google Scholar]
- 17.Hall SH, Hamil KG, French FS. Host defense proteins of the male reproductive tract. J Androl. 2002;23:585–97. [PubMed] [Google Scholar]
- 18.Zhang YL, Zhang JS, Zhou YC, Zhao Y, Ni MJ. Identification of microRNAs and application of RNA interference for gene targeting in vivo in the rat epididymis. J Androl. 2011;32:587–91. doi: 10.2164/jandrol.111.013060. [DOI] [PubMed] [Google Scholar]
- 19.Avellar MC, Honda L, Hamil KG, Radhakrishnan Y, Yenugu S, et al. Novel aspects of the sperm-associated antigen 11 (SPAG11) gene organization and expression in cattle (Bos taurus) Biol Reprod. 2007;76:1103–16. doi: 10.1095/biolreprod.106.059626. [DOI] [PubMed] [Google Scholar]
- 20.Dorin JR, Barratt CL. Importance of β-defensins in sperm function. Mol Hum Reprod. 2014;20:821–6. doi: 10.1093/molehr/gau050. [DOI] [PubMed] [Google Scholar]
- 21.Radhakrishnan Y, Fares MA, French FS, Hall SH. Comparative genomic analysis of a mammalian beta-defensin gene cluster. Physiol Genomics. 2007;30:213–22. doi: 10.1152/physiolgenomics.00263.2006. [DOI] [PubMed] [Google Scholar]
- 22.Radhakrishnan Y, Hamil KG, Yenugu S, Young SL, French FS, et al. Identification, characterization, and evolution of a primate beta-defensin gene cluster. Genes Immun. 2005;6:203–10. doi: 10.1038/sj.gene.6364184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augustin DK, Heimer SR, Tam C, Li WY, Le Due JM, et al. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect Immun. 2011;79:595–605. doi: 10.1128/IAI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison G, Kilanowski F, Davidson D, Dorin J. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect Immun. 2002;70:3053–60. doi: 10.1128/IAI.70.6.3053-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navid F, Boniotto M, Walker C, Ahrens K, Proksch E, et al. Induction of regulatory T cells by a murine β-defensin. J Immunol. 2012;188:735–43. doi: 10.4049/jimmunol.1100452. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YS, Webb S, Lettice L, Tardif S, Kilanowski F, et al. Partial deletion of chromosome 8 β-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 2013;9:e1003826. doi: 10.1371/journal.pgen.1003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Dong J, Gu Y, Liu H, xin A, et al. The novel human β-defensin 114 regulates lipopolysaccharide (LPS)-mediated inflammation and protects sperm from motility loss. J Biol Chem. 2013;288:12270–82. doi: 10.1074/jbc.M112.411884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigues A, Queiróz DB, Honda L, Silva EJ, Hall SH, et al. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia coli. Biol Reprod. 2008;79:1135–47. doi: 10.1095/biolreprod.108.069930. [DOI] [PubMed] [Google Scholar]
- 30.Da Silva N, Smith T. Exploring the role of mononuclear phagocytes in the epididymis. Asian J Androl. 2015;17:591–6. doi: 10.4103/1008-682X.153540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedger MP. The Immunophysiology of male reproduction. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. San Diego: Academic Press; 2015. pp. 805–92. [Google Scholar]
- 32.Michel V, Pilatz A, Hedger MP, Meinhardt A. Epididymitis: revelations at the convergence of clinical and basic sciences. Asian J Androl. 2015;17:756–63. doi: 10.4103/1008-682X.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Chan HC, He B, So SC, Chung YW, et al. An antimicrobial peptide gene found in the male reproductive system of rats. Science. 2001;291:1783–5. doi: 10.1126/science.1056545. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Cx, Zhang YL, xiao L, Zheng M, Leung KM, et al. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat Cell Biol. 2004;6:458–64. doi: 10.1038/ncb1127. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Diao H, Ni Z, Hu S, Yu H, et al. The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus) Cell Mol Life Sci. 2011;68:697–708. doi: 10.1007/s00018-010-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tollner TL, Yudin AI, Tarantal AF, Treece CA, Overstreet JW, et al. Beta-defensin 126 on the surface of macaque sperm mediates attachment of sperm to oviductal epithelia. Biol Reprod. 2008;78:400–12. doi: 10.1095/biolreprod.107.064071. [DOI] [PubMed] [Google Scholar]
- 37.Yudin AI, Tollner TL, Li MW, Treece CA, Overstreet JW, et al. ESP13.2, a member of the beta-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol Reprod. 2003;69:1118–28. doi: 10.1095/biolreprod.103.016105. [DOI] [PubMed] [Google Scholar]
- 38.Palladino MA, Mallonga TA, Mishra MS. Messenger RNA (mRNA) expression for the antimicrobial peptides β-defensin-1 and β-defensin-2 in the male rat reproductive tract: β-defensin-1 mRNA in initial segment and caput epididymidis is regulated by androgens and not bacterial lipopolysaccharides. Biol Reprod. 2003;68:509–15. doi: 10.1095/biolreprod.102.008953. [DOI] [PubMed] [Google Scholar]
- 39.Biswas B, Yenugu S. Antimicrobial responses in the male reproductive tract of lipopolysaccharide challenged rats. Am J Reprod Immunol. 2011;65:557–68. doi: 10.1111/j.1600-0897.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 40.Cao D, Li Y, Yang R, Wang Y, Zhou Y, et al. Lipopolysaccharide-induced epididymitis disrupts epididymal beta-defensin expression and inhibits sperm motility in rats. Biol Reprod. 2010;83:1064–70. doi: 10.1095/biolreprod.109.082180. [DOI] [PubMed] [Google Scholar]
- 41.Lang T, Dechant M, Sanchez V, Wistuba J, Boiani M, et al. Structural and functional integrity of spermatozoa is compromised as a consequence of acute uropathogenic E. coli-associated epididymitis. Biol Reprod. 2013;89:59, 1–10. doi: 10.1095/biolreprod.113.110379. [DOI] [PubMed] [Google Scholar]
- 42.Fei Z, Hu S, xiao L, Zhou J, Diao H, et al. mBin1b transgenic mice show enhanced resistance to epididymal infection by bacteria challenge. Genes Immun. 2012;13:445–51. doi: 10.1038/gene.2012.13. [DOI] [PubMed] [Google Scholar]
- 43.Yeung AY, Gellatly S, Hancock RW. Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci. 2011;68:2161–76. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribeiro CM, Queiróz DB, Patrão MT, Denadai-Souza A, Romano RM, et al. Dynamic changes in the spatio-temporal expression of the β-defensin SPAG11C in the developing rat epididymis and its regulation by androgens. Mol Cell Endocrinol. 2015;404:141–50. doi: 10.1016/j.mce.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Yenugu S, Chintalgattu V, Wingard CJ, Radhakrishnan Y, French FS, et al. Identification, cloning and functional characterization of novel beta-defensins in the rat (Rattus norvegicus) Reprod Biol Endocrinol. 2006;4:7. doi: 10.1186/1477-7827-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yenugu S, Hamil KG, Grossman G, Petrusz P, French FS, et al. Identification, cloning and functional characterization of novel sperm associated antigen 11 (SPAG11) isoforms in the rat. Reprod Biol Endocrinol. 2006;4:23. doi: 10.1186/1477-7827-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jalkanen J, Huhtaniemi I, Poutanen M. Discovery and characterization of new epididymis-specific beta-defensins in mice. Biochim Biophys Acta. 2005;1730:22–30. doi: 10.1016/j.bbaexp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Hamil KG, Sivashanmugam P, Richardson RT, Grossman G, Ruben SM, et al. HE2beta and HE2gamma, new members of an epididymis-specific family of androgen-regulated proteins in the human. Endocrinology. 2000;141:1245–53. doi: 10.1210/endo.141.3.7389. [DOI] [PubMed] [Google Scholar]
- 49.Ibrahim NM, Young LG, Frohlich O. Epididymal specificity and androgen regulation of rat EP2. Biol Reprod. 2001;65:575–80. doi: 10.1095/biolreprod65.2.575. [DOI] [PubMed] [Google Scholar]
- 50.Pujianto DA, Loanda E, Sari P, Midoen YH, Soeharso P. Sperm-associated antigen 11A is expressed exclusively in the principal cells of the mouse caput epididymis in an androgen-dependent manner. Reprod Biol Endocrinol. 2013;11:59. doi: 10.1186/1477-7827-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q, Hamil KG, Sivashanmugam P, Grossman G, Soundararajan R, et al. Primate epididymis-specific proteins: characterization of ESC42, a novel protein containing a trefoil-like motif in monkey and human. Endocrinology. 2001;142:4529–39. doi: 10.1210/endo.142.10.8422. [DOI] [PubMed] [Google Scholar]
- 52.Fröhlich O, Po C, Young LG. Organization of the human gene encoding the epididymis-specific EP2 protein variants and its relationship to defensin genes. Biol Reprod. 2001;64:1072–9. doi: 10.1095/biolreprod64.4.1072. [DOI] [PubMed] [Google Scholar]
- 53.Hu SG, Zou M, Yao Gx, Ma WB, Zhu QL, et al. Androgenic regulation of beta-defensins in the mouse epididymis. Reprod Biol Endocrinol. 2014;12:76. doi: 10.1186/1477-7827-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murashima A, xu B, Hinton BT. Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J Androl. 2015;17:749–55. doi: 10.4103/1008-682X.155540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warren DW, Haltmeyer GC, Eik-Nes KB. Synthesis and metabolism of testosterone in the fetal rat testis. Biol Reprod. 1972;7:94–9. doi: 10.1093/biolreprod/7.1.94. [DOI] [PubMed] [Google Scholar]
- 56.Ward IL, Ward OB, Affuso JD, Long WD, 3rd, French JA, et al. Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Horm Behav. 2003;43:531–9. doi: 10.1016/s0018-506x(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 57.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–16. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 58.Hannema SE, Hughes IA. Regulation of wolffian duct development. Horm Res Paediatr. 2007;67:142–51. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- 59.Welsh M, Saunders PT, Marchetti NI, Sharpe RM. Androgen-dependent mechanisms of wolffian duct development and their perturbation by flutamide. Endocrinology. 2006;147:4820–30. doi: 10.1210/en.2006-0149. [DOI] [PubMed] [Google Scholar]
- 60.Welsh M, Saunders PT, Sharpe RM. The critical time window for androgen-dependent development of the wolffian duct in the rat. Endocrinology. 2007;148:3185–95. doi: 10.1210/en.2007-0028. [DOI] [PubMed] [Google Scholar]
- 61.Bentvelsen FM, Brinkmann AO, van der Schoot P, van der Linden JE, van der Kwast TH, et al. Developmental pattern and regulation by androgens of androgen receptor expression in the urogenital tract of the rat. Mol Cell Endocrinol. 1995;113:245–53. doi: 10.1016/0303-7207(95)03593-v. [DOI] [PubMed] [Google Scholar]
- 62.You L, Sar M. Androgen receptor expression in the testes and epididymides of prenatal and postnatal Sprague-Dawley rats. Endocrine. 1998;9:253–61. doi: 10.1385/ENDO:9:3:253. [DOI] [PubMed] [Google Scholar]
- 63.Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys. 1992;100:127–31. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- 64.Queiróz DB, Mendes FR, Porto CS, Avellar MC. Alpha1-adrenoceptor subtypes in rat epididymis and the effects of sexual maturation. Biol Reprod. 2002;66:508–15. doi: 10.1095/biolreprod66.2.508. [DOI] [PubMed] [Google Scholar]


