Abstract
A total of 27 children with esotropia (mean age, 3.9 years; range, 9 months to 13.8 years) were enrolled in a 9-month observational study following botulinum toxin A (BTX-A) injection of one (n = 7) or both (n = 20) medial rectus muscles. BTX-A dosage ranged from 3.0 to 6.0 units per muscle. Three participants developed tonic pupil, noted at the first follow-up visit, occurring 12-19 days after injection. All 3 cases occurred in the left eye of participants who underwent bilateral BTX-A injection by the same surgeon. Anisocoria diminished from a maximum of 4 mm at the 2-week visit to 1–2 mm in all patients over the 9-month postinjection data collection period. No adverse visual outcomes were noted. Tonic pupil is an infrequently reported complication of BTX-A injection for strabismus. The experience of our investigator group suggests the need for careful injection technique and thorough preinjection counseling.
Botulinum toxin A (BTX-A) has been used successfully in the management of strabismus, both as a primary therapeutic intervention1-3 and as adjunctive treatment with incisional strabismus surgery.4 Because it is administered by injection, treatment complications relate principally to trauma from the needle or unintended effects of toxin leakage outside the targeted extraocular muscle capsule. It is not surprising, then, that ptosis and vertical strabismus are commonly reported,5 presumably due to chemodenervation of the levator muscle or one or both vertical rectus muscles. The pharmacological effect of BTX-A on other intraconal and extraconal structures is less well known but may contribute to an infrequently reported complication, tonic pupil.6-10
Our investigator group undertook a 9-month observational pilot study to assess recruitment potential for a randomized controlled trial comparing BTX-A to incisional surgery for treatment of esotropia. Participants <17 years of age with esotropia were enrolled following BTX-A injection of one or both medial rectus muscles. Children who had incisional extraocular muscle surgery at the time of injection were excluded. Because the BTX-A injection was performed as part of routine patient care and was not part of the study, the injection technique was not standardized. Most injections were performed using a closed conjunctival technique without electromyography control; a single investigator used an electromyography needle for the injection for 1 participant. Participants were managed according to investigators’ routine clinical practice. Demographic, historical, and preinjection clinical data as well as details of the BTX-A injection and perioperative complications were collected at enrollment. Transient ptosis, consecutive exodeviations, and vertical deviations are common occurrences following BTX-A injection and were not considered complications for this study. Postinjection data from each visit were collected retrospectively after 9 months. Safety data are reported herein but postoperative alignment outcomes are not included because of substantial missing data, a small cohort, multiple etiologies of esotropia, and lack of a comparison group.
At 13 sites (13 surgeons), 27 participants aged 9 months to 13.8 years (mean, 3.9 ± 3.3) were enrolled; 11 (41%) were female and 19 (70%) were white. Esotropia onset occurred before 6 months of age in 13 participants (48%) and after 6 months of age in 14 (52%). Eight (30%) participants had prior strabismus surgery, and 1 (4%) had prior BTX-A injection. Mean age at injection was 3.9 ± 3.3 years (range, 9 months to 13.8 years). BTX-A injection was unilateral (n = 7) or bilateral (n = 20), and the dosage ranged from 3.0 to 6.0 units per muscle under general anesthesia. Preoperative esodeviation, measured by prism and alternate cover test, ranged from 10Δ to 65Δ (mean, 26Δ) at distance and from 12Δ to 65Δ (mean, 28Δ) at near (distance and near data reported for 20 and 22 participants, resp.). Of 27 participants, 4 (15%; 95% CI, 4%-34%) experienced a complication from the injection. One participant (4%; 95% CI, 0%-19%) had a left eye scleral perforation, which was noted at the time of injection and treated by laser retinopexy the same day with good visual and motor outcomes. Three participants (11%; 95% CI, 2%-29%) developed tonic pupil (3 of 5 participants with BTX-A injection by the same surgeon).
The 3 cases of tonic pupil were noted at the first postinjection visit occurring from 12-19 days after treatment. All 3 cases occurred in the left eye of participants who underwent bilateral BTX-A injection by the same surgeon, under general anesthesia. BTX-A, 4 units, in 0.1 cc of nonpreserved normal saline was administered through a 30-gauge, 16 mm needle without incising conjunctiva. Motility testing at the first postinjection visit showed minimal to no adduction weakness of the affected eye for all 3 participants. Initial pupil testing showed left eye mydriasis and minimal reactivity to light or an accommodative target. The only symptom reported in any subject was mild photosensitivity. Subsequent testing of affected eyes showed dilated pupils that were unresponsive to light but constricted to a near accommodative target. Two of the 3 participants had pharmacological testing with pilocarpine 0.125% within 4 weeks of injection; reversal of anisocoria did not occur in either participant. In all 3 cases, however, denervation hypersensitivity to pilocarpine 0.125% with reversal of the anisocoria was evident by 6-8 weeks post-BTX-A injection (Figure 1). The anisocoria gradually diminished during the 9-month data collection period, from 2.5, 3.5, and 4 mm differences at 2 weeks to 2.5, 2, and 2 mm differences at 2 months, and 1, 2, and 1 mm differences at 9 months. The photosensitivity that was present initially resolved by 9 months in all 3 cases. Visual acuity remained stable in all 3 participants.
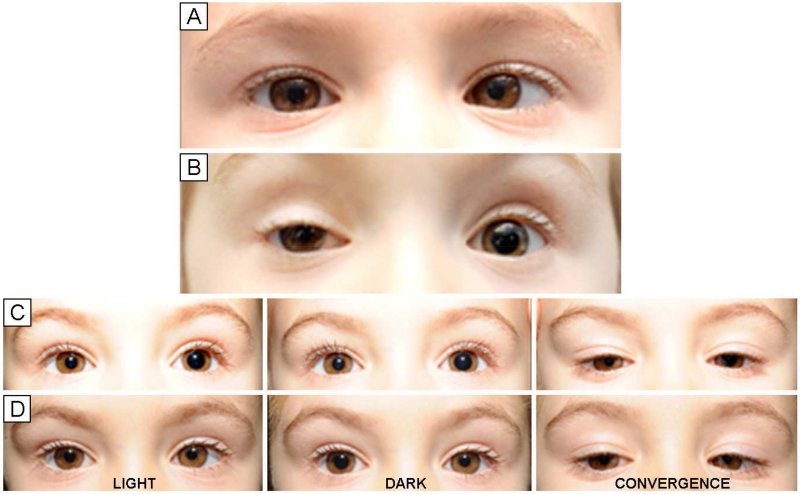
FIG 1.
Pre- and postinjection photographs showing pupil findings in a patient who developed a tonic left pupil after botulinum toxin-A injection of both medial rectus muscles for treatment of strabismus. A, Preinjection. B, 2 weeks after injection. C, 55 days after injection. D, 55 days after injection, post-pilocarpine. In this patient, as in all cases, affected pupils showed minimal responsiveness to light but constriction with fixation on a near accommodative target. Anisocoria was reversed after treatment of both eyes with pilocarpine 0.125%, diagnostic for tonic pupil.
Our investigator group experienced a somewhat higher-than-expected rate of complications following BTX-A treatment of children with esotropia. In a previous report of 5,587 BTX-A injections in 3,104 patients treated for horizontal strabismus, 9 patients had scleral perforations (0.28%).7 In the same series, 5 patients (0.16%) developed pupillary changes consistent with tonic pupil. This rate of tonic pupil is far lower than we report. One possible explanation for the higher incidence of tonic pupil in our series may have been inaccurate needle placement in the muscle. The tonic pupil occurred in the left eye of bilaterally treated patients. The surgeon for all cases was right-handed and reported that the left eye injection was more technically challenging than the right eye injection. The lack of significant adduction weakness following injection suggests that the BTX-A injection occurred outside the muscle capsule, either superficial to the muscle or, less likely, through the muscle. This could give rise to pharmacological blockade of the cholinergic terminals in the iris,10 either by means of trans-scleral diffusion or by ciliary artery perfusion if the injection was superficial to the muscle or to anticholinergic toxicity6 or direct injury at the level of the ciliary ganglion if the injection was deep to the muscle. While the tonic pupil occurred acutely after injection, denervation hypersensitivity to dilute pilocarpine was a delayed response. This is characteristic of tonic pupils after acute injury11 and probably results from up-regulation of acetylcholine receptors in the iris.
In older patients and adults, the risk of inaccurate needle placement may be mitigated by electromyography control. At the time of the Allergan series,7 injection with an electromyography needle was the clinical standard and may have ensured more reliable placement of the needle intramuscularly. Differences in level of investigator experience with BTX-A injections for strabismus may also have been a factor.
Supplementary Material
Acknowledgments
Supported by National Eye Institute of National Institutes of Health, Department of Health and Human Services EY011751, EY018810, and EY023198. The funding organization had no role in the design or conduct of this research.
Footnotes
Presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Denver, Colorado, May 3-7, 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044–9. doi: 10.1016/s0161-6420(80)35127-0. [DOI] [PubMed] [Google Scholar]
- 2.McNeer KW, Tucker MG, Guerry CH, Spencer RF. Incidence of stereopsis after treatment of infantile esotropia with botulinum toxin A. J Pediatr Ophthalmol Strabismus. 2003;40:288–92. doi: 10.3928/0191-3913-20030901-10. [DOI] [PubMed] [Google Scholar]
- 3.de Alba Campomanes AG, Binenbaum G, Campomanes Eguiarte G. Comparison of botulinum toxin with surgery as primary treatment for infantile esotropia. J AAPOS. 2010;14:111–16. doi: 10.1016/j.jaapos.2009.12.162. [DOI] [PubMed] [Google Scholar]
- 4.Ozkan SB, Topaloğlu A, Aydin S. The role of botulinum toxin A in augmentation of the effect of recession and/or resection surgery. J AAPOS. 2006;10:124–7. doi: 10.1016/j.jaapos.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Sener EC, Sanaç AS. Efficacy and complications of dose increments of botulinum toxin-A in the treatment of horizontal comitant strabismus. Eye (Lond) 2000;14:873–8. doi: 10.1038/eye.2000.240. [DOI] [PubMed] [Google Scholar]
- 6.Hemmerdinger C, Srinivasan S, Marsh IB. Reversible pupillary dilation following botulinum toxin injection to the lateral rectus. Eye (Lond) 2006;20:1478–9. doi: 10.1038/sj.eye.6702366. [DOI] [PubMed] [Google Scholar]
- 7.Botox [package insert] Allergan Inc; Irvine, CA: 2009. [Google Scholar]
- 8.Speeg-Schatz C. Persistent mydriasis after botulinum toxin injection for congenital esotropia. J AAPOS. 2008;12:307–8. doi: 10.1016/j.jaapos.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Akkaya S, Kökcen HK, Atakan T. Unilateral transient mydriasis and ptosis after botulinum toxin injection for a cosmetic procedure. Clin Ophthalmol. 2015;9:313–15. doi: 10.2147/OPTH.S76054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy Y, Kremer I, Shavit S, Korczyn AD. The pupillary effects of retrobulbar injection of botulinum toxin A (oculinum) in albino rats. Invest Ophthalmol Vis Sci. 1991;32:122–5. [PubMed] [Google Scholar]
- 11.Thompson HS. Adie’s syndrome: some new observations. Trans Am Ophthalmol Soc. 1977;75:587–626. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.