Abstract
Despite a well-developed and growing body of work in copper catalysis, the potential of copper to serve as a photocatalyst remains underexplored. Herein, we describe a photoinduced, copper-catalyzed method for coupling readily available racemic tertiary alkyl chloride electrophiles with amines to generate fully substituted stereocenters with high enantioselectivity. The reaction proceeds at –40 °C under excitation by a blue light-emitting diode and benefits from the use of a single, Earth-abundant transition metal acting as both the photocatalyst and the source of asymmetric induction. An enantioconvergent mechanism transforms the racemic starting material into a single product enantiomer.
Photochemistry can furnish reactive intermediates that would otherwise be difficult to access under synthetically useful conditions. Its application in organic synthesis has therefore expanded rapidly during the past decades (1), most recently in the context of enantioselective photoredox catalysis with transition metals (2,3,4). With several recent noteworthy exceptions, each of which involves the α-functionalization of carbonyl compounds by a chiral iridium catalyst (5,6,7), the metal-catalyzed methods require two catalysts, a transition metal complex that undergoes photoexcitation and serves as a site for redox chemistry, as well as a separate chiral catalyst that effects enantioselective bond formation. Transition metal-free asymmetric photoredox catalysis has also been reported (8,9).
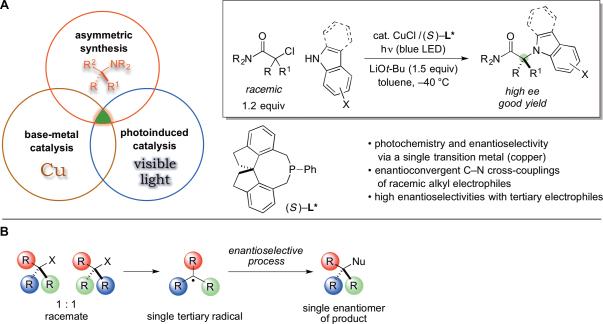
We have been interested in photocatalytic approaches to the construction of C–N bonds, given the high value of amines in fields ranging from biology to chemistry to materials science (10). Whereas initial efforts to develop transition metal-catalyzed C–N cross-coupling reactions focused on the use of aryl and alkenyl halides as the electrophilic coupling partner (11,12), during the past few years, alkyl halides that are not suitable substrates for classic SN2 reactions have emerged as useful coupling partners under the combined action of light and copper catalysis (13,14). To date progress has not yet been reported in the development of an asymmetric variant of these reactions, and the use of copper as a photoredox catalyst (15) is uncommon by comparison with precious metals such as iridium and ruthenium. Here we describe a copper-catalyzed enantioconvergent cross-coupling of racemic tertiary alkyl halides that is induced by visible light, a process that lies at the intersection of several important dimensions of modern chemical catalysis (Fig. 1A).
Fig. 1. A photocatalytic approach to the asymmetric synthesis of amines.
(A) Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. (B) Outline of a strategy for the enantioconvergent cross-coupling of a racemic tertiary alkyl halide via a radical intermediate.
Although considerable advances have recently been reported in the development of enantioconvergent cross-couplings of racemic secondary alkyl electrophiles with carbon nucleophiles to form C–C bonds (16,17,18), no highly effective methods have yet been described for tertiary alkyl halides, which require differentiation by the catalyst of three distinct carbon substituents in order to furnish high enantioselectivity. Indeed, in the field of asymmetric synthesis as a whole, highly stereoselective reactions of tertiary electrophiles are relatively uncommon, despite the fact that fully substituted carbons are a common motif in organic molecules (19). We anticipated that the radical mechanism that we have postulated for C–X bond cleavage in the presence of copper and light (vide infra) (13,14) might enable us to surmount this challenge, because a single, comparatively stable tertiary radical could be formed from a racemic mixture of electrophiles (Fig. 1B).
Another issue was whether common chiral ligands such as phosphines would even bind to copper, much less induce high enantioselectivity in the C–N bond-forming process, in the presence of a much more abundant nucleophilic coupling partner. Indeed, the previously described methods for photoinduced, copper-catalyzed N-alkylation had employed CuI as a pre-catalyst with no added ligand (13,14).
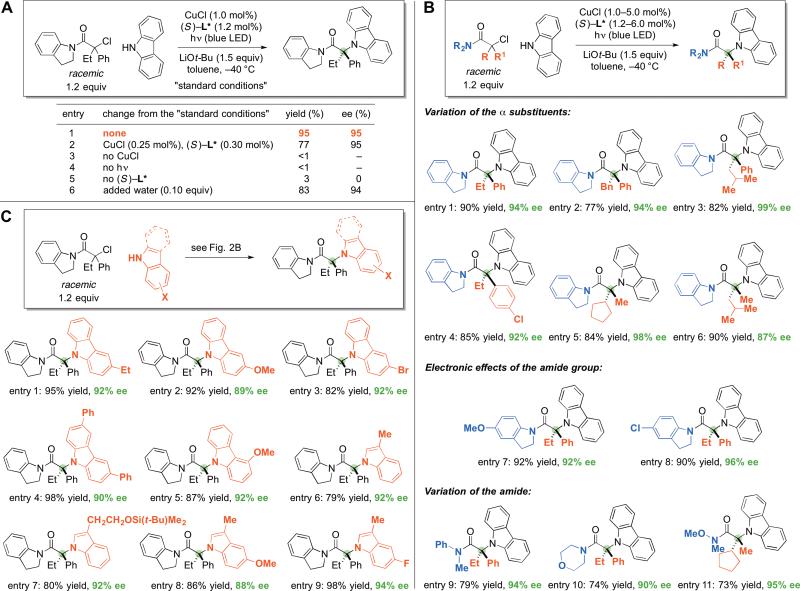
As a model coupling process, we examined the reaction of carbazole, a heterocycle that occurs in bioactive molecules, including N-tert-alkyl-substituted compounds (20,21), with an α-halocarbonyl compound, a class of electrophiles that has not previously been employed in photoinduced, copper-catalyzed cross-couplings. Upon investigating a range of reaction parameters, we discovered that irradiation of the cross-coupling partners at –40 °C for 16 hours in the presence of CuCl, a chiral phosphine (L*), and a Brønsted base provides the desired product in 95% yield and 95% enantiomeric excess (ee) (Fig. 2A, entry 1). In contrast to our earlier studies of photoinduced, copper-catalyzed N-alkylations, this process operates under visible light from a blue LED (rather than an ultraviolet source) and at relatively low catalyst loading (1.0 mol% rather than 10 mol%); a catalyst loading of 0.25 mol% led to only a modest loss in yield and no erosion in ee (entry 2: ~300 turnovers; previously, the highest turnover number for a photoinduced, copper-catalyzed N-alkylation was ~9) (13,14).
Fig. 2. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light.
(A) Effect of changes in the reaction parameters (yields were determined through analysis by 1H NMR spectroscopy with the aid of an internal standard). (B) Scope with respect to the electrophile (yields were determined by isolation after chromatographic purification). (C) Scope with respect to the nucleophile (yields were determined by isolation after chromatographic purification).
Control experiments established that copper (Fig. 2A, entry 3; the alkyl halide is recovered quantitatively) and light (entry 4) are necessary to achieve C–N bond formation under these conditions. Furthermore, essentially no C–N coupling (<1%) occurs when the tertiary alkyl chloride, carbazole, and LiOt-Bu are heated at 80 °C in toluene for 16 hours. Our concern that a phosphine (e.g., L*) might not bind effectively to copper in the presence of a stoichiometric quantity of the nucleophile appears to be unfounded, as evidenced by our observation of high enantiomeric excess in the C–N coupling (entry 1) and of enhanced rate in the presence of the ligand (ligand-accelerated catalysis (22): entry 1 vs. entry 5). From a practical point of view, it is worth noting that CuCl and the chiral phosphine are commercially available and that the process is not highly moisture-sensitive (entry 6).
We have examined the scope of this photoinduced, copper-catalyzed method for enantioconvergent N-alkylation by racemic tertiary alkyl halides (Fig. 2B). For couplings of carbazole with N-acylindoline-derived electrophiles, good to excellent yields and enantioselectivities are observed with a range of substituents in the α position of the electrophile (entries 1–6). In the case of α,α-dialkyl-substituted electrophiles (entries 5 and 6), the catalyst selectively discriminates between two alkyl groups, including a methyl and an isobutyl group (entry 6), to furnish high ee.
To gain insight into the compatibility of various functional groups with these conditions for enantioconvergent C–N cross-couplings of tertiary alkyl halides, we have examined the impact of additives (1.0 equivalent) on the course of the coupling process depicted in Fig. 2B, entry 1. We determined that an unactivated secondary alkyl bromide (cyclohexyl bromide), a ketone (2-nonanone), a secondary alcohol (5-nonanol), an ester (methyl octanoate), an alkene (cis- or trans-5-decene), an alkyne (5-decyne), and a nitrile (valeronitrile) have no adverse impact on the yield/enantioselectivity and can be recovered intact at the end of the cross-coupling, whereas a primary amine (3-phenylpropylamine) and an aldehyde (octanal) impede N-alkylation.
The introduction of an electron-donating or an electron-withdrawing substituent onto the indoline does not compromise the efficiency of the cross-coupling (Fig. 2B, entries 7 and 8). If desired, N-acylindolines can be transformed into primary alcohols or carboxylic acids (23). A variety of other α-haloamides are also suitable electrophilic cross-coupling partners (entries 9–11), including a Weinreb amide (entry 11), which is important in synthesis because it serves as a useful precursor to ketones (24).
With respect to the nucleophilic coupling partner, substituted carbazoles are also suitable substrates (Fig. 2C, entries 1–5); the enantioconvergent C–N cross-coupling can be conducted on a gram-scale with a similar outcome (entry 2: 1.29 g of product, 94% yield, 94% ee). Indoles can also be employed as nucleophiles in these photoinduced, copper-catalyzed couplings, furnishing the desired product with good yield and enantioselectivity (entries 6–9). Because indoles are common subunits in bioactive compounds (25), and natural products with a tertiary N-alkyl substituent are known (26,27), these represent a useful addition to the limited families of nitrogen nucleophiles compatible with metal-catalyzed C–N alkylations (13,14).
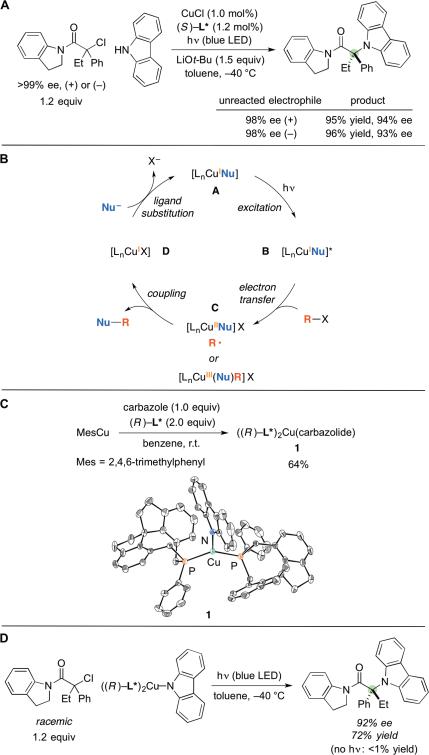
Because we are able to obtain the cross-coupling product in high yield and ee when using only 1.2 equivalents of a racemic electrophile, it is evident that both enantiomers of the electrophile can be transformed under the reaction conditions into a particular enantiomer of the product (enantioconvergence), although not necessarily at identical rates (kinetic resolution (28)). To gain insight into whether a kinetic resolution was occurring, we measured the ee of the unreacted tertiary alkyl halide at the end of the cross-coupling depicted in Fig. 2B, entry 1. Our observation that the recovered electrophile is racemic suggests either that the enantiomeric substrates are reacting at essentially identical rates (i.e., no kinetic resolution) or that in situ racemization of the electrophile is occurring. Through the use of enantiopure alkyl halides, we established that virtually no racemization takes place under the reaction conditions (Fig. 3A). These couplings with enantiopure electrophiles further establish that the chiral ligand very effectively controls the absolute configuration of the product, regardless of the stereochemistry of the starting electrophile, and that C–Cl bond cleavage is essentially irreversible.
Fig. 3. Mechanistic studies.
(A) Investigation of kinetic resolution. (B) Outline of a possible pathway for photoinduced, copper-catalyzed C–N cross-couplings of alkyl halides (for the sake of simplicity, all copper complexes are illustrated as neutral species, and all processes are depicted as being irreversible; X may be serving as an inner- or an outer-sphere ligand). (C) Synthesis and structural characterization of (L*)2Cu(carbazolide) (thermal ellipsoids drawn at 50% probability and H atoms omitted for clarity). (D) Stoichiometric cross-coupling reaction with isolated (L*)2Cu(carbazolide).
An outline of a possible mechanism for photoinduced, copper-catalyzed C–N couplings of alkyl halides is illustrated in Fig. 3B (13,14). Irradiation of a copper–nucleophile complex (A) could lead to an excited-state adduct (B) that would then engage in electron transfer with the alkyl halide (R–X) to generate an alkyl radical; next, Nu–R bond formation could occur through an inner-sphere pathway involving a copper–nucleophile complex (C). In contrast to most asymmetric photoredox reactions catalyzed by transition metals (2,3,4), a single metal (copper) appears to be responsible for both the photochemistry and the enantioselective bond-forming process. The binding of the nucleophile to copper in situ to form a copper complex that can serve as a photoreductant is important in this outline.
We have synthesized and crystallographically characterized a copper complex that includes the chiral phosphine and the carbazolide nucleophile, (L*)2Cu(carbazolide) (1; Fig. 3C). The three ligands are arranged in a trigonal planar geometry around copper. When complex 1 (1.0 mol%) is employed in place of CuCl/L* under our standard reaction conditions, the yield and the ee of the C–N cross-coupling product are essentially unchanged (92% yield, 94% ee; cf. Fig. 2A, entry 1: 95% yield, 95% ee). Furthermore, irradiation of complex 1 in the presence of a stoichiometric amount of a racemic tertiary alkyl halide leads to C–N bond formation in good yield and with enantioselectivity that is comparable to the catalyzed process (Fig. 3D; cf. Fig. 2A, entry 1: 95% ee); no coupling occurs in the absence of light. Collectively, these observations are consistent with the suggestion that complex 1, or a copper/carbazolide/L* species that can be derived from 1, is a plausible intermediate in the catalytic cycle.
Whereas enantioconvergent metal-catalyzed cross-couplings of racemic secondary alkyl halides have recently emerged as powerful tools for C–C bond construction, there has been little progress in corresponding C–heteroatom bond-forming processes or in the use of tertiary alkyl halides as coupling partners. Herein, we have established that, with the aid of visible light, a copper-based chiral catalyst derived from commercially available components can achieve enantioconvergent C–N cross-coupling reactions of racemic tertiary alkyl chlorides with good to excellent enantioselectivity. In contrast to nearly all metal-catalyzed asymmetric photoredox methods described to date, which employ separate catalysts to effect redox chemistry and bond formation, in this method a single catalyst is responsible for the photochemistry and for the enantioselective bond construction. This work stands at a previously unexplored intersection of asymmetric synthesis, catalysis with Earth-abundant metals, photoinduced processes, and cross-coupling reactions of alkyl electrophiles, each of which represents an important current theme in chemical synthesis. We anticipate that our observations comprise the initial advances in a fertile area of asymmetric catalysis: the enantioconvergent synthesis of secondary and tertiary C–heteroatom bonds through photoinduced transition metal-catalyzed couplings of alkyl halides.
Supplementary Material
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences: R01–GM109194), the Gordon and Betty Moore Foundation, the Alexander von Humboldt Foundation (fellowship for Q.M.K.), and the Bengt Lundqvist Memorial Foundation of the Swedish Chemical Society (fellowship for A.B.). We thank Jun Myun Ahn, Lawrence M. Henling (X-ray Crystallography Facility), Dr. Miles W. Johnson, Dr. Nathan D. Schley, Dr. Mona Shahgholi (Mass Spectrometry Facility), Dr. Michael K. Takase (X-ray Crystallography Facility), Naseem Torian (Mass Spectrometry Facility), Dr. David G. VanderVelde (NMR Facility), and Dr. Scott C. Virgil (Center for Catalysis and Chemical Synthesis) for assistance and helpful discussions. Experimental procedures and characterization data are provided in the Supplementary Materials. Metrical parameters for the structures of compounds 1-4 are available free of charge from the Cambridge Crystallographic Data Centre under accession numbers CCDC 1435979, 1435978, 1435977, 1435980.
Footnotes
Supplementary Materials:
Materials and Methods
Supplementary Text
Figures S1-S5
Tables S1-S23
References (29-36)
X–Ray Diffraction Data
NMR Spectra
References and Notes
- 1.Albini A, Fagnoni M, editors. Photochemically-Generated Intermediates in Synthesis. Wiley; Hoboken, NJ: 2013. [Google Scholar]
- 2.Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brimioulle R, Lenhart D, Maturi MM, Bach T. Angew. Chem. Int. Ed. 2015;54:3872–3890. doi: 10.1002/anie.201411409. [DOI] [PubMed] [Google Scholar]
- 4.Schultz DM, Yoon TP. Science. 2014;343:1239176. doi: 10.1126/science.1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huo H, et al. Nature. 2014;515:100–103. doi: 10.1038/nature13892. [DOI] [PubMed] [Google Scholar]
- 6.Huo H, Wang C, Harms K, Meggers E. J. Am. Chem. Soc. 2015;137:9551–9554. doi: 10.1021/jacs.5b06010. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, et al. Chem. Eur. J. 2015;21:7355–7359. [Google Scholar]
- 8.Romero NA, Margrey KA, Tay NE, Nicewicz DA. Science. 2015;349:1326–1330. doi: 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]
- 9.Bauer A, Westkämper F, Grimme S, Bach T. Nature. 2005;436:1139–1140. doi: 10.1038/nature03955. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence SA. Amines: Synthesis, Properties and Applications. Cambridge Univ. Press; Cambridge, UK: 2004. [Google Scholar]
- 11.Jiang L, Buchwald SL, Metal-Catalyzed Cross-Coupling Reactions . In: de Meijere A, Diederich F, editors. Vol. 2. Wiley–VCH; Weinheim, Germany: 2004. pp. 699–760. [Google Scholar]
- 12.Hartwig JF, Shekhar S, Shen Q, Barrios-Landeros F. In: Chemistry of Anilines. Rappoport Z, editor. Vol. 1. Wiley; New York: 2007. pp. 455–536. [Google Scholar]
- 13.Bissember AC, Lundgren RJ, Creutz SE, Peters JC, Fu GC. Angew. Chem. Int. Ed. 2013;52:5129–5133. doi: 10.1002/anie.201301202. [DOI] [PubMed] [Google Scholar]
- 14.Do H-Q, Bachman S, Bissember AC, Peters JC, Fu GC. J. Am. Chem. Soc. 2014;136:2162–2167. doi: 10.1021/ja4126609. [DOI] [PubMed] [Google Scholar]
- 15.Paria S, Reiser O. ChemCatChem. 2014;6:2477–2483. [Google Scholar]
- 16.Fischer C, Fu GC. J. Am. Chem. Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Fu GC. J. Am. Chem. Soc. 2015;137:9523–9526. doi: 10.1021/jacs.5b04725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin M, Adak L, Nakamura M. J. Am. Chem. Soc. 2015;137:7128–7134. doi: 10.1021/jacs.5b02277. [DOI] [PubMed] [Google Scholar]
- 19.Quasdorf KW, Overman LE. Nature. 2014;516:181–191. doi: 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuster C, et al. J. Org. Chem. 2015;80:5666–5673. doi: 10.1021/acs.joc.5b00630. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt AW, Reddy KR, Knölker H-J. Chem. Rev. 2012;112:3193–3328. doi: 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- 22.Berrisford DJ, Bolm C, Sharpless KB. Angew. Chem. Int. Ed. 1995;34:1059–1070. [Google Scholar]
- 23.Lundin PM, Fu GC. J. Am. Chem. Soc. 2010;132:11027–11029. doi: 10.1021/ja105148g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balasubramaniam S, Aidhen IS. Synthesis 3707–3738. 2008 [Google Scholar]
- 25.Gribble GW, editor. Heterocyclic Scaffolds II: Reactions and Applications of Indoles. Springer; Heidelberg, Germany: 2010. [Google Scholar]
- 26.Vallakati R, May JA. J. Am. Chem. Soc. 2012;134:6936–6939. doi: 10.1021/ja301387k. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt EK, et al. Angew. Chem. Int. Ed. 2011;50:5889–5891. doi: 10.1002/anie.201101740. [DOI] [PubMed] [Google Scholar]
- 28.Pellissier H. In: Separation of Enantiomers. Todd M, editor. Wiley–VCH; Weinheim, Germany: 2014. pp. 75–122. [Google Scholar]
- 29.Kramer S, Fu GC. J. Am. Chem. Soc. 2015;137:3803–3806. doi: 10.1021/jacs.5b01944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Z, Wilsily A, Fu GC. J. Am. Chem. Soc. 2011;133:8154–8157. doi: 10.1021/ja203560q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harpp DN, Bao LQ, Black CJ, Smith RA, Gleason JG. Tetrahedron Lett. 1974;15:3235–3238. [Google Scholar]
- 32.Schwenk E, Papa D. J. Am. Chem. Soc. 1948;70:3626–3627. doi: 10.1021/ja01191a026. [DOI] [PubMed] [Google Scholar]
- 33.Du Y, Huang H-Y, Liu H, Ruan Y-P, Huang P-Q. Synlett. 2011:565–568. [Google Scholar]
- 34.Cheng H-G, et al. Angew. Chem. Int. Ed. 2013;52:3250–3254. doi: 10.1002/anie.201209998. [DOI] [PubMed] [Google Scholar]
- 35.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. J. Appl. Crystallogr. 2009;42:339–341. doi: 10.1107/S0021889811041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheldrick GM. Acta. Crystallogr. 2008;A 64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.