Introduction
Renal cell carcinoma (RCC) is the third most common cancer of the genitourinary system and in 2015 will account for an estimated 61,560 new cases and 14,080 deaths in the United States 1. Over the past several decades, the incidence of RCC has risen steadily by approximately 2-4% annually 2. Imaging plays an integral role in the evaluation and management of a patient with a renal mass, from the preoperative workup to the postoperative surveillance. Unfortunately, in clinical practice the urologist is often faced with imaging dilemmas that lack definitive answers. Herein we explore the current data behind contemporary imaging topics, including imaging a patient with renal insufficiency, establishing a surveillance protocol after RCC therapy, minimizing radiation therapy during surveillance, and emerging imaging trends.
Imaging in the Setting of Renal Insufficiency
Contrast-enhanced studies are a crucial part of the evaluation of a renal mass. Contrast administration, however, is associated with various patient risks. One of the primary risks associated with iodinated contrast is contrast-induced nephropathy (CIN). (Table 1) CIN is the acute deterioration of renal function after the administration of IV iodinated contrast. There is no consensus definition of CIN though the Acute Kidney Injury Network (AKIN) definition includes one of the following criteria: absolute increase in serum creatinine of 0.3 mg/dL from baseline, a 50% increase in serum creatinine from baseline, or urine output less than 0.5mL/kg/hour for at least six hours3.
Table 1.
Commonly Used Iodinated Contrast Agents
| Name | Compound | Iodine Content (mgl/mL) | Osmolality (mOsm/kg H2O) | |
|---|---|---|---|---|
| Hexabrix (Covidien) | Ionic | 320 | 600 | Low |
| Conray 43 (Covidien) | Ionic | 202 | 1000 | High |
| Hypaque 50 (Nycomed) | Ionic | 300 | 1550 | High |
| Visipaque 320 (GE Healthcare) | Nonionic | 320 | 290 | Low |
| Omnipaque 140 (GE Healthcare) | Nonionic | 140 | 322 | Low |
| Ultavist 300 (Bayer) | Nonionic | 300 | 607 | Low |
It is widely agreed upon that past a certain degree of baseline renal insufficiency, iodinated contrast should not be administered. Unfortunately, there is poor evidence for defining this exact threshold. One survey of 420 radiologists revealed the three most common serum creatinine thresholds for avoiding iodinated contrast were 1.5, 1.7, and 2.0 mg/dL used by 35%, 27%, and 31% of radiologists, respectively4. The American College of Radiology Committee on Drugs and Contrast Media, however, notes that eGFR provides the best level of evidence for risk stratification of CIN and suggests that iodinated contrast can be safely administered in patients with eGFR ≥30 mL/min/1.73m23.
Prevention of CIN is important to the urologist, especially given the anticipated nephron loss associated with many RCC treatments. Several preventative measures may be employed to help mitigate the risk of CIN. Intravenous hydration is the principle intervention shown to reduce the incidence of CIN and should be part of any mitigation protocol for at-risk patients receiving iodinated contrast5. Further, some data shows hydration with IV 0.9% saline is superior to 0.45% saline5. Another important principle is avoiding the use of high osmolality contrast media in patients with renal dysfunction, as level I evidence demonstrates its greater nephrotoxicity compared to low osmolality contrast media6 (Table 1). Two other methods used to reduce the incidence of CIN, sodium bicarbonate and N-acetylcysteine, have had conflicting meta-analysis findings and consequently have significant variability in their clinical use. Given the clinical equipoise of these interventions, a prospective, randomized trial (The Prevention of Serious Adverse Events following Angiography (PRESERVE)) involving enrollment of 8680 patients is currently underway to provide definitive conclusions on the efficacy of sodium bicarbonate and N-acetylcysteine. Other interventions (e.g. endothelin-1, theophylline) are theoretically renoprotective yet have no data supporting their clinical use.
In patients at high-risk of developing CIN, efforts should be made to utilize alternative imaging including non-contrast CT, ultrasound, or MRI with gadolinium-based contrast agents (GBCAs) when possible. GBCAs, however, carry their own risk in patients with renal insufficiency, as they may develop nephrogenic systemic fibrosis (NSF). In the past, renal insufficiency was an absolute contraindication to receiving GBCAs. However, as the data associated with NSF was more carefully analyzed, it became clear that many patients with renal insufficiency could receive GBCAs with minimal risk. For instance, NSF in patients with eGFR > 30 ml/min/1.73 m2 is exceptionally rare and GBCAs can be safely administered3. The only caveat is that patients with eGFR of 30-40 should be treated similarly to those with eGFR <30, as eGFR may fluctuate on a day-to-day basis.
Patients with eGFR <30, and especially those with eGFR <15, are most at risk for NSF and so GBCA administration is not recommended in most cases. However, one literature review analyzed risk factor data based on 290 NSF cases and determined several key risk factors increased the incidence of NSF by approximately ten-fold each7. The most important were high dosage (>0.1 mmol/Kg) of GBCA, a delay in dialysis post-GBCA administration (for patients already on dialysis), and GBCA use during acute kidney injury. If these risk factors can all be avoided, the risk of NSF can be reduced by a thousand-fold. Another reported risk factor is the specific agent used, as three particular GBCAs (gadopentetate dimeglumine (Magnevist), gadodiamide (Omniscan), and gadoversetamide (Optimark)) are responsible for the majority of NSF cases and are contraindicated in at-risk patients8.
In summary, caution should be exercised when administering GBCA in patients with GFR <30. For those in whom GBCA-enhanced MRI is deemed necessary, only low-dose GBCA should be administered, hemodialysis should be initiated immediately following the procedure for patients on renal replacement therapy, injection of high-risk GBCAs should be avoided, and the study should not be performed in the setting of acute kidney injury. Moreover, alternative contrast-free methods, such as arterial spin labeling (ASL) perfusion MRI or diffusion MRI, can be employed to provide useful diagnostic information.
Post-surgical Surveillance Imaging
Although surgical excision of organ-confined kidney cancer is often curative, local and distant recurrence rates vary by stage and histology 9. Thus, the goals of surveillance imaging include detection of both metastasis and local recurrence at an early time point. Follow-up after RCC resection is individualized and based on the patient's risk factors for recurrence, which in turn can be predicted by several different models.
Both the 2015 NCCN and AUA guidelines on follow-up after treatment (PN or RN) of RCC use only TNM stage to stratify patients into risk groups 10,11 with subsequent follow-up regimens tailored to the specific groups. An as example, in both the NCCN and AUA guidelines, follow-up of a low risk pT1N0M0 patient entails baseline abdominal imaging (CT, MRI or US) within 3-12 months of surgery. Thereafter, patients treated with PN may optionally receive yearly abdominal imaging (CT, MRI, or US) for three years based on the presence of additional risk factors, while RN-treated patients need only undergo further abdominal imaging at the urologist's discretion. Finally, annual chest imaging is recommended for three years in all low risk patients. Another important consideration is surveillance following ablative therapies (i.e. cryoablation, radiofrequency ablation, and microwave ablation). Given that local recurrence is higher with ablative therapies, patients need to be followed more closely12. Current NCCN guidelines suggest baseline abdominal CT or MRI followed by five years of abdominal (CT, MRI, or US) and chest (CT or CXR) imaging. Finally, although non-ccRCC has very different outcomes compared to ccRCC, surveillance protocols are independent of histology. Thus, the onus is on the clinician to institute less rigorous surveillance for more indolent tumors (e.g. chromophobe) or more vigilant follow-up for more aggressive tumors (e.g. papillary type 2).
While stage-based surveillance protocols are straightforward and benefit from relative ease of use, alternative surveillance scoring systems and nomograms have been developed that utilize both clinical and pathological variables to stratify patients and predict the likelihood of tumor recurrence. For instance, the UCLA Integrated Staging System (UISS) places postoperative RCC patients into low, intermediate, and high-risk strata based on Fuhrman nuclear grade, ECOG PS, and T stage 13, while the Leibovich model uses tumor stage, regional lymph node status, tumor size, Fuhrman nuclear grade, and histologic tumor necrosis to predict metastatic recurrence after radical nephrectomy for ccRCC. However, none of the proposed models in the literature is free from error in delineating high-risk from low-risk patients, as a review of all postoperative models assessing recurrence showed C-indices range from 74%-82.2%14. Despite the accuracy limitations of the various models, the 2014 EAU Guidelines on Renal Cell Carcinoma recommend that the clinician choose a risk-stratifying model for use in practice15.
Importantly, however, no level I evidence exists on which to base surveillance protocols, as the literature is based only on observational and case study data. The AUA surveillance guideline notes inconsistent outcomes when attempting to incorporate grade or other prognostic factors, and therefore settled on using TMN stage as the sole risk stratification metric. Data, however, indicate that urologists often do not follow a risk-adapted approach to surveillance imaging as suggested by the guidelines16. An analysis of the Surveillance, Epidemiology and End Results (SEER)-Medicare database revealed that surveillance imaging is over-utilized in low risk patients (e.g. pT1) while under-utilized in high risk patients (e.g. pT3) following nephrectomy17. Moreover, a recent study by Stewart et al. suggests that the current AUA and NCCN guideline recommendations may be inadequate for detecting recurrences18. They analyzed 3,651 patients who underwent RN or PN for M0 RCC and determined the number of recurrences when following the 2014 NCCN and AUA guidelines for surveillance. At a median of 9 years, almost one third of patients will have developed a recurrence that was missed by the 2014 NCCN and AUA guidelines. These findings suggest that current surveillance guidelines should become more intensive. On the other hand, as Smith et al. pointed out in an editorial response, extending the surveillance guidelines based on this study might be premature19. The most important reason is that the overall survival benefit of increased surveillance after RCC therapy is unproven. Further, there are multiple drawbacks to increased surveillance, including increased cost, effect on quality of life, and the risks of radiation exposure. In particular, the Medicare costs of surveillance based on current guidelines range from from $898 to $3,701, yet would rise to over $10,000 or more if surveillance were lengthened to capture 95% of RCC recurrences.
One response to the acknowledged inadequacies of the current guidelines is a novel, risk-based surveillance model that balances the risk of recurrence with the risk of non-RCC death. The Mayo Clinic developed a model that incorporates Charlson comorbidity index (CCI), pathologic tumor stage, and relapse location-specific data to predict the optimal duration of surveillance20. For instance, in an 80 year-old patient with pT1Nx-0 RCC and CCI of 1 or less, the risk of abdominal recurrence only exceeds the risk of non-RCC for a six-month period postoperatively. Therefore, in this example, surveillance is not warranted for more than six months and excessive costs, radiation exposure, etc. are avoided. Conversely, in a 50 year-old patient with pT1Nx-0 disease and a CCI of 1 or less, the risk of abdominal recurrence exceeded the risk of non-RCC for a 20-year period, indicating surveillance for longer than current guideline recommendations is warranted.
Another promising alternative to more intensive or lengthier surveillance methods is tailoring recurrence risk and surveillance to the individual patient's RCC tumor biology rather than TNM stage as used in AUA/NCCN guidelines. For example, one large retrospective analysis of 472 total patients with sporadic ccRCC showed median overall survival was significantly shorter in the BAP1-mutant group compared to the PBRM1-mutant group (4.6 vs. 10.6 years, p=0.044)21. Further, a 16-gene signature (Oncotype DX) recurrence score was recently validated in 626 patients as a predictor of recurrence after nephrectomy in stage I-III ccRCC. Knowing that different ccRCC gene mutations have different survival profiles may lead to better recurrence risk stratification and future surveillance guidelines.
Another challenge related to post-RCC treatment surveillance is balancing the need for intensive surveillance with the attendant risks of radiation exposure including the development of radiation-induced malignancies. The lifetime risk of a secondary malignancy related to surveillance after RCC treatment is largely unexamined. However, the risk is likely non-trivial. For instance, an estimation of lifetime cancer risk was calculated by Tarin et al. based on a five-year NCCN surveillance protocol for stage I nonseminomatous germ cell tumors of the testis22. By their calculations, a 40-year-old patient has a lifetime cancer risk of 1 in 61 (1.6%) after undergoing sixteen CTs of the chest/abdomen/pelvis in a five-year period. By comparison, an intermediate risk RCC patient following the UISS surveillance protocol would undergo thirteen chest CTs and five abdominal CTs over a ten-year period. Moreover, one study retrospectively analyzed the postsurgical surveillance of 315 patients with a pT1a RCC and found the relative risks of radiation-related solid cancers and leukemia were 1.05 and 1.12, respectively23. Again, these are small but non-negligible risks, especially in younger patients with RCC. Additionally, the absence of uniform surveillance regimens further complicates the issue of defining radiation risk. One review revealed that twelve total surveillance regimens exist in the literature with widely varying levels of radiation. For example, in a pT1b RCC lesion, if surveillance protocols were strictly followed a sample patient would receive anywhere from 0.5-450 mSv of cumulative radiation depending on the specific protocol24. Overall, it is clear that surveillance protocols pose a small but non-trivial risk of secondary malignancy, though the exact risk is poorly defined and protocol-dependent. Given the available data, modalities that lack ionizing radiation (e.g. MRI and US) should be considered in surveillance, especially in those patients with a long life expectancy and those with a low-risk of recurrence (e.g. T1a tumors).
In short, current guidelines and the majority of urologists favor the TNM staging system for its simplicity, though more sophisticated tools (e.g. nomograms, gene signatures, etc.) may ultimately play a larger role in the future given recent data on missed recurrences. The most important questions requiring further study include whether surveillance impacts overall survival and the optimal timing and duration of surveillance to best detect metastases. Finally, it should be noted that the above strategies are applicable to surgical extirpation of RCC. Less data is available for surveillance after ablative therapies, though theoretically surveillance should be more rigorous given the higher rate of local recurrence in these treatments.
Contemporary Trends and Future Investigation
An important point to note is that renal masses represent a heterogeneous group of tumors that may be subdivided into various histological entities with different survival and oncologic outcomes. For instance, up to 30% of surgically resected kidney tumors less than 4cm in size will have a benign pathology (e.g. oncocytoma, angiomyolipoma)25-27. Further, a significant portion of small renal masses (SRMs) are of the chromophobe or papillary type I RCC subtype, both of which portend a significantly better disease specific survival compared to clear cell RCC (ccRCC) histology28,29. There is thus a definite advantage to preoperatively identifying the histology of a SRM, as both the benign and less aggressive tumors (i.e. low-grade clear cell, papillary type I and chromophobe) could potentially be managed with active surveillance whereas more aggressive tumors should be surgically removed. However, no imaging modality has yet proven capable of reliably differentiating benign from malignant tumors or distinguishing between the histologic subtypes of the malignant tumors 30. Of note, biopsy-based risk stratification is emerging as a potentially viable option to determine active surveillance versus surgical excision, but biopsy remains an inherently invasive procedure with a risk of morbidity31. Ideally, a patient could preoperatively undergo a non-invasive imaging study to ascertain the histology of the renal mass. Molecular imaging modalities may be able to help bridge the gap between structural imaging (CT/MRI) and histologic diagnosis (biopsy).
Molecular Imaging
The paradigm may be changing with the introduction of iodine-124 (124I)– cG250 PET/CT, a novel molecular imaging biomarker specific for ccRCC. This modality takes advantage of the fact that clear cell RCC overexpresses the enzyme carbonic anhydrase IX (CAIX), while non-clear cell RCC and normal tissues do not. Furthermore, the chimeric monoclonal antibody cG250 (girentuximab) specifically targets CAIX, allowing the radiotracer 124I-girentuximab to localize in ccRCC on PET/CT.
Two clinical trials thus far have investigated the potential of 124I-girentuximab PET/CT to preoperatively detect ccRCC. The first was a phase I pilot study, in which 26 patients with renal masses scheduled to undergo surgical resection were given 124I-girentuximab 32. The preliminary results were quite favorable: 15/16 ccRCC and 9/9 non-ccRCC masses were correctly identified on preoperative PET/CT, with 94% and 100% sensitivity and specificity, respectively. A phase III open-label trial (REnal Masses: Pivotal Study to DETECT Clear Cell Renal Cell Carcinoma With Pre-Surgical PET/CT [REDECT]) was subsequently conducted at fourteen centers 33. In this trial, 195 patients with renal masses were administered (124)I-girentuximab and preoperative PET/CT was then performed. The imaging findings were then compared to the histopathology. The results echoed those of the phase I trial: average sensitivity, specificity, positive predictive value, and negative predictive value of (124)I-cG250 PET for preoperative identification of ccRCC was 86.2% (95% CI, 75.3% to 97.1%), 85.9% (95% CI, 69.4% to 99.9%), 94.4%, and 69.4% respectively. The implications of these trials are far-reaching. As described above, the indeterminate SRM poses a clinical dilemma with multiple management options, including active surveillance, biopsy, ablation, and surgical excision34. Preoperative knowledge of the histology could reduce a number of unnecessary surgeries for benign renal masses and indolent RCCs and could ultimately supplant the renal mass biopsy.
While the results of the phase III trial is certainly optimistic, as Khandani et al. pointed out in their review of the data, important questions must be answered before this test plays a role in the routine management of the indeterminate SRM [37]. First, the study did not examine just SRMs but also included renal masses up to 22cm. Moreover, analysis of the T1a subgroup showed a sensitivity of just 70.8% for masses less than or equal to 2cm, while failing to supply PPV, NPV, or specificity values for this subgroup. The main utility of this imaging modality is in the workup of the SRM and so more essential data related to SRMs is needed before this molecular imaging test reaches routine clinical practice. In addition, a technical concern raised by Khandani et al. is that the PET/CT scanners currently utilized by hospitals are inadequately equipped for adjustments related to optimal imaging of SRMs; that is, prompt γ correction and longer acquisition times may be needed for proper image quality but simply are not available on the typical hospital's PET/CT machine 35. Finally, in the event that 124I-girentuximab PET/CT does not detect ccRCC, the histology and malignant potential of the mass remains unknown. This may be a common scenario given that non-ccRCC accounts for approximately 25% of kidney tumors. In short, this technology is promising and may significantly alter the clinical practice of a SRM but both technical considerations and the need for additional data may limit its immediate impact.
Perfusion MRI and Diffusion MRI
Like (124I)– cG250 PET/CT, perfusion MRI and diffusion MRI are contemporary imaging technologies that may provide information about tumor histology as well as physiology. Perfusion MRI examines the microcirculation at the capillary level. There are three perfusion MRI methods: Dynamic Contrast Enhanced (DCE), Dynamic Susceptibility Contrast (DSC) and Arterial Spin Labeling (ASL). The former two require the administration of a gadolinium-based contrast agent, while ASL uses blood as an endogenous contrast material. Using corresponding imaging protocols and postprocessing techniques 36, various perfusion parameters, such as transfer constant (Ktrans), blood flow, and blood volume, can be obtained.
Perfusion MRI has been applied in the characterization of renal masses, providing histologic information such as subtype and grade of tumor (Figure 1)37. For instance, Lanzman et al. prospectively obtained preoperative ASL MRI scans in 34 patients with renal masses and compared the results to the postoperative histopathology 38. Notably, their results showed that oncocytomas demonstrate both higher peak and mean levels of perfusion than all types of RCC, including chromophobe. Oncocytoma is often indistinguishable from chromophobe RCC and this imaging modality may provide a way to avoid surgery and/or biopsy when the preoperative suspicion for oncocytoma is high. Sun et al. used DCE MRI to retrospectively examine the enhancement patterns of pathology-proven clear cell, papillary, and chromophobe RCCs masses. They concluded that each subtype has a characteristic signal intensity change, and this allowed, for example, distinguishing ccRCC from papillary RCC with a 93% sensitivity and 96% specificity39. However, the overall applicability of both ASL and DCE MRI to a SRM needs further validation, as neither of the two discussed studies provided T1a subgroup analysis nor relevant statistics such as positive and negative predictive value.
Figure 1. Perfusion MRI.
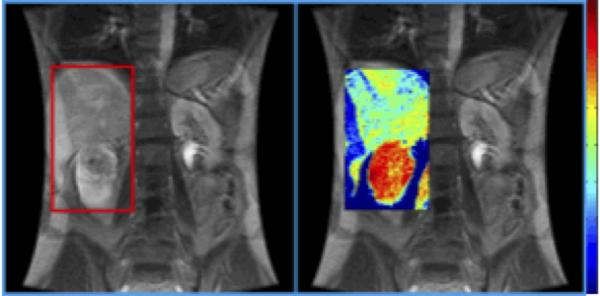
Coronal T1-weighted MRI (left) and Dynamic contrast-enhanced (DCE) MRI (right) of a right renal mass. 3D perfusion parametric map was obtained showing the microcirculation of the mass. Red color indicates a high level of perfusion. Pathology revealed clear cell RCC, Fuhrman grade 4.
Reproduced from Wu et al. with permission.37
Diffusion MRI reflects random thermal motion of water molecules and can be used to detect and characterize diffusion restricting lesions (Figure 2)37. Diffusion weighted imaging (DWI) with Apparent Diffusion Coefficient (ADC) map can be obtained using a diffusion weighted sequence with a b factor 40. Images acquired with a low b factor have higher signal to noise ratio and perform well in lesion detection, whereas images acquired with a higher b factor have better contrast and perform better in lesion characterization.
Figure 2. Diffusion Weighted MRI.
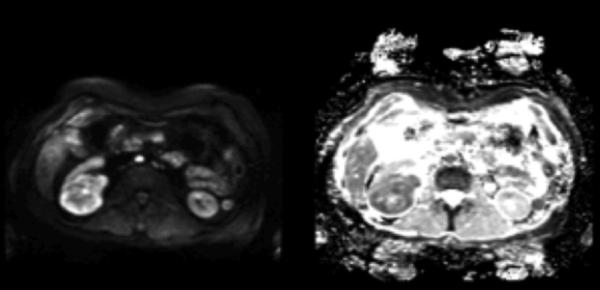
Axial DWI (left) and ADC (right) images of the same right renal mass with a b value of 800 s/mm2. On DWI, the high-grade clear cell RCC appears hyperintense, showing restricted diffusion, while the ADC map shows hypointensity, confirming this finding. Reproduced from Wu et al. with permission.37
Wang et al. retrospectively evaluated 85 renal masses imaged with DWI and assessed the ability of ADCs to predict RCC subtype 41. The findings showed that a high b value (of 800 sec/mm2) allowed statistically significant differentiation of clear cell, papillary, and chromophobic RCCs. Further, ccRCC could be differentiated from non-ccRCC with high sensitivity (95.9%) and specificity (94.4%), suggesting that DWI could possibly be a useful modality for preoperative characterization of a SRM. Limitations include the retrospective nature of the study, median mass size of 4.4cm, and absence of T1a subgroup data. Similarly, Taouli et al. retrospectively analyzed 109 renal lesions with DWI and concluded that imaging based diagnosis of solid RCC versus oncocytoma can be accomplished with an area under the curve of 0.85442.
In contrast to the work of Wang et al. and Taouli et al., a retrospective study by Sandrasegaran et al. using DWI for characterization of renal masses had differing results. With a sample size of 42 patients, preoperative ADC measurements of renal masses (using a b value of 800 sec/mm2) were compared to postoperative pathology 43. The ADC values of the benign cystic lesions were significantly higher than those of the cystic malignant lesions, suggesting that this modality may help reliably differentiate between malignant and non-malignant cysts. The study did not detect a significant difference in ADC values between the different RCC subtypes or tumor grade.
Radiomics
Radiomics is an emerging form of automated image analysis that acquires large amounts of data from images in order to make quantitative decisions about defined tumor regions37. The underlying hypothesis is that tumor genomic and proteomic heterogeneity is expressed as intra-tumoral heterogeneity on imaging44. Thus, this type of quantitative analysis has the potential to non-invasively predict tumor phenotypes. Gaing et al. performed heterogeneity analysis (mean, standard deviation, skewness and kurtosis) of intravoxel incoherent motion imaging (IVIM) parameters (perfusion fraction (fp), tissue diffusivity (Dt), and pseudodiffusivity (Dp)) from DWI MRI preoperatively performed on 44 patients with histopathology proven renal cell carcinomas45. They reported that IVIM parameters fp and Dt differentiated 8 of 15 subtype pairs of renal tumors, while histogram analysis differentiated 9 of 15 subtype pairs. These results demonstrate that histogram analysis of IVIM parameters may add complementary value to routine MRI measurements and is a feasible way of distinguishing between renal subtypes.
Conclusions
A number of topics related to kidney cancer imaging are evolving or lack consensus answers and are of great contemporary interest to the field of urology. Safely obtaining contrast-enhanced imaging in patients with renal insufficiency is a topic that plagues all clinicians, though there are a number of proven interventions to ameliorate the risk of CIN. Surveillance protocols are currently stage based, though more sophisticated models employing clinical, pathologic, and genetic variables offer promise for better risk stratification. Finally, novel imaging techniques such as molecular imaging, perfusion/diffusion MRI, and radiomics show great promise in revealing histologic diagnosis of tumors.
Acknowledgments
Funding:
This work is supported by a grant from the National Cancer Institute (P30CA072720).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015 Jan-Feb;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr. Rising incidence of renal cell cancer in the United States. Jama. 1999 May 5;281(17):1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Media ACoDaC. ACR Manual on Contrast Media. 10.1 ed2015:33-44 [Google Scholar]
- 4.Elicker BM, Cypel YS, Weinreb JC. IV contrast administration for CT: a survey of practices for the screening and prevention of contrast nephropathy. AJR. American journal of roentgenology. 2006 Jun;186(6):1651–1658. doi: 10.2214/AJR.05.0407. [DOI] [PubMed] [Google Scholar]
- 5.Weisbord SD, Palevsky PM. Prevention of contrast-induced nephropathy with volume expansion. Clinical journal of the American Society of Nephrology : CJASN. 2008 Jan;3(1):273–280. doi: 10.2215/CJN.02580607. [DOI] [PubMed] [Google Scholar]
- 6.Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993 Jul;188(1):171–178. doi: 10.1148/radiology.188.1.8511292. [DOI] [PubMed] [Google Scholar]
- 7.Prince MR, Zhang HL, Roditi GH, Leiner T, Kucharczyk W. Risk factors for NSF: a literature review. Journal of magnetic resonance imaging : JMRI. 2009 Dec;30(6):1298–1308. doi: 10.1002/jmri.21973. [DOI] [PubMed] [Google Scholar]
- 8.Broome DR. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. European journal of radiology. 2008 May;66(2):230–234. doi: 10.1016/j.ejrad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. The Journal of urology. 2007 Jul;178(1):41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Donat SM, Diaz M, Bishoff JT, et al. Follow-up for Clinically Localized Renal Neoplasms: AUA Guideline. The Journal of urology. 2013 Aug;190(2):407–416. doi: 10.1016/j.juro.2013.04.121. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2015. Journal of the National Comprehensive Cancer Network : JNCCN. 2015 Feb;13(2):151–159. doi: 10.6004/jnccn.2015.0022. [DOI] [PubMed] [Google Scholar]
- 12.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. The Journal of urology. 2009 Oct;182(4):1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. The Journal of urology. 2005 Aug;174(2):466–472. doi: 10.1097/01.ju.0000165572.38887.da. discussion 472; quiz 801. [DOI] [PubMed] [Google Scholar]
- 14.Sun M, Shariat SF, Cheng C, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011 Oct;60(4):644–661. doi: 10.1016/j.eururo.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 15.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015 May;67(5):913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Leibovich BC. Deconstructing RCC Surveillance Guidelines: How to Close the Gaps to Improve Detection of Recurrences. Kidney Cancer Journal. 2015;13(2):38–40. [Google Scholar]
- 17.Feuerstein MA, Atoria CL, Pinheiro LC, Huang WC, Russo P, Elkin EB. Patterns of surveillance imaging after nephrectomy in the Medicare population. BJU international. 2014 Nov 10; doi: 10.1111/bju.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart SB, Thompson RH, Psutka SP, et al. Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Dec 20;32(36):4059–4065. doi: 10.1200/JCO.2014.56.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AB, Milowsky MI. Is extending surveillance guidelines for renal cell carcinoma without understanding patient outcomes putting the cart before the horse? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Dec 20;32(36):4031–4032. doi: 10.1200/JCO.2014.58.3195. [DOI] [PubMed] [Google Scholar]
- 20.Stewart-Merrill SB, Thompson RH, Boorjian SA, et al. Oncologic Surveillance After Surgical Resection for Renal Cell Carcinoma: A Novel Risk-Based Approach. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Sep 8; doi: 10.1200/JCO.2015.61.8009. [DOI] [PubMed] [Google Scholar]
- 21.Kapur P, Pena-Llopis S, Christie A, et al. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. The Lancet. Oncology. 2013 Feb;14(2):159–167. doi: 10.1016/S1470-2045(12)70584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarin TV, Sonn G, Shinghal R. Estimating the risk of cancer associated with imaging related radiation during surveillance for stage I testicular cancer using computerized tomography. The Journal of urology. 2009 Feb;181(2):627–632. doi: 10.1016/j.juro.2008.10.005. discussion 632-623. [DOI] [PubMed] [Google Scholar]
- 23.Lipsky MJ, Shapiro EY, Hruby GW, McKiernan JM. Diagnostic radiation exposure during surveillance in patients with pT1a renal cell carcinoma. Urology. 2013 Jun;81(6):1190–1195. doi: 10.1016/j.urology.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 24.Lin YK, Gettle L, Raman JD. Significant variability in 10-year cumulative radiation exposure incurred on different surveillance regimens after surgery for pT1 renal cancers: yet another reason to standardize protocols? BJU international. 2013 May;111(6):891–896. doi: 10.1111/j.1464-410X.2012.11531.x. [DOI] [PubMed] [Google Scholar]
- 25.Akdogan B, Gudeloglu A, Inci K, Gunay LM, Koni A, Ozen H. Prevalence and predictors of benign lesions in renal masses smaller than 7 cm presumed to be renal cell carcinoma. Clinical genitourinary cancer. 2012 Jun;10(2):121–125. doi: 10.1016/j.clgc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Pahernik S, Ziegler S, Roos F, Melchior SW, Thuroff JW. Small renal tumors: correlation of clinical and pathological features with tumor size. The Journal of urology. 2007 Aug;178(2):414–417. doi: 10.1016/j.juro.2007.03.129. discussion 416-417. [DOI] [PubMed] [Google Scholar]
- 27.Corcoran AT, Russo P, Lowrance WT, et al. A review of contemporary data on surgically resected renal masses--benign or malignant? Urology. 2013 Apr;81(4):707–713. doi: 10.1016/j.urology.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. The American journal of surgical pathology. 2002 Mar;26(3):281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. 2000 Aug 1;89(3):604–614. [PubMed] [Google Scholar]
- 30.Millet I, Doyon FC, Hoa D, et al. Characterization of small solid renal lesions: can benign and malignant tumors be differentiated with CT? AJR. American journal of roentgenology. 2011 Oct;197(4):887–896. doi: 10.2214/AJR.10.6276. [DOI] [PubMed] [Google Scholar]
- 31.Halverson SJ, Kunju LP, Bhalla R, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. The Journal of urology. 2013 Feb;189(2):441–446. doi: 10.1016/j.juro.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 32.Divgi CR, Pandit-Taskar N, Jungbluth AA, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. The Lancet. Oncology. 2007 Apr;8(4):304–310. doi: 10.1016/S1470-2045(07)70044-X. [DOI] [PubMed] [Google Scholar]
- 33.Divgi CR, Uzzo RG, Gatsonis C, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Jan 10;31(2):187–194. doi: 10.1200/JCO.2011.41.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. The Journal of urology. 2008 Apr;179(4):1227–1233. doi: 10.1016/j.juro.2007.11.047. discussion 1233-1224. [DOI] [PubMed] [Google Scholar]
- 35.Khandani AH, Rathmell WK, Wallen EM, Ivanovic M. PET/CT with (124)I-cG250: great potential and some open questions. AJR. American journal of roentgenology. 2014 Aug;203(2):261–262. doi: 10.2214/AJR.14.12490. [DOI] [PubMed] [Google Scholar]
- 36.Zaharchuk G. Theoretical basis of hemodynamic MR imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. AJNR. American journal of neuroradiology. 2007 Nov-Dec;28(10):1850–1858. doi: 10.3174/ajnr.A0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Kwon YS, Labib M, Foran DJ, Singer EA. Magnetic Resonance Imaging as a Biomarker for Renal Cell Carcinoma. Disease Markers. 2015;2015:9. doi: 10.1155/2015/648495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanzman RS, Robson PM, Sun MR, et al. Arterial spin-labeling MR imaging of renal masses: correlation with histopathologic findings. Radiology. 2012 Dec;265(3):799–808. doi: 10.1148/radiol.12112260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun MR, Ngo L, Genega EM, et al. Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes--correlation with pathologic findings. Radiology. 2009 Mar;250(3):793–802. doi: 10.1148/radiol.2503080995. [DOI] [PubMed] [Google Scholar]
- 40.Mori S, Barker PB. Diffusion magnetic resonance imaging: its principle and applications. The Anatomical record. 1999 Jun 15;257(3):102–109. doi: 10.1002/(SICI)1097-0185(19990615)257:3<102::AID-AR7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Cheng L, Zhang X, et al. Renal cell carcinoma: diffusion-weighted MR imaging for subtype differentiation at 3.0 T. Radiology. 2010 Oct;257(1):135–143. doi: 10.1148/radiol.10092396. [DOI] [PubMed] [Google Scholar]
- 42.Taouli B, Thakur RK, Mannelli L, et al. Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology. 2009 May;251(2):398–407. doi: 10.1148/radiol.2512080880. [DOI] [PubMed] [Google Scholar]
- 43.Sandrasegaran K, Sundaram CP, Ramaswamy R, et al. Usefulness of diffusion-weighted imaging in the evaluation of renal masses. AJR. American journal of roentgenology. 2010 Feb;194(2):438–445. doi: 10.2214/AJR.09.3024. [DOI] [PubMed] [Google Scholar]
- 44.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. European journal of cancer. 2012 Mar;48(4):441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaing B, Sigmund EE, Huang WC, et al. Subtype differentiation of renal tumors using voxel-based histogram analysis of intravoxel incoherent motion parameters. Investigative radiology. 2015 Mar;50(3):144–152. doi: 10.1097/RLI.0000000000000111. [DOI] [PubMed] [Google Scholar]


