To the Editor
We present the case of a 4-month Saudi Arabian female infant born to consanguineous parents diagnosed shortly after birth with T-B+NK- severe combined immunodeficiency (SCID) of unknown molecular type. Of note, the patient had been vaccinated with Bacille-Calmette Guerrin (BCG) on day 1 of life, despite a family history of early death in a sibling due to infection and a sister with SCID who is status post a hematopoietic stem cell transplantation (HSCT) performed in Germany. Our patient was noted at presentation to have erythema at the site of her BCG vaccination. She was placed on oral isoniazid and clarithromycin. Initial Duke immunologic evaluation revealed serum IgG 511 (on IG replacement), IgA 0, IgM 21 mg/dl, and IgE <5 IU/ml. Flow cytometry confirmed the absence of T and NK cells, and proliferation studies demonstrated no responses to mitogens or antigens. Given the risk for disseminated BCG, she was given an unfractionated bone marrow transplant from an HLA identical normal sister. She did not receive pre-transplant conditioning or post-transplant graft versus host disease prophylactic immunosuppressive agents.
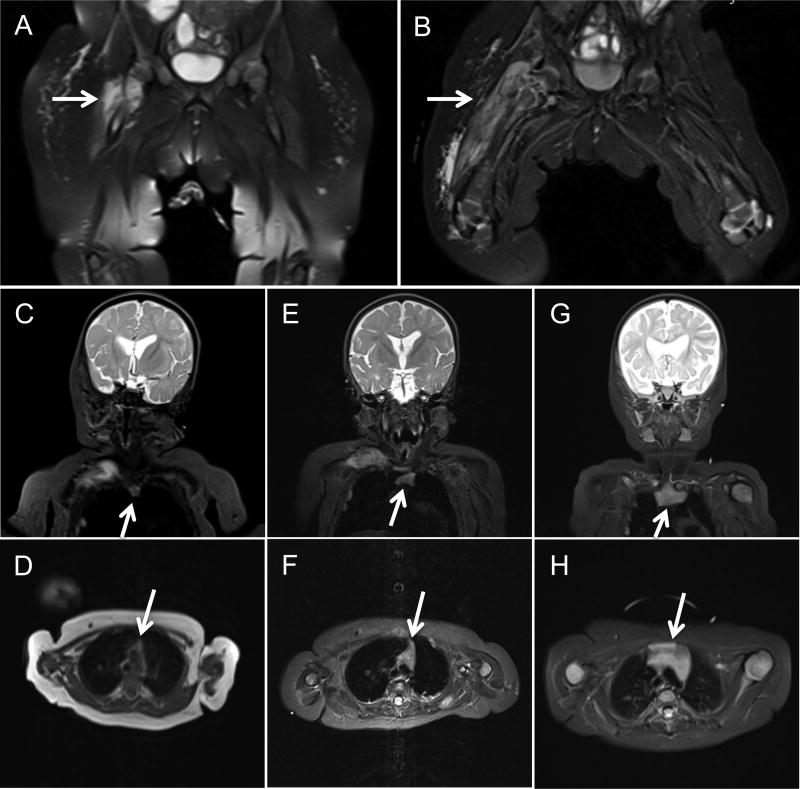
Fifteen days post-transplantation, she developed fever, an ulcerated BCG vaccination site, and multiple subcutaneous abscesses. Rifampin and ethambutol were added to her anti-mycobacterial regimen, and multiple skin biopsies eventually grew Mycobacterium bovis. An MRI of her pelvis approximately one month post-transplantation showed a right hip osteomyelitis (Figure 1AB). Aspiration and culture were positive for Mycobacterium bovis, resistant to pyrazinamide, streptomycin and standard doses of isoniazid. A whole body MRI showed extensive multifocal osteomyelitis, multiple abscesses including a large paraspinal abscess with cord impingement, pleural lesions and pulmonary microabscesses, and a solid mass within the abdomen. All immunologic and chimerism studies performed post-transplantation were consistent with successful engraftment of her sister's adoptively transferred mature immune cells and, with time, engraftment of donor stem cells resulting in normal thymic output. She has poor B cell function despite donor B cell chimerism, so continues to receive IVIG infusions.
The patient underwent multiple surgical interventions, with repeated biopsies revealing acid-fast bacilli. She was treated with a multidrug anti-mycobacterial treatment (MAT) regimen consisting of high dose isoniazid, amikacin, ethambutol, levofloxacin, and rifampin. Interferon gamma (IFN-γ) was started at a dose of 50 mcg/m2 and, subsequently, increased to 200 mcg/m2 over several weeks. Due to the burden of BCG disease we were concerned that engrafted donor T cells would become anergic. In view of this, she was infused biweekly with fresh whole blood from her BCG-immunized HLA identical donor to adoptively transfer BCG-specific T cells. Seven months status post HSCT, T cells from the patient (of donor origin) demonstrated a strong lymphoproliferative response to purified protein derivative (187,678 cpm) compared to controls. Serial whole body MRIs obtained every 4-8 weeks showed gradual improvement of her lesions, as well as progressive thymic enlargement until normalization (Figure 1), correlating with successful engraftment of her sister's bone marrow stem cells.
Figure 1.
Coronal sections from serial MRI 1 and 2 months status post HSCT demonstrate severe osteomyelitis and myositis of the right lower extremity (panels A, B). Coronal and axial sections from serial MRI shows progressive thymic enlargement at 1 month (panels C, D), 2 months (panels E, F) and 5 months (panels G, H) status post HSCT.
Four months post-transplantation, she was noted to have very good T cell function with normal responses to phytohemagglutinin, IL-2, concavalin A, pokeweed mitogen, anti-CD3, tetanus and candida; as well as a normal percentage of recent thymic emigrants (28% CD4+,CD45RA+ CD62L+). At approximately 5 ½ months post-transplantation, she was clinically stable with radiographic improvement of all of her lesions and became an outpatient. IFN-γ was continued for approximately 18 months; she continues to receive whole blood infusions from her donor every two weeks. She suffered hearing loss from aminoglycoside toxicity and has required reconstructive orthopedic procedures for her extensive osteomyelitis, but is, otherwise, doing well. Testing for known SCID-causing gene mutations, including Janus kinase 3, was negative. Thus far, whole exome sequencing has been unrevealing, so whole genome sequencing will be performed.
Complications of BCG vaccination occur often in SCID patients and include localized and disseminated disease, with the most frequent sites of dissemination being lymph nodes, skin, or lungs.1 While lesions commonly develop at BCG vaccination sites, multiple recurring subcutaneous abscess are uncommon.2 BCG osteitis has been reported in healthy children receiving the vaccination and has been shown to occur less frequently than other manifestations in SCID patients.1, 3 A recent retrospective, multicenter study showed complication rates of 51% among SCID patients receiving BCG.1 A majority of these patients developed disseminated disease associated with increased risk of death.1, 2, 4
This case illustrates several critical points associated with the management of disseminated BCG in SCID including the following: 1] comprehensive radiologic and microbiologic screening is indicated even in asymptomatic patients; 2] early and aggressive, parenteral MAT should be considered; 3] disseminated BCGosis should be presumed in all BCG-vaccinated patients with SCID until proven otherwise. Finally, immune reconstitution syndrome (IRS), marked by systemic inflammation due to “cytokine storm” is commonly reported after HSCT in BCG-infected patients with SCID. Treatment for IRS is high dose steroids. While glucocorticoids may dampen an overwhelming immune response they also suppress donor T cell-mediated immunity, critical for combating mycobacterial disease. Our patient's disease progression may have had an IRS component, but given the presence of acid-fast bacilli in repeated biopsies we posit the benefits of adoptive immunity and engraftment outweighed the potential benefits of immunosuppression.
To the best of our knowledge, the unique combination of treatments employed has not previously been described. Standard treatment consists of MAT and immune reconstitution with HCST1, 2, 4 High dose IFN-γ has been used for disseminated BCG infection in two patients with a deficiency of the interleukin-12/23 receptor β1, as well as for patients with disseminated mycobacterial infection, CD4 lymphopenia, and dominant partial interferon gamma receptor deficiency.5-7 Interestingly, this is the first case at our institution to document progressive thymic enlargement by serial MRI following stem cell engraftment in a SCID infant (Figure 1). While our patient survived it was not without significant cost and morbidity; underscoring the need to screen for primary immunodeficiency prior to administration of live vaccines.8
Clinical Implications.
This case highlights the importance of screening infants for primary immunodeficiency prior to immunization with live vaccines. It also suggests aggressive and novel management strategies for patients with SCID who develop disseminated BCG including: non-ablative unfractionated HLA identical HSCT; multidrug anti-mycobacterial treatment; high dose IFN-γ; and donor lymphocyte infusions.
Acknowledgments
Declaration of funding: none
Footnotes
Current address: DUMC 2898, Durham, NC 27710, Telephone: 919-684-3204, Telefax: 919-681-7979
Contributor Information
Lauren L. Smith, Children's Hospital of the King's Daughters, Eastern Virginia School of Medicine, Norfolk, VA
Benjamin L. Wright, Fellow in Allergy and Clinical Immunology, Duke University Medical Center, Durham, NC
Rebecca H. Buckley, Email: rebecca.buckley@dm.duke.edu, J. Buren Sidbury Professor of Pediatrics and Professor of Immunology, Duke University Medical Center, Durham, NC.
References
- 1.Marciano BE, Huang CY, Joshi G, Rezaei N, Carvalho BC, Allwood Z, et al. BCG vaccination in patients with severe combined immunodeficiency: complications, risks, and vaccination policies. J Allergy Clin Immunol. 2014;133:1134–41. doi: 10.1016/j.jaci.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzucchelli JT, Bonfim C, Castro GG, Condino-Neto AA, Costa NM, Cunha L, et al. Severe combined immunodeficiency in Brazil: management, prognosis, and BCG-associated complications. J Investig Allergol Clin Immunol. 2014;24:184–91. [PubMed] [Google Scholar]
- 3.Kroger L, Korppi M, Brander E, Kroger H, Wasz-Hockert O, Backman A, et al. Osteitis caused by bacille Calmette-Guerin vaccination: a retrospective analysis of 222 cases. J Infect Dis. 1995;172:574–6. doi: 10.1093/infdis/172.2.574. [DOI] [PubMed] [Google Scholar]
- 4.Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin Infect Dis. 1997;24:1139–46. doi: 10.1086/513642. [DOI] [PubMed] [Google Scholar]
- 5.Ulrichs T, Fieschi C, Nevicka E, Hahn H, Brezina M, Kaufmann SH, et al. Variable outcome of experimental interferon-gamma therapy of disseminated Bacillus Calmette-Guerin infection in two unrelated interleukin-12Rbeta1-deficient Slovakian children. Eur J Pediatr. 2005;164:166–72. doi: 10.1007/s00431-004-1599-2. [DOI] [PubMed] [Google Scholar]
- 6.Holland SM, Eisenstein EM, Kuhns DB, Turner ML, Fleisher TA, Strober W, et al. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report N Engl J Med. 1994;330:1348–55. doi: 10.1056/NEJM199405123301904. [DOI] [PubMed] [Google Scholar]
- 7.Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364:2113–21. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 8.Buckley RH. The long quest for neonatal screening for SCID. J Allerg Clin Immunol. 2012;129:597–604. doi: 10.1016/j.jaci.2011.12.964. [DOI] [PMC free article] [PubMed] [Google Scholar]