Abstract
Our environment and internal states are frequently complex, ambiguous and dynamic, meaning we need to have selection mechanisms to ensure we are basing our decisions on currently relevant information. Here, we review evidence that orbitofrontal (OFC) and ventromedial prefrontal cortex (VMPFC) play conserved, critical but distinct roles in this process. While OFC may use specific sensory associations to enhance task-relevant information, particularly in the context of learning, VMPFC plays a role in ensuring irrelevant information does not impinge on the decision in hand.
Introduction
It is an obvious, but sometimes overlooked, fact that it frequently takes many weeks to get an experimental animal to perform a task that could be explained to a human participant in a matter of minutes. From one perspective, this neatly encapsulates how useful language is to communicate information. However, it also highlights just how important, and often difficult, it can be without such input to determine which specific elements of a complex environment should be used to guide and update behaviour. This is particularly evident in situations where stimuli and rewards are separated in space and time, can have different meanings depending on the external context or internal state, and can also provide several different types of information (for instance, a food or fluid reward might both satisfy an internal need and provide information that the correct response has been made) [1].
One pressing question is therefore what neural structures help select relevant information and inhibit irrelevant information for the task in hand and how these relate to neural mechanisms implicated in value-guided decision making [2-6••,7]. A related issue concerns the mechanisms that allow us to determine, and potentially seek out, information relevant to satisfy a current need, and also how these systems interrelate with circuits implicated in reward seeking [8].
While these are complex topics, in this brief review we will focus on converging evidence that the lateral parts of orbitofrontal cortex (OFC) and ventromedial prefrontal cortex (VMPFC) play key roles in these faculties.
Anatomical considerations
OFC and VMPFC are large structures consisting of multiple distinct areas. Nonetheless, there are anatomical similarities between certain regions, which has allowed Price to define two distinct, though interconnected, networks in rodents, monkeys and humans [9]. First, an “orbital sensory” network, including Walker’s areas 11, 12 and 13 and parts of anterior insula in primates, receives rich sensory information from all sensory modalities and also projects back to sensory structures. The equivalent network in the rat would include LO, VLO and AIv. By contrast, a “medial visceromotor” network, including medial OFC area 14 as well as areas 25 and 32 and medial area 10, is characterised by strong connections with the medial temporal lobe as well as projections to limbic regions such as ventral striatum and lateral hypothalamus. In the rat, this network is likely made up of MO (medial orbital), prelimbic and infralimbic cortex. This network has access to information about motivational states and the current context, and can influence arousal states through connections to regions such as the hypothalamus.
Therefore, for the purposes of this review, we will refer to the former set of areas collectively as “OFC” and the latter, including medial OFC as “VMPFC”. However, we acknowledge that the information encoded and specific function of particular structures within these general areas may have important differences [cf.7].
OFC and specific reward representations
There is general agreement from both single unit [10,11] and fMRI [12•] studies that parts of OFC encode the precise identity of rewards, and can represent specific associations between stimuli and economic parameters such as reward size, probability and delay [12•, 13-14]. Arguably the most reliable effect of disruption to this region is to reduce the influence of reinforcer devaluation on subsequent choices [15,16].
What remains a matter of much debate is the function these signals play during learning and decision making. One possibility is this information is used to construct an integrated value signal that could underpin “goods-based” decision making [4]. OFC represents the value of options (large negative < neutral < large positive) rather than their salience as defined by their divergence from indifference (large negative > neutral < large positive) [17,18].
However, the interpretation of such value coding has been challenged. Schoenbaum and colleagues have demonstrated in a series of elegant studies that cells in rat OFC are sensitive to parameters such as identity or associative salience even when reward value is carefully controlled for [19,20]. Perhaps most compellingly, McDannald and colleagues [21••] recently showed that a population of OFC cells would increase their firing when a new stimulus combination was followed by either an increase in reward magnitude or a different, but equally-preferred, flavour of reward. In fact, these cells would generally signal the degree of sensory and outcome divergence from the original learned state, a finding that chimes with several other studies showing rich, rapid sensory encoding in OFC [22,23].
Indeed, outside of the domain of reward quality and quantity, few OFC neurons encode combinations of economic parameters; instead, individual value parameters are encoded in overlapping small populations of neurons [13,14,24•,25]. Given that OFC can encode information about the specific association between a stimulus and the sensory properties of a reward separate to any information about value, this implies that OFC’s role in the decision process is better described as the formation of stimulus-based predictions based on the attributes of rewards and the information to be gained from their outcomes, key inputs for a decision process.
Crediting outcomes to the correct choices
Another way of considering the functional significance of specific representations of expected outcomes is that they can facilitate appropriate updating of value estimates [6••,26,27]. Walton, Noonan and colleagues [28,29•] showed that lesions to lateral OFC, but not to medial OFC, in macaque monkeys caused a specific and selective deficit in crediting a particular stimulus choice with its consequent outcome in a 3-option probabilistic decision making task.
While the precise locus of such an effect is a matter of current debate [30], under this perspective, it seems plausible that specific types of outcome are not represented in OFC to control choices directly, but instead to facilitate rapid updating of stimulus-based associations by allowing animals to accurately assign credit to a particular stimulus or choice that produced them. This in turn will enable accurate stimulus-based value estimates to be passed on to structures involved in choosing what option to select.
OFC, task structure and selecting how to learn
If correct, the next pressing question is to determine what exact computations OFC performs and how the OFC resolves which elements of the world are relevant for learning. Some potential clues can be found in the study by Walton and colleagues discussed above [28]. One consequence of the loss in appropriate credit assignment observed in the OFC-lesioned animals was that it unmasked a separate, intact learning mechanism that could approximate stimulus-outcome associations by using recent choice and reward histories. It is important to note that this faculty was not a novel learning strategy acquired after the lesion; logistic regression analyses showed that these recency-weighted choice and reward histories affected choices to an almost equal extent pre- and post-operatively in the control and lesion groups. However, in the absence of an OFC lesion, their impact on behaviour was dwarfed by the much stronger influence of specific stimulus-outcome pairings. This implies that the way the OFC promotes appropriate credit assignment might therefore be to enhance current task-relevant associations rather than to suppress irrelevant ones.
A number of studies have provided evidence for a role of OFC in such a faculty. For instance, excitotoxic OFC lesions in rats cause them to have abnormally persistent latent inhibition [31]. The lesion rendered them slower to respond to a stimulus relative to unlesioned control animals when it switched from being neutral to becoming reinforced; in other words, the OFC group were impaired at upregulating attention to a familiar but previously behaviourally irrelevant stimulus once it became a useful predictor of future events. By contrast, there is little evidence that OFC lesions that spare medial OFC directly disrupt extinction learning, implying no role for this region in disengaging with a stimulus when it no longer predicts reward [15,32].
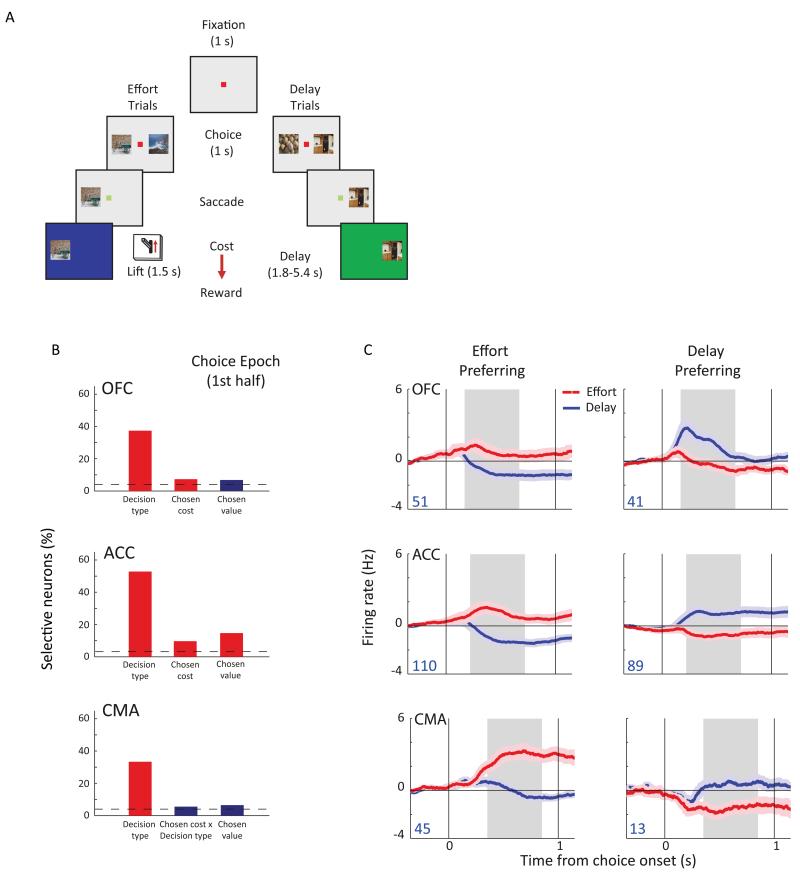
There is also evidence that OFC might play a role in identifying the type of decision environment the agent currently faces, a sort of ‘relevance filter’ over the vast stimulus (decision) space available to an agent at any given time [6••]. For instance, in a task where monkeys were trained to make decisions on separate trials between either delay- or effort-discounted rewards (Fig. 1A), the single biggest factor explaining OFC neuronal variance was the type of decision presented on that trial rather than the value of the options [24•; See Fig. 1B, C].
Figure 1. OFC neurons signal task context.
A) Task design in [24•]. Subjects made choices between pairs of presented pictures. On effort trials, they had to lift a lever and hold it for 1.5-s to earn the reward. On delay trials, they simply had to wait for a specific delay before they received the reward. The amount of reward they received, the force to lift the lever and the length of delay varied depending on the picture they chose. Effort and delay trials were intermingled in the same session. B) Percentage of neurons that were identified in OFC, the anterior cingulate cortex (ACC) and the cingulate motor area (CMA), using stepwise regression, as encoding specific variables during the first half of the choice epoch. For OFC, the 3 variables that were most frequently encoded are shown. The horizontal dotted line represents chance levels of neuronal selectivity. Red bars indicate that the proportion of selective neurons is significantly higher than the average proportion of selective neurons in the fixation period, assessed using a binomial test at p < 0.05, with a Bonferroni correction for multiple comparisons. C) Time course of activity encoding the type of decision for effort-preferring and delay-preferring neuronal populations. Each plot shows the mean firing rate relative to baseline ± S.E.M. The grey shading illustrates the 500-ms epoch used for analysis which is centered on the mean latency at which the information is encoded in each area. The blue numbers indicate the number of neurons included in each plot. OFC neurons were modulated primarily by delay- but not effort-based decisions. This is consistent with studies showing effects of OFC lesions on delay- but not effort-related decision making in situations where there is no bridging cue between the choice point and the delayed reward [63,64]. Adapted from [24•].
Whether OFC is able to select the appropriate task structure or just applies this information computed by other frontal cortical regions is not yet known; as is shown in Fig. 1B, encoding of decision type predominated across multiple regions of frontal cortex and was not unique to OFC. What is evident is that OFC can utilise information about task structure to promote rapid contingent learning.
VMPFC, valuation and value comparison
Unlike research into OFC function, evidence for the role of VMPFC in value-guided decision making has to date been largely driven by human studies. The BOLD signal in this region has often been shown to correlate with the current subjective value of various different types of options [33-35]. This holds true even in the case where the particular item has never previously been directly experienced [36].
However, as with the OFC, the functional role of VMPFC value signals remains disputed. Representations of decision value are evident in many brain regions [37], thus an important question is to identify a neural signature of a decision. A version of a biophysically plausible attractor network model of a binary probabilistic choice process [38] suggests decision inputs (values) are initially summed, and then compete via mutual inhibition, producing a later, second signal reflecting the difference in value between the chosen and unchosen options [39••]. Critically, VMPFC activity contained both such signatures in the correct timeframe [39••].
In fact, in many situations when two choice options are presented, the BOLD signal in this region not only correlates positively with the subjective value of a chosen, attended option, but also negatively with the value of the next best, but rejected option [40-42]. Recently, Strait and colleagues have reported comparable antagonistic effects between the values of two sequentially presented options in area 14 in macaques [43•]. Together, this evidence points towards an important role for VMPFC in a competitive value comparison necessary for decision making [3,39••].
When and why does value comparison take place in VMPFC?
Nonetheless, while VMPFC activation is common to a range of studies (outside the domain of decision making as well as within), it is not a signature of all decisions and is instead critically dependent on the local context. For instance, VMPFC value comparison signals not observed when selecting whether to take an available option or to forego this to search for something better in the environment; only when a decision is made to engage with the current option does the VMPFC BOLD signal represent the value of this chosen item [44].
VMPFC also only appears involved in the context of “difficult” choices, such as when choosing between two options that are close in value or when different decision variables advocate opposing choices, and even this may depend on how unfamiliar subjects are with such decisions [29•,39••,45]. Monkeys with medial, but not lateral, OFC lesions also exhibit irrational context-dependence of their choices in a 3-option probabilistic decision making task; after surgery, logistic functions describing the pattern of choices between pairs of options became affected by the value of the 3rd available option in these animals, violating normative models of rational choice [29•]. Such effects were particularly prominent during difficult choices.
VMPFC, attention and selection
What is common to situations that recruit or require VMPFC during value-guided decision making is that, first, the goal is clearly selectable from currently presented stimuli and, second, the task environment requires relevant information to be sampled and selected for an optimal choice to be made. Indeed, an alternative account of the chosen minus unchosen comparison signal in VMPFC is that it instead reflects the difference between an attended and an unattended option, especially as chosen items generally are attended longer than unchosen ones [46,47•]. Neurons in dorsal parts of VMPFC encode value information particularly around attentional shifts, suggesting integration between the allocation of attention and valuation processes [48].
A change in the way information is attended to and acquired following VMPFC damage [49] might explain why the predominant deficit observed experimentally in monkeys and humans with VMPFC damage is an increased tendency for inconsistent choices [15,50,51]. Unlike the maladaptive increase in exploratory choices seen following OFC lesions [28], this cannot be explained by impaired value learning [29•].
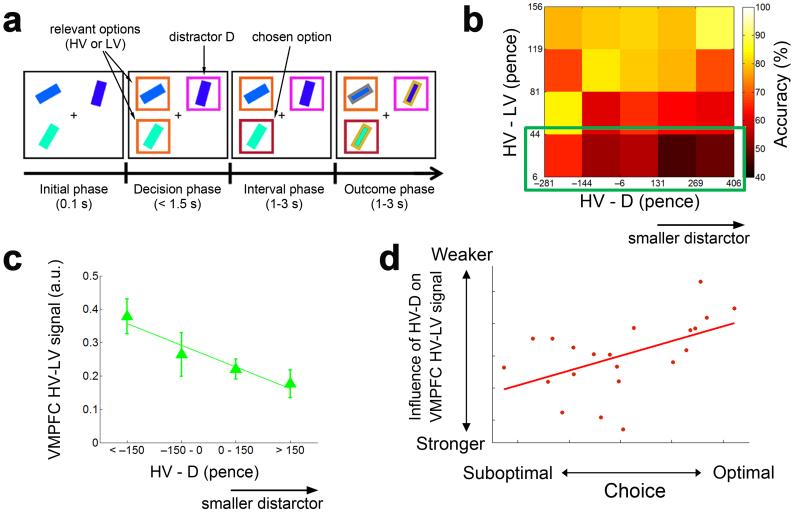
One way of integrating these ideas is to suggest that VMPFC does not just mediate value comparison, but is also required to maintain selective focus on information that is most relevant to the current goal. Chau and colleagues [52••] investigated how the presence of a third, but unavailable and therefore irrelevant, alternative would influence speeded choices between two other relevant options (Figure 2A). They found that people would on average make more suboptimal choices during difficult decisions when the value of the unavailable distractor was comparatively low and the presence of such a low value distractor reduced the VMPFC value comparison signal (Figure 2B-C). Moreover, subjects who showed the greatest influence of the distractor on the VMPFC value comparison signal also made fewer choices of the best option (Figure 2D). There was also evidence that this process was influenced by interactions with OFC. The value comparison signal in VMPFC was positively coupled with activity in lateral OFC whereas the influence of the distractor on the VMPFC signal was negatively coupled with a similar part of lateral OFC.
Figure 2. The behavioural and neural influence of an irrelevant distractor on decision making.
A) Task design in [52••]. Subjects were presented with three options each consisting of a rectangular bar of a particular colour presented at a particular orientation. Each colour and orientation combination was associated with specific reward magnitude and probability. 100ms after presentation of the 3 options, one of the options became an unavailable distractor, D, that could not be selected (indicated by the purple box). Participants had to make speeded decisions between the 2 relevant, available options (higher value option, HV, and lower value option, LV). B) During difficult trials when the two available options were close in value (green box), more suboptimal decisions were paradoxically made when the difference between the value of the best available option and the distractor (HV – D) was larger. C - D) The VMPFC HV-LV value comparison signal was also attenuated with increasing size of HV – D (panel C) and individuals who exhibited a stronger influence of the distractor on the VMPFC signals (i.e., a more negative HV-D effect) resulted in more suboptimal decisions (panel D). Adapted from [52••].
This could be interpreted to suggest that VMPFC might help facilitate context-appropriate value comparison in part through mechanisms that suppress information that is not currently relevant for their current motivational needs [44,53-55]. Such an account is congruent with recent evidence in rodents that stimulus-selective cells in medial OFC, unlike in lateral OFC, show a small but significant increase in firing to odours associated with the least valuable option in a delay/reward decision task [56]. Lesions to an adjacent structure – prelimbic cortex – also cause rats to fail to downregulate attention to a novel cue in a blocking paradigm even though it provides no new information to guide predictions and choice [57]. It will be interesting to determine whether a similar process of competition by mutual inhibition, which can successfully account for VMPFC value comparison signals and even the paradoxical effects of a distracting alternative [39••,52••], might be extended to generally predict such a function.
Conclusions
In this brief review, we have outlined ideas that suggest that OFC and VMPFC have key complimentary roles in selecting the appropriate information to allow appropriate value learning and value comparison to occur. OFC, through interactions with sensory cortex, can use stimulus-reward associations to enhance attention towards specific, task-relevant environmental information, which in turn can allow rapid contingent learning when new information is acquired; VMPFC, with access to information about the current motivational goals, can help suppress irrelevant value information impinging on an ongoing decision. These regions clearly do not perform these functions in isolation [cf. 52••] and it will be critical in the coming years to investigate how these two networks cooperate to promote selection. This will also require a comparison between OFC and VMPFC signals with interconnected brain areas [14,24•,48,52••], examining interactions between structures [52••,58,59], and particularly looking at how interference in one part of the network affects coding elsewhere [27,60]. Moreover, understanding the way in which these or other regions determine current task relevance and gather information in a dynamic setting is of primary importance [61,62].
Acknowledgements
MEW is supported by a Wellcome Trust Research Career Development Fellowship (090051) and SWK by a Wellcome Trust New Investigator Award. Many of the ideas in this article were initiated through work with Matthew Rushworth, Jon Wallis, Tim Behrens and MaryAnn Noonan, as well as from lengthy discussions with Laurence Hunt, Erie Boorman, and Nils Kolling.
References
- 1.White NM. Reward: What Is It? How Can It Be Inferred from Behavior? In: Gottfried JA, editor. Neurobiology of Sensation and Reward. Frontiers in Neuroscience; 2011. [Google Scholar]
- 2.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Padoa-Schioppa C. Neurobiology of economic choice: a good-based model. Annu Rev Neurosci. 2011;34:333–359. doi: 10.1146/annurev-neuro-061010-113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fellows LK. Orbitofrontal contributions to value-based decision making: evidence from humans with frontal lobe damage. Ann N Y Acad Sci. 2011;1239:51–58. doi: 10.1111/j.1749-6632.2011.06229.x. [DOI] [PubMed] [Google Scholar]
- ••6.Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–279. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This comprehensive review brings together a range of evidence to suggest that a key contribution of OFC is to encode the current task state.
- 7.Murray EA, Rudebeck PH. The drive to strive: goal generation based on current needs. Front Neurosci. 2013;7:112. doi: 10.3389/fnins.2013.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessup RK, O'Doherty JP. Distinguishing informational from value-related encoding of rewarding and punishing outcomes in the human brain. Eur J Neurosci. 2014;39:2014–2026. doi: 10.1111/ejn.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 10.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stalnaker TA, Cooch NK, McDannald MA, Liu TL, Wied H, Schoenbaum G. Orbitofrontal neurons infer the value and identity of predicted outcomes. Nat Commun. 2014;5:3926. doi: 10.1038/ncomms4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •12.Klein-Flugge MC, Barron HC, Brodersen KH, Dolan RJ, Behrens TE. Segregated encoding of reward-identity and stimulus-reward associations in human orbitofrontal cortex. J Neurosci. 2013;33:3202–3211. doi: 10.1523/JNEUROSCI.2532-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using repetition suppression, this was the first study to demonstrate with fMRI that representations of specific stimulus-outcome pairs, as well as of reward types, can be observed in different parts of OFC. This approach has also since been fruitfully used to explore how novel values are constructed in VMPFC-centred networks [36]
- 13.Roesch MR, Taylor AR, Schoenbaum G. Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron. 2006;51:509–520. doi: 10.1016/j.neuron.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •15.Rudebeck PH, Murray EA. Dissociable Effects of Subtotal Lesions within the Macaque Orbital Prefrontal Cortex on Reward-Guided Behavior. J Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study finds a double dissociation between the effects of lesions to macaque lateral OFC (areas 11/13) and medial OFC (area 14) on reward devaluation and both choice consistency respectively.
- 16.Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahnt T, Park SQ, Haynes JD, Tobler PN. Disentangling neural representations of value and salience in the human brain. Proc Natl Acad Sci U S A. 2014;111:5000–5005. doi: 10.1073/pnas.1320189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 19.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Ann N Y Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa M, van der Meer MA, Esber GR, Cerri DH, Stalnaker TA, Schoenbaum G. Risk-responsive orbitofrontal neurons track acquired salience. Neuron. 2013;77:251–258. doi: 10.1016/j.neuron.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••21.McDannald MA, Esber GR, Wegener MA, Wied HM, Liu TL, Stalnaker TA, Jones JL, Trageser J, Schoenbaum G. Orbitofrontal neurons acquire responses to 'valueless' Pavlovian cues during unblocking. Elife (Cambridge) 2014:e02653. doi: 10.7554/eLife.02653. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using an unblocking paradigm, the authors report strong evidence that a population of OFC cells respond to the sensory qualities of a cue and the predicted outcome, especially when this engenders new learning, even when controlling for reward value.
- 22.Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA. Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci. 2009;12:932–938. doi: 10.1038/nn.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci U S A. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •24.Hosokawa T, Kennerley SW, Sloan J, Wallis JD. Single-neuron mechanisms underlying cost-benefit analysis in frontal cortex. J Neurosci. 2013;33:17385–17397. doi: 10.1523/JNEUROSCI.2221-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compared single neuron responses in four different frontal regions, including OFC, as monkeys made binary choices between either delay- or effort-discounted rewards. A value comparison signal was not evident in any of the areas; only a small fraction of neurons in ACC reflected an integrated cost-benefit signal.
- 25.O'Neill M, Schultz W. Coding of reward risk by orbitofrontal neurons is mostly distinct from coding of reward value. Neuron. 2010;68:789–800. doi: 10.1016/j.neuron.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Walton ME, Behrens TE, Noonan MP, Rushworth MF. Giving credit where credit is due: orbitofrontal cortex and valuation in an uncertain world. Ann N Y Acad Sci. 2011;1239:14–24. doi: 10.1111/j.1749-6632.2011.06257.x. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi YK, Roesch MR, Wilson RC, Toreson K, O'Donnell P, Niv Y, Schoenbaum G. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nat Neurosci. 2011;14:1590–1597. doi: 10.1038/nn.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walton ME, Behrens TE, Buckley MJ, Rudebeck PH, Rushworth MF. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]; In a companion paper to [28], the authors found that the choices of monkeys with medial, but not lateral, OFC lesions were irrationally affected by the least valuable option in a 3-option decision task; by contrast lateral OFC lesions exhibited impairments in specific learning not shown by the medial OFC group.
- 30.Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiller D, Weiner I. Lesions to the basolateral amygdala and the orbitofrontal cortex but not to the medial prefrontal cortex produce an abnormally persistent latent inhibition in rats. Neuroscience. 2004;128:15–25. doi: 10.1016/j.neuroscience.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Panayi MC, Killcross S. Orbitofrontal cortex inactivation impairs between- but not within-session Pavlovian extinction: an associative analysis. Neurobiol Learn Mem. 2014;108:78–87. doi: 10.1016/j.nlm.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 33.McNamee D, Rangel A, O'Doherty JP. Category-dependent and category-independent goal-value codes in human ventromedial prefrontal cortex. Nat Neurosci. 2013;16:479–485. doi: 10.1038/nn.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barron HC, Dolan RJ, Behrens TE. Online evaluation of novel choices by simultaneous representation of multiple memories. Nat Neurosci. 2013;16:1492–1498. doi: 10.1038/nn.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallis JD, Kennerley SW. Heterogeneous reward signals in prefrontal cortex. Curr Opin Neurobiol. 2010;20:191–198. doi: 10.1016/j.conb.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XJ. Probabilistic decision making by slow reverberation in cortical circuits. Neuron. 2002;36:955–968. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- ••39.Hunt LT, Kolling N, Soltani A, Woolrich MW, Rushworth MF, Behrens TE. Mechanisms underlying cortical activity during value-guided choice. Nat Neurosci. 2012;15:470–476. S471–473. doi: 10.1038/nn.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using magnoencephalography, the authors showed that VMPFC neural dynamics exhibited signatures reflecting the overall decision value followed subsequently by a difference between the chosen and unchosen option. These signals are consistent with predictions from a biophysically plausible attractor network model of probabilistic 2-option decision making adapted from [38].
- 40.Boorman ED, Behrens TE, Woolrich MW, Rushworth MF. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 41.FitzGerald TH, Seymour B, Dolan RJ. The role of human orbitofrontal cortex in value comparison for incommensurable objects. J Neurosci. 2009;29:8388–8395. doi: 10.1523/JNEUROSCI.0717-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philiastides MG, Biele G, Heekeren HR. A mechanistic account of value computation in the human brain. Proc Natl Acad Sci U S A. 2010;107:9430–9435. doi: 10.1073/pnas.1001732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •43.Strait CE, Blanchard TC, Hayden BY. Reward Value Comparison via Mutual Inhibition in Ventromedial Prefrontal Cortex. Neuron. 2014;82:1357–1366. doi: 10.1016/j.neuron.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study to show that single VMPFC neurons not only encode decision offers as an integrated value signal, but that both offers are represented antagonistically as if reflecting a value comparison process.
- 44.Kolling N, Behrens TE, Mars RB, Rushworth MF. Neural mechanisms of foraging. Science. 2012;336:95–98. doi: 10.1126/science.1216930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt LT, Woolrich MW, Rushworth MF, Behrens TE. Trial-type dependent frames of reference for value comparison. PLoS Comput Biol. 2013;9:e1003225. doi: 10.1371/journal.pcbi.1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim SL, O'Doherty JP, Rangel A. The decision value computations in the vmPFC and striatum use a relative value code that is guided by visual attention. J Neurosci. 2011;31:13214–13223. doi: 10.1523/JNEUROSCI.1246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •47.Krajbich I, Armel C, Rangel A. Visual fixations and the computation and comparison of value in simple choice. Nat Neurosci. 2010;13:1292–1298. doi: 10.1038/nn.2635. [DOI] [PubMed] [Google Scholar]; The authors used a modified version of a drift diffusion model (DDM) to explore how visual fixations bias choice. The model predicted two important choice biases: choice probability is increased the longer an item is fixated and if the item is fixated last.
- 48.Kaping D, Vinck M, Hutchison RM, Everling S, Womelsdorf T. Specific contributions of ventromedial, anterior cingulate, and lateral prefrontal cortex for attentional selection and stimulus valuation. PLoS Biol. 2011;9:e1001224. doi: 10.1371/journal.pbio.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fellows LK. Deciding how to decide: ventromedial frontal lobe damage affects information acquisition in multi-attribute decision making. Brain. 2006;129:944–952. doi: 10.1093/brain/awl017. [DOI] [PubMed] [Google Scholar]
- 50.Henri-Bhargava A, Simioni A, Fellows LK. Ventromedial frontal lobe damage disrupts the accuracy, but not the speed, of value-based preference judgments. Neuropsychologia. 2012;50:1536–1542. doi: 10.1016/j.neuropsychologia.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Camille N, Griffiths CA, Vo K, Fellows LK, Kable JW. Ventromedial frontal lobe damage disrupts value maximization in humans. J Neurosci. 2011;31:7527–7532. doi: 10.1523/JNEUROSCI.6527-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••52.Chau BK, Kolling N, Hunt LT, Walton ME, Rushworth MF. A neural mechanism underlying failure of optimal choice with multiple alternatives. Nat Neurosci. 2014;17:463–470. doi: 10.1038/nn.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors extend the biophysical model described in [38,39••] to examine how speeded binary choices might be made in the presence of a 3rd unavailable, though potentially behaviourally-relevant, cue. They found a paradoxical effect in both the model and in human subjects’ behaviour of more suboptimal choices when the distractor value was particularly low, an effect that was also reflected in VMPFC BOLD signals.
- 53.Bouret S, Richmond BJ. Ventromedial and orbital prefrontal neurons differentially encode internally and externally driven motivational values in monkeys. J Neurosci. 2010;30:8591–8601. doi: 10.1523/JNEUROSCI.0049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hare TA, Malmaud J, Rangel A. Focusing Attention on the Health Aspects of Foods Changes Value Signals in vmPFC and Improves Dietary Choice. J Neurosci. 2011;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boorman ED, Rushworth MF, Behrens TE. Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. J Neurosci. 2013;33:2242–2253. doi: 10.1523/JNEUROSCI.3022-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burton AC, Kashtelyan V, Bryden DW, Roesch MR. Increased Firing to Cues That Predict Low-Value Reward in the Medial Orbitofrontal Cortex. Cereb Cortex. 2013 doi: 10.1093/cercor/bht189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharpe MJ, Killcross S. The prelimbic cortex contributes to the down-regulation of attention toward redundant cues. Cereb Cortex. 2014;24:1066–1074. doi: 10.1093/cercor/bhs393. [DOI] [PubMed] [Google Scholar]
- 58.Lim SL, O'Doherty JP, Rangel A. Stimulus value signals in ventromedial PFC reflect the integration of attribute value signals computed in fusiform gyrus and posterior superior temporal gyrus. J Neurosci. 2013;33:8729–8741. doi: 10.1523/JNEUROSCI.4809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark AM, Bouret S, Young AM, Murray EA, Richmond BJ. Interaction between orbital prefrontal and rhinal cortex is required for normal estimates of expected value. J Neurosci. 2013;33:1833–1845. doi: 10.1523/JNEUROSCI.3605-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudebeck PH, Mitz AR, Chacko RV, Murray EA. Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron. 2013;80:1519–1531. doi: 10.1016/j.neuron.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mante V, Sussillo D, Shenoy KV, Newsome WT. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature. 2013;503:78–84. doi: 10.1038/nature12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gershman SJ, Niv Y. Learning latent structure: carving nature at its joints. Curr Opin Neurobiol. 2010;20:251–256. doi: 10.1016/j.conb.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 64.Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, Rawlins JN, Walton ME, Rushworth MF, Baxter MG, et al. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci. 2009;30:472–484. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]