Abstract
We explored the changes in multi-finger synergies in patients after a single cortical stroke with mild motor impairments. We hypothesized that both synergy indices and anticipatory synergy adjustments prior to the initiation of a self-paced quick action would be diminished in the patients compared to age-matched controls. The patients with history of cortical stroke, and age-matched controls (n = 12 in each group) performed one-finger and multi-finger accurate force production tasks involving both steady-state and quick force pulse production. Finger interdependence (enslaving) and multi-finger synergies stabilizing total force were quantified. The stroke patients showed lower maximal finger forces, in particular in the contralesional hand, which also showed increased enslaving indices. Multi-finger synergies during steady-state force production were, however, unchanged after stroke. In contrast, a drop in the synergy index prior to the force pulse generation was significantly delayed in the stroke patients. Our results show that mild cortical stroke leads to no significant changes in multifinger synergies, but there is impairment in feed-forward adjustments of the synergies prior to a quick action, a drop in the maximal force production, and an increase in enslaving. We conclude that studies of synergies reveal two aspects of synergic control differentially affected by cortical stroke.
Keywords: Stroke, hand, synergy, feed-forward control, anticipatory synergy adjustments
1. Introduction
Sensorimotor impairment of the hand happens commonly after a unilateral cortical stroke affecting a range of activities of daily living. Typical consequences of stroke that affect the extremities contralateral to the lesion include weakness, predominance of fixed patterns of muscle activations (abnormal synergies, resulting in losses of independent joint control, Bobath, 1978; Dewald et al., 1995), spasticity, and intersegmental coordination deficits (Wyke, 1967; Beer et al., 2000). In cases of mild stroke, impairments in the coordination among motor elements, such as arm joints and digits, become important factors affecting functional independence, even in the ipsilesional arm (Schaefer et al., 2009a,b; Wetter et al., 2005; Rinehart et al., 2009).
Recently, an approach to the neural coordination of multiple effectors has been developed based on the principle of abundance (Gelfand and Latash, 1998; Latash, 2012). According to this principle, the apparently redundant design of the human body is not a source of computational problems for the central nervous system (CNS), but a rich and flexible apparatus that allows the CNS to organize stable performance in the varying and unpredictable environment. Neural organizations that ensure stable performance by co-varied contributions of elements (muscles, joints, digits, etc.) have been referred to as “synergies” (Latash et al., 2007). This term has been used in the literature in different meanings. As mentioned, abnormal synergies describe stereotypic pattern of muscle activations that interfere with intentional movements (Twitchell 1951; Brunnström 1970). This term has also been used to imply parallel changes in variables produced by effectors, kinetic, kinematic, or electromyographic, across task parameters or over the time course of movement execution (D'Avella et al., 2003; Ivanenko et al., 2004; Ting and Macpherson, 2005). We use this term to reflect stability of natural movements with respect to salient variables, which is crucial for success given the natural variability in body states and unpredictable changes in external forces (reviewed in Latash et al., 2007; Latash, 2008).
This important aspect of coordination can be quantified using a method that has been developed within the uncontrolled manifold (UCM) hypothesis (Scholz and Schöner, 1999). This analysis is based on the idea that repeating actions from slightly different initial conditions is expected to lead to diverging trajectories in unstable directions and converging trajectories in stable directions. Hence, analysis of inter-trial variance in different directions in the space of elemental variables (those produced by elements involved in the action) provides an index that can be used as a proxy of stability in those directions within the multi-dimensional space of elemental variables. The analysis quantifies inter-trial variance in directions that lead to no changes in a potentially important performance variable (along the UCM for that variable, VUCM) and in directions orthogonal to the UCM (VORT). If VUCM > VORT, quantified per degree of freedom in the corresponding sub-spaces, a conclusion is drawn that a synergy stabilizes that performance variable. A synergy index, ΔV, reflecting relative amount of VUCM in total variance has been used as a metric reflecting stability of the performance variable (reviewed in Latash, 2008). Recently, relations of the ΔV index to stability have been studied in a number of experiments with controlled perturbations of ongoing actions (Yang et al. 2007; Wilhelm et al., 2013; Reschechtko et al., 2014; Zhou et al., 2015).
In more intuitive terms, this definition of synergy is related to inter-compensation of errors among the contributions of elements to a salient performance variable (cf. principle of error compensation, Latash et al. 1998). For example, carrying a cup of coffee while walking requires co-variation of joint rotations to keep the cup vertical. A strong joint configuration synergy implies that spontaneous variations in joint angles or a perturbation applied to the arm would lead to changes in joint configuration primarily within the UCM for the vertical cup orientation, i.e., cup orientation would show dynamic stability. This is expected to lead to relatively high VUCM values and positive ΔV values.
In patients with mild to severe contralesional impairment, deficits in intersegmental coordination during reaching movements of the contralesional arm have been documented (Beer et al., 2000), and have been shown to vary with the side of the lesion. In addition, coordination deficits in the ipsilesional arm have been documented as early as 1967 (Wyke 1967), and have been shown to differ, depending on the side of the lesion (Winstein et al., 1999; Haaland et al., 2004). While these studies have addressed the coordination of joint motions to produce a given trajectory within a reaching trial, they have not addressed the ability to stabilize performance as reflected in the two aforementioned indices of inter-trial variance, VUCM and VORT, and in the synergy index ΔV.
Only one group has so far applied this method of analysis to arm movements of post-stroke patients (Reisman and Scholz, 2003, 2006). The results of the first study were surprising: While the patients with mild-to-moderate hemiparesis displayed abnormal patterns of coordination in the contralesional arm that were notably more deficient than those of the ipsilesional arm, the relative amount of VUCM and VORT computed in the joint configuration space (these are addressed as goal-equivalent and non-goal-equivalent solutions in the Reisman and Scholz studies) was about the same in the contralesional and ipsilesional arms and also similar to the data in age-matched controls. This was accompanied by a proportional increase in both variance components, VUCM and VORT, in the contralesional arms of the patients. The follow-up study (Reisman and Scholz 2006) confirmed these findings for reaches of the paretic hand to contralateral targets, while during reaches to ipsilateral targets VORT showed a disproportional increase. These findings suggest that the pronounced effects of stroke on reaching movements, which are seen in the averaged across-trial kinematics, are not necessarily reflected in patterns of joint movement covariation across trials. The small number of subjects in the two studies (eight and seven per group, respectively) makes these conclusions tentative.
In contrast, studies of finger coordination in patients with subcortical disorders (Parkinson's disease, PD, and multi-system atrophy, Park et al., 2012, 2013a,b; Jo et al., 2015) have shown a consistent, significant drop in the synergy index during accurate steady-state multi-finger force production and prehensile tasks. These changes were significant in both hands, even in patients with Hoehn-Yahr stage-I of PD who showed clinical symptoms on one side of the body only. The contrast between the findings in PD and stroke patients suggests that subcortical loops, rather than cortical structures, might be crucial for synergic control. However, since the stroke study quantified arm reaching movements, and the PD studies quantified finger coordination, a comparison of the results cannot differentiate between the effect of the disorder (PD or Stroke) and the limb effectors (proximal joint coordination vs. finger coordination).
In order to directly address this ambiguity, we quantified multi-finger synergies in a group of stroke survivors with unilateral hemisphere damage and mild contralesional impairment, using the same procedure as that of several earlier studies of PD patients (Park et al., 2012, 2014). Based on the mentioned earlier study (Reisman and Scholz, 2003), we hypothesize that the stroke group should show differences from control subjects and between the ipsilesional and contralesional hands in overall performance indices, but not in the multi-finger synergy index computed with respect to the salient performance task variable, namely total force (Hypothesis-1). As in many earlier studies (Latash et al., 2001; Scholz et al., 2002; reviewed in Latash et al., 2007), we analyzed multi-finger synergies in the space of finger modes as elemental variables. Finger modes are defined as commands to individual fingers that can be modified by the subject one at a time; each mode, however, leads to force production by all the fingers of the hand because of the phenomenon of enslaving (Zatsiorsky et al., 2000; Danion et al., 2003b). Due to enslaving, finger forces are expected to co-vary across tasks, and the amount of co-variation may differ across groups. Therefore, analysis of synergies in a finger force space can potentially lead to false conclusions on stronger or weaker synergies stabilizing specific performance variables. Analysis in the mode space eliminates this confound.
We also explored feed-forward adjustment of synergies in preparation for quick action (anticipatory synergy adjustments, ASAs, Olafsdottir et al., 2005; Shim et al., 2005). ASAs reflect an important mechanism of gradually reducing stability of a variable in preparation to its quick change. ASAs are delayed and decreased in magnitude in PD patients (Park et al., 2012; Jo et al., 2015). Following the prediction of no changes in synergies after stroke, we hypothesized that no changes in ASAs would take place (Hypothesis-2).
2. Methods
2.1. Subjects
Twelve patients with unilateral stoke (aged 64.58 ± 12.05; 10 males) and 12 healthy control subjects (CS; aged 62.18 ± 7.65; 9 males) were tested. All participants were right-handed according to their preferential hand use during writing and eating. None of the CS had any known neurological disorders or arthritis in their upper extremities. Descriptive data for stroke patients are presented in Table 1. Six patients with right-hemisphere damage (RHD) and 6 patients with left-hemisphere damage (LHD) were recruited. The median time since stroke was 3.5 years and patients were mildly impaired, with average Upper Limb Fugl-Meyer motor scores of 61.8 ± 4.3 (out of a possible 66 points). The hand Fugl-Meyer score was 14 out of 14 in all patients. Eight patients showed no sensory deficit in the hands (2 points out of 2), while three patients showed dysesthesia (1 out of 2 points), and one patient showed anesthesia (0 points) according to the Fulg-Meyer hand sensory score.
Table 1.
Descriptive data of stroke patients
| Subject | Sex, M/F |
Age, yr |
Handedness, R/L |
Stroke side, R/L |
Years post- stroke |
UE FM score |
FM- Hand |
FM- Hand Sensory |
Pegboard Ipsi/Contra (s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 73 | R | R | 1.0 | 61 | 14 | 2 | 54/46 |
| 2 | M | 80 | R | L | 1.2 | 61 | 14 | 2 | 48/48 |
| 3 | M | 66 | R | L | 4.5 | 62 | 14 | 2 | 14/14 |
| 4 | M | 75 | R | R | 3.6 | 62 | 14 | 1 | 49/45 |
| 5 | M | 56 | R | R | 3.8 | 56 | 14 | 1 | 35/12 |
| 6 | M | 69 | R | L | 1.2 | 66 | 14 | 2 | 62/62 |
| 7 | M | 75 | R | R | 3.5 | 60 | 14 | 2 | 55/50 |
| 8 | M | 50 | R | L | 2.4 | 66 | 14 | 2 | 36/36 |
| 9 | F | 54 | R | L | 2.5 | 65 | 14 | 2 | 44/44 |
| 10 | M | 56 | R | R | 18.9 | 53 | 14 | 1 | 30/dnc |
| 11 | M | 77 | R | R | 1.6 | 65 | 14 | 2 | 57/51 |
| 12 | M | 44 | R | L | 5.1 | 65 | 14 | 0 | 27/27 |
Abbreviations: M/F - male/female; R/L - right/left, UE –upper extremity, FM – Fugl-Meyer, dnc – did not complete, the last column presents the data for the Grooved Pegboard test performed by the ipsilesional (Ispi) and contralesional (Contra) hands.
The lesion distribution of the patients is illustrated in Figure 1, which shows a multi-slice representation of the damaged areas manually traced by a trained technician on T1 or T2 weighted brain images using MRIcron (version 6 June 2013; Chris Rorden) software (Rorden and Brett 2000). Unified segmentation and normalization routines found in the Clinical Toolbox in SPM8 were used to normalize the T1 or T2 images and corresponding lesion maps to a clinical template created specifically for older adults (Rorden et al., 2012). Binarized, warped, and smoothed lesion maps created from the normalization process were then overlaid onto a brain template in MRIcron. While there was substantial variability among the patients in the lesion volume, no difference was seen between the RHD and LHD groups, 29.78 ± 37.13 and 42.73 ± 41.52 cm3, respectively. The locations of the lesions are described in more detail in Table 2. One RHD patient had the lesion extended to subcortical regions, with involvement of the basal ganglia. The results were qualitatively similar with and without that patient's data. Hence, we present results across the whole stroke group.
Figure 1.
A multi-slice representation of the damaged areas manually traced by a trained technician on T1 or T2 weighted brain images.
Table 2.
Location of lesions in stroke patients
| Subject | Location of lesions |
|---|---|
| 1 | R Frontal lobe, parieto-occipital lobe |
| 2 | L Parietal lobe |
| 3 | L Parietal and temporal white matter, centrum semiovale |
| 4 | R Frontoparietal |
| 5 | R Frontoparietal |
| 6 | L Frontal lobe, insula, frontal periventricular white matter |
| 7 | R Frontal lobe |
| 8 | L Parieto-temporal lobe, posterior frontal lobe |
| 9 | L Inferior frontal lobe, superior temporal lobe |
| 10 | R Frontoparietal |
| 11 | R Frontoparietal, centrum semiovale, insula |
| 12 | L Frontoparietal, temporal lobe, insular, caudate nucleus, putamen |
Abbreviations: R, right; L,left.
The study protocol followed the Helsinki principles and was reviewed and approved by the Pennsylvania State University-Hershey Medical Center Institutional Review Board. Written informed consent was obtained from all subjects.
2.2. Experimental Setup
Four piezoelectric force sensors (model 208A03; PCB Piezotronics, Depew, NY) were used to measure vertical forces produced by the fingers. The sensors were placed into slots in a panel that allowed adjusting the sensor position in the anterior-posterior direction. The subjects determined the most comfortable location for each sensor. The distance between the centers of the sensors in the medio-lateral direction was 3 cm. Each sensor was covered with sandpaper (300-grit) to increase the friction between the fingertips and the top surface of the sensor. An illustration of the setup is presented in Figure 2A.
Figure 2.
An illustration of the experimental setup (A) and typical feedback provided to the subjects for the single-finger ramp task (B) and for the accurate force pulse task (C).
The subjects sat in a chair facing the 19-inch computer screen and positioned an upper arm on a wrist-forearm brace that was fixed to the table. The forearm was held stationary with Velcro straps to prevent forearm and wrist movement. A custom-made wooden piece was placed underneath the subject's palm to help maintain a constant hand and finger configuration during the tests. The metacarpophalangeal joints were at about 120°, and all the inter-phalangeal joints were slightly flexed so that the hand formed a dome. The four force signals were digitized at 200 Hz with a 16-bit resolution with a customized LabView program.
2.3. Experimental Procedures
During the experiment, the subjects sat in a chair with the shoulder at ~30° of abduction and ~45° of flexion. The elbow was flexed at ~135°. The monitor was at subject's eye level and showed real-time finger force feedback. Prior to each trial, all sensor signals were set to zero when subjects placed their fingertips on the sensor centers and relaxed their hand. As a result, the sensors measured only active downward forces. The other hand rested on the lap of the subject.
The experiment involved three tasks: 1) Maximal voluntary contraction (MVC) task; 2) single-finger ramp tasks; and 3) quick force pulse production task. The subjects performed all three tasks with the left and right hands separately in a balanced across subjects order. Subjects were given an instruction before each task and a few practice trials until they acquired a reasonable level of accuracy and consistency. Typically, 1-2 trials were given prior to the ramp task and 5-8 trials prior to the force pulse production task; only one practice trial was performed prior to the MVC task. There were 5-min breaks after testing one hand. Subjects were offered rest at any time if they felt fatigued. The entire experiment lasted ~1 h.
Clinical tests, including the Upper limb component of the Fugl-Meyer test Assessment and Grooved Pegboard test, were scored with standard procedures that had been tested earlier for reliability (Ruff and Parker 1993). Raw scores (total time in sec) in the Grooved Pegboard test were converted to age-, education-, and gender-adjusted T scores using normative data from Halstead-Reitan battery (Heaton et al. 2004). All clinical tests were administered during a separate visit prior to the experiment.
2.3.1. MVC task
In the MVC task, subjects were instructed to press on the sensors with the four fingers together as hard as possible in a self-paced manner and achieve maximal total force level within 8 s. The subjects were instructed to relax immediately after reaching a maximal force. The feedback showed the sum of the four finger forces (FTOT). The maximal total force (MVCTOT) and the forces of individual fingers (MVCi; i = I, index; M, middle; R, ring; and L, little) at the time of MVCTOT were measured and used to determine the target force levels for the next two tasks. The subjects performed two consecutive attempts and the trial with the higher MVCTOT was selected to set further tasks.
2.3.2. Single-finger ramp tasks
The purpose of this task was to quantify the interdependence among finger forces. In these trials, subjects were required to press with one of the fingers (the task finger) and match its force with the template shown on the screen. The 20-s template consisted of a horizontal segment at zero force for the first 4 s, followed by a slanted line from 0% to 40% of MVCi of the task finger measured in the MVC test over the next 12 s, and a horizontal segment at 40% of MVCi for the last 4 s. The monitor in front of the subject was used to provide task-specific feedback as illustrated in Figure 2B for this task as well as for the accurate force production task (see below). Subjects were asked to pay no attention to possible force production by other fingers (non-task fingers) and to keep all the fingers on the sensors at all times. This instruction has been used in many earlier studies of enslaving (e.g., Zatsiorsky et al. 2000; Latash et al. 2001; Shinohara et al. 2003); we used it to be able to compare our findings with earlier publications.
2.3.3. Accurate force pulse production task
In this task, subjects were asked to produce a quick force pulse from a steady-state level of force into the target shown on the screen by pressing with all four fingers (Figure 2C). During each trial, the feedback on FTOT was provided on the computer screen. Two horizontal lines showed an initial steady-state force level (set at 8% of MVCTOT) and a target level (set at 25% of MVCTOT; with ±5% error margins). The instruction was to press on the sensors with all four fingers and match FTOT during the steady state as accurately as possible. A vertical line was shown corresponding to 5 s after the trial initiation. Once the cursor crossed the vertical line, the subjects were required to produce a very quick force pulse into the target at a self-selected time within the next 5 s. Quickness of action was emphasized while accuracy constraints were purposefully made relatively mild. Each subject performed at least 25 trials and additional trials (over the minimum 25) were given if the subject made a major mistake (for example, pressing before the cursor reached the vertical line, pressing several times within 1 trial, or changing the baseline force slowly in preparation to pressing).
2.4. Data Analysis
The force data were digitally low-pass filtered with a zero-lag, fourth-order Butterworth filter at 10 Hz. The data processing was done using a customized Matlab code.
2.4.1. Single-finger ramp tasks
During these tasks, non-task fingers always produced force that increased in parallel with the force of the task finger (enslaving, Li et al., 1998). The enslaving matrix (E) was computed reflecting the involuntary force productions by non-task fingers when an instructed finger produces force (Zatsiorsky et al., 2000). For each single-finger trial, linear regressions of the force produced by individual fingers against FTOT over a 10-s time interval were computed. The first and last 1-s intervals were excluded to avoid edge effects. The regression coefficients in were used to construct:
Where i, j = {I, M, R, L}; j represents a task finger; Fi,j and FTOT,j indicate the individual i-finger force and FTOT, respectively, when j-finger was the task-finger. An overall index of enslaving, ENj, was computed for each finger as the average ki,j across the non-task fingers when j-finger was the task-finger: (i ≠ j).
2.4.2. Accurate force pulse production tasks
The trials with the following errors were excluded from further analysis: the peak force was outside the ±5% error margins of the target force, the time to peak force was over 1 s, the baseline force was not stabilized prior to pressing, and/or the force pulse showed multiple peaks. Overall, the total number of excluded trials varied between 3 and 12 among subjects. The number of included trials was 22±1.5 for all subjects since we collected extra trials (over the minimum 25) in case the subject made mistakes that could be recognized during the testing procedure. The following variables were computed only for the accepted trials.
The time (t0) of initiation of FTOT change was defined as the time when the first derivative of force (dF/dt) reached 5% of its peak value in that particular trial. All the accepted trials for each hand and each subject were aligned with respect to t0. The time to reach peak force (Tpeak) was defined as the time of peak force with respect to t0.
An index of multi-finger force stabilizing synergy was computed within the framework of the uncontrolled manifold (UCM) hypothesis (Scholz and Schöner, 1999; for computational details see Latash et al. 2001). Finger forces were transformed into finger modes (m) with the help of the E matrix. Mode is a hypothetical neural variable reflecting the intentional force production by a finger leading to forces by all the fingers of the hand due to enslaving. The variance in the mode space across all the accepted trials was quantified separately in two subspaces for each time sample. The first sub-space (UCM) corresponded to no changes in FTOT. The second sub-space was the orthogonal complement (ORT) to the UCM; variance within ORT changed FTOT. The two variance components (VUCM and VORT) were further combined into a single metric, a synergy index, ΔV, which was computed for each time sample: ΔV = (VUCM – VORT)/VTOT, where each variance index is normalized by the number of degrees-of-freedom in the corresponding spaces, 3 for UCM, 1 for ORT, and 4 for TOT; VTOT stands for total variance.
We interpret ΔV > 0 as sign of a FTOT – stabilizing synergy (see Latash et al. 2001; reviewed in Latash et al. 2007; Latash 2008); a higher ΔV implies a stronger synergy. For further statistical analysis, ΔV was log-transformed (ΔVZ) using the Fischer transformation applied for the computational boundaries, from −4 to +1.333.
The average value and standard deviation (SD) of ΔVZ were computed for the steady-state interval (between −600 and −400 ms prior to t0). Anticipatory synergy adjustment (ASA) was quantified using two indices, the difference in the ΔVZ between steady state and t0 (ΔVSS–t0) and the time of initiation of the ΔVZ drop (tASA). The time of initiation of ΔVZ change was defined as the time when ΔVZ dropped below its average steady-state value (ΔVSS) by 2 SDs. Negative values of tASA mean that ΔVZ started to drop before the initiation of FTOT changes.
2.5. Statistics
Standard descriptive statistics were used, and the data are presented as means and standard errors (SE). Mixed-design ANOVAs with repeated measures were used to explore how outcome variables (EN, Tpeak, ΔVSS, ΔVSS–t0, and tASA) were affected by factors Group (stroke and CS), Finger (I, M, R, and L), and Hand (right and left). We also used the factor Side (affected and less-affected), which compared the data for the ipsilesional hands of the stroke patients (less-affected) to the data for the contralesional hands of both groups (affected). The data were checked for violations of sphericity and Greenhouse-Geisser criterion was used to adjust the degrees-of-freedom when necessary. Pair-wise comparisons with Bonferroni corrections were used to explore significant ANOVA effects. Pearson correlation coefficients were used to determine significant relationships between variables. All statistical tests were performed with SPSS 19.0 (SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Performance indices
Stroke patients produced significantly lower MVC forces with both hands compared to CS. The MVC values (mean ± SE) for the stroke patients were 60.9 ± 5.2, whereas for the CS group these values were 85.4 ± 8.1 N. A two-way repeated measures ANOVA on Group and Hand showed only a main effect of Group [F[1,22] = 7.29, p < 0.05]. The comparison between the more affected, contralesional, and less-affected, ipsilesional, hands of the stoke patients showed that the MVC was significantly lower in the contralesional hand (paired t-test, p < 0.05). The values were 56.2 ± 5.6 N for the affected hand and 65.7 ± 4.8 N for the less-affected hand.
During the single-finger ramp force production tasks, unintended force production by non-task fingers (EN; enslaving) was seen in all subjects and the summed EN of all individual fingers (ENsum) was not different between groups (0.30 ± 0.04 in CS; 0.29 ± 0.04 in stroke patients). Whereas different fingers showed different amount of enslaving, the overall pattern was similar in both groups; EN was smallest when index finger was the task finger, while the ring finger caused the largest enslaving. A three-way Group × Hand × Finger ANOVA on EN showed a main effect of Finger [F[3,66] = 19.59, p < 0.001] without other effects. Post hoc comparisons confirmed that ENI < ENM < ENL < ENR (p < 0.05).
Further comparison within the stroke group showed that ENsum was larger in the contralesional hand compared to the ipsilesional hand (0.35 ± 0.05 and 0.23 ± 0.02 for the affected and less-affected side, respectively) while EN indices for individual fingers showed similar overall patterns for the contralesional and ipsilesional hands (Figure 3). A two-way repeated measures ANOVA on EN with factors Side and Finger showed significant main effects for Side [F[1,22] = 4.93, p < 0.05] and Finger [F[3,66] = 8.19, p < 0.001] without an interaction. Post-hoc comparisons confirmed that ENI < ENR, ENL (p < 0.05).
Figure 3.
The enslaving indices (EN) for the index (I), middle (M), ring (R), and little (L) fingers of the affected (filled bars) and less-affected (open bars) hands for the stroke patients. Average values are presented with standard error bars. Note the larger enslaving in the affected (contralesional) hand.
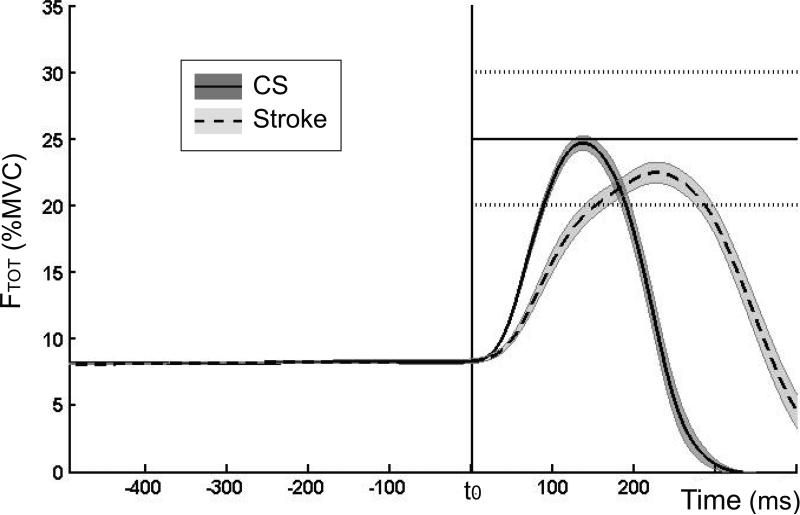
Figure 4 shows the averaged performance of representative subjects from each group for the quick force pulse production. Stroke patients were slower than CS in reaching the force peak (Tpeak; 0.21 s in the stroke patients and 0.15 s in the CS) but there were no noticeable differences between the hands. These results were supported by a two-way repeated-measures ANOVA on Tpeak with factors Group and Hand which only showed significant main effect for Group [F[1,22] = 6.97, p < 0.05]. There was no significant difference for Tpeak between the ipsilesional and contralesional hands (Table 3).
Figure 4.
Time profiles of the averaged total force with SE shades computed across trials for typical subjects of each group during the quick force pulse task. The trials were aligned by the initiation of the force pulse (t0). The dashed line shows the data for the left hand (affected side) of a stroke subject (Tpeak = 0.22 s) and the solid line shows the data for the left hand of a CS (Tpeak = 0.14 s).
Table 3.
Performance and synergy indices for the quick force production test
| T Fpeak | Δ V SS | Δ V SS–t0 | tASA (S) | ||
|---|---|---|---|---|---|
| CS (n=12) | Right | 0.16 ± 0.02 | 2.22 ± 0.13 | 0.53 ± 0.08 | −0.23 ± 0.03 |
| Left | 0.15 ± 0.01 | 2.63 ± 0.13 | 0.70 ± 0.14 | −0.29 ± 0.03 | |
| Stroke (n=12) | Contra | 0.21 ± 0.02 | 2.43 ± 0.13 | 0.37 ± 0.09 | −0.10 ± 0.03 |
| Ipsi | 0.21 ± 0.02 | 2.48 ± 0.15 | 0.45 ± 0.08 | −0.17 ± 0.04 | |
Means ± SE of time to force peak (TFpeak), variance indices at steady-state (ΔVSS), magnitude (ΔVSS–t0) and time of anticipatory synergy adjustments (tASA) are presented for stroke patients and CS. Abbreviations: Contra - contralesional side; Ipsi - ipsilesional side.
3.2. Multi-digit synergies and ASA in quick force pulse production
During the steady-state phase of the main task, both stroke and CS groups demonstrated stable task performance, which kept FTOT close to the target level before the pulse initiation. This was reflected in consistently positive synergy indices during steady states (ΔVSS) in both groups. These values reflected the larger amounts of inter-trial variance in the space of commands to fingers (modes, see Methods) that kept FTOT unchanged. There was no significant group difference in ΔVSS. These results were supported by a two-way Group × Hand ANOVA on ΔVSS that showed a significant main effect of Hand [F[1,22] = 9.24, p < 0.05] without other effects.
Before the initiation of the force pulse, there was a drop in the synergy index (ΔVZ). The initiation of the ΔVZ drop was delayed in stroke patients, on average by 48% for both hands. These results were supported by two-way repeated-measures ANOVA on tASA with factors Group [main effect: F[1,22] = 10.49, p < 0.05 for tASA] and Hand. There were no interaction effects. The stroke group, on average, showed a smaller magnitude of the ΔVZ drop (see Table 3 and Figure 5) but the group difference did not reach significance level.
Figure 5.
The average across subjects time profiles of the synergy index (z-transformed, ΔVZ) with standard error shades. The data for the stroke group are shown with the dashed line and light shade; the data for the control group (CS) are shown with the solid line and darker shade. The data for the contralesional hand of the stroke patients and the average data across both hands of CS are presented. Note the very similar steady-state values and the much earlier drop in ΔVZ in preparation to the force pulse.
All outcome variables from the synergy analysis are presented for each group in Table 3. When we compared the contralesional and ipsilesional hands of stroke patients, the delay in ASA was more prominent in the contralesional hand (Figure 5). The tASA values for the contralesional hands and ipsilesional hands in stroke patients and the average values between the right and left hands in CS were compared with one-way ANOVA. There was a significant difference among hands [F = 6.03, p < 0.05] and post-hoc comparisons confirmed that tASA was later (closer to t0) in the contralesional hand of stroke patients than in CS (p < 0.05).
Lastly, we explored the correlations among the mentioned outcome variables from the synergy index and clinical test scores. The index of synergy (ΔVSS) correlated positively with the Grooved Pegboard score in stroke patients when the Grooved Pegboard test was performed by the left hand (r = 0.68; p < 0.05). The data for patient #10 were excluded because she was unable to perform the Pegboard test with the left hand. No other significant correlations were found. We also performed correlation analyses between lesion volume and outcome variables (including synergy indices and clinical test scores) for the ipsilesional side and contralesional side separately. However, no significant correlation was found.
Discussion
The main hypothesis of the study that stroke patients would show significant differences from the control group in indices of performance but not in synergy indices (Hypothesis-1) has been largely supported. Indeed, stroke patients differed significantly from the control group in such indices as MVC (lower in stroke) and time to peak force (longer in stroke). While group difference in the enslaving index was not significant, the index was significantly higher in the contralesional hand of the stroke patients compared to the ipsilesional hand. In addition, the contralesional hand showed lower MVC compared to the ipsilesional hand. In contrast, the synergy index during the steady-state phase of the task showed no effects of stroke and no difference between the ipsilesional and contralesional hands. Hypothesis-2, however, was not supported: Anticipatory synergy adjustments (ASAs) started significantly later in the stroke patients, and the delay in ASA was more prominent in the contralesional hand of the stroke patients.
Effects of stroke on finger interdependence
When humans try to move a finger or press with a finger, other fingers of the hand move and press unintentionally (Kilbreath and Gandevia, 1994; Li et al., 1998; Lang and Schieber, 2003). This phenomenon, addressed as lack of independence or enslaving (Zatsiorsky et al., 2000), is due to many factors including peripheral connective tissue links between fingers, multi-digit extrinsic hand muscles, and overlapping cortical representations of fingers (reviewed in Schieber and Santello, 2004). Studies with transcranial magnetic stimulation have suggested a substantial degree of physiological independence among the compartments of the external hand flexors (Danion et al., 2003a). In addition, experiments comparing the patterns of finger interdependence during pressing tasks with fingertips and with proximal phalanges showed similar patterns of enslaving in both conditions (Latash et al., 2002). Note that pressing with proximal phalanges is produced by intrinsic hand flexors, which are finger-specific. Overall, these observations suggest that the patterns of finger enslaving are primarily defined by central, neural factors. This conclusion is also supported by the observations of strong and quick effects of practice on the enslaving indices (Wu et al., 2013).
It is not surprising, therefore, that even mild cortical stroke has significant effects on finger individuation resulting in higher indices of finger enslaving (Li et al., 2003), likely with an important contribution from the lost cortico-spinal projections. Our experiment corroborated this conclusion by showing significantly higher enslaving indices in the contralesional hand of the stroke patients compared to the ipsilesional hand. The increased enslaving is likely to contribute to poor finger coordination and impaired hand function typical of stroke (cf. Schieber et al., 2009). We would like to emphasize, however, that relations between changes in enslaving and dexterity are not unambiguous. For example, healthy older adults show lower enslaving indices (Shinohara et al. 2003, 2004), while a drop in hand dexterity with advanced age is also well documented (Hackel et al. 1992; Hughes et al. 1997). It is possible that a certain amount of enslaving is optimal for performance of the typical range of everyday tasks given that enslaving contributes to stabilization of the total moment of force exerted on hand-held object (Zatsiorsky et al. 2000).
Note that the magnitude of enslaving is an important factor in the analysis of multi-finger synergies. High enslaving is expected to lead to predominantly positive co-variation among finger forces, which act to weaken synergies stabilizing total force. To remove these effects of enslaving, analysis of finger synergies is performed in the space of finger modes, hypothetical commands to individual fingers (Danion et al., 2003b). Each mode produces forces in all four fingers due to enslaving. Note that in persons with high enslaving, analysis in the finger mode space is more conservative: It leads to indices that are smaller than those in the force space. This means that low synergy indices in the finger mode space in persons/hands with higher enslaving imply even lower indices in the space of forces.
Finger synergies and their changes in neurological disorders
The definition of synergies accepted in this paper differs from two commonly used definitions. In clinics, abnormal synergy is a term used to describe stereotypical patterns of muscle activation interfering with intentional movements (Bobath, 1978; Dewald et al., 1995). In the motor control literature, synergies commonly mean proportional involvement of elemental variables, for example muscle activations, joint rotations, forces, etc. (d'Avela et al., 2003; Krishnamoorthy et al., 2003; Ivanenko et al., 2004; Ting and Macpherson, 2005). We link synergies to a crucial feature of all meaningful movements performed by redundant (more exactly, abundant, Latash, 2012) systems, that is, their stability. Two characteristics of synergies are usually studied, sharing and co-variation. The former reflects the average involvement of elements in a task; commonly, patterns of sharing are studied using optimization methods (reviewed in Prilutsky and Zatsiorsky, 2002). The latter implies that, when one element in a particular trial deviates from its average trajectory, other elements are also likely to show deviations organized to keep an important performance variable relatively unchanged. A method to study this feature quantitatively has been developed within the UCM hypothesis (reviewed in Latash et al., 2007).
Characteristics of synergies show significant changes in atypical development, fatigue, and healthy aging (reviewed in Latash, 2008). Recently, significant changes in multi-digit synergies have been documented for patients with subcortical disorders such as Parkinson's disease and multi-system atrophy (Park et al., 2012, 2013b). In particular, synergy changes in PD could be quantified even in patients who, according to the clinical evaluation, showed no signs of the disease in the studied extremities. Note that these patients were tested on their optimal medication and, as a result, they displayed minimal functional differences from healthy controls in overall characteristics of performance.
An earlier study of synergies during reaching in stroke survivors showed a different picture: Overall patterns of joint coordination during reaching movements were affected in the patients, while the relative amounts of VUCM and VORT computed in the joint configuration space with respect to the endpoint trajectory were unchanged (Reisman and Scholz, 2003) or showed changes during reaching to ipsilateral targets only (Reisman and Scholz, 2006). This was accompanied by an increase in VORT in both studies with a parallel increase in VUCM in all the conditions except during the reaches to ipsilateral targets in Reisman and Scholz (2006). These study emphasized the importance of quantifying the two variance components separately because changes in the synergy index could reflect changes in either VUCM or VORT or both. Our study differed from those by Reisman and Scholz in presenting visual feedback and explicit target for the salient performance variable (total force). As a result, VORT was forced to be very low across subjects (see Figure 3). Hence, we reduced the analysis to the single metric, ΔV. In future, studies of more natural multi-digit tasks, e.g. static prehensile tasks, are warranted to provide a better comparison to the studies of reaching.
The contrast between the results in stroke patients and those in PD patients could be due to the difference in the task (multi-digit pressing and prehension in PD vs. multi-joint reaching in stroke) or to qualitatively different effects on synergies of a disorder in different brain structures. The results from the current study favor the latter hypothesis. Indeed, we observed significant changes in performance variables in the stroke group and between the contralesional and ipsilesional hands while no changes in the synergy index could be seen during the steady-state force production. This conclusion fits well the hypothesis of Houk (2005) on the role of neural circuits involving the basal ganglia and the cerebellum in the so-called distributed processing modules, hypothetical neural operators that participate in the coordination of natural movements. Given that there have been so far only a few studies exploring synergies after stroke and that they involved relatively small cohorts of patients (eight in Reisman and Scholz 2003, seven in Reisman and Scholz 2006, and twelve in the current study), these conclusions should be viewed as tentative.
Effects of stroke on feed-forward control of synergies
Anticipatory synergy adjustments (ASAs) represent a drop in a synergy index in anticipation of a quick change of the performance variable, for which the index has been computed (Olafsdottir et al., 2005; Shim et al., 2005). As a result, ASAs lead to relative loss of stability of the performance variable. ASAs are functionally important because they allow the central nervous system to avoid fighting its own synergies. In fact, phenomena similar to ASAs can be observed in some of the everyday and athletic actions; consider, for example, the increased body sway of a tennis player who is getting ready for a fast serve by the opponent.
Earlier studies of various populations provided evidence for parallel changes in synergies and ASAs. In particular, synergy and ASA indices are both decreased in healthy elderly, in patients with PD, and in patients with multi-system atrophy (Shinohara et al., 2004; Olafsdottir et al., 2007; Park et al., 2012, 2013a,b). Taken together, these changes have been addressed as impaired control of stability of movement and posture (Latash and Huang, 2015). Our current study provides the first evidence for dissociation between changes in synergies and ASAs. Indeed, stroke patients showed no differences in the steady-state synergy index but significantly delayed ASAs (see Figure 3). These results suggest that while cortical structures may not be important to ensure the stability of performance (as reflected in synergy indices), they do contribute (at least in part) to agility of performance, as reflected in ASAs.
Problems with feed-forward movement control are well documented in patients with a variety of neurological disorders such as stroke, PD, cerebellar disorders, and even in healthy older adults (reviewed in Lemon and Griffiths, 2005; Latash and Huang, 2015). Examples include anticipatory postural adjustments (APAs, Massion 1992) and grip adjustments to manipulation of a hand-held object: Both are reduced in all the mentioned populations as compared to control groups. Note that ASAs and APAs represent very different phenomena with respect to their function (synergy adjustments vs. generation of net forces/moments) and timing (ASAs are seen 100-200 ms prior to the first signs of APAs). But they both are examples of feed-forward control, and it is possible that similar neural mechanism are involved in these two phenomena.
The documented loss of ASAs may be causally related to the loss of hand dexterity after stroke (Hackel et al. 1992; Hughes et al. 1997), in particular in tasks that require quick finger actions. We would like to emphasize that the arm of our subjects was supported during the testing procedure so that possible abnormal upper-arm synergies were not expected to interfere with the required finger actions.
Concluding comments
Our study has a number of limitations. Among the obvious drawbacks is the relatively small cohort of subjects and the unbalanced male-female composition. Another problem typical of stroke studies is the diverse etiology and location of hemisphere lesions. Despite these drawbacks, the significant findings carry two strong messages. First, in contrast to patients with subcortical disorders, survivors of cortical stroke show relative unaffected synergies stabilizing steady-state performance during accurate multi-finger force production, even though indices of performance are changed significantly. Second, feed-forward adjustments of synergies in cortical stroke patients suffer similar impairment as in patients with Parkinson's disease and multi-system atrophy.
It is notable that our clinical test of hand impairment, the UEFM, indicated that all subjects were unimpaired in hand function. However, our higher-resolution tests of enslaving and synergy indicated increased enslaving, or reduced finger individuation, and impaired anticipatory aspects of coordination. While clinical tests of impairment are often not strong predictors of functional independence, especially in cases of mild impairment (Chen et al., 2015) more precise measures of finger coordination such as enslaving and synergy indices might prove more predictive of functional independence. Further research is necessary to test this hypothesis.
Highlights.
Mild unilateral cortical stroke survivors show lower maximal finger forces and higher enslaving.
No changes in multi-finger synergies during steady-state tasks are seen in mild unilateral cortical.
Mild unilateral cortical stroke leads to significantly delayed anticipatory synergy adjustments.
Two aspects of synergic control are differentially affected by mild unilateral cortical stroke.
Acknowledgments
The present work was partially supported by National Institutes of Health (NIH) grants HD059783, NS035032, and AR048563.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Hang Jin Jo: developed apparatus and methods; performed the experiment; designed, performed, and reviewed statistical analysis; wrote the first draft; and reviewed the manuscript.
Candice Maenza: performed the experiment; reviewed statistical analysis; and reviewed the manuscript.
David Good: designed the study; supervised patient selection; reviewed the manuscript.
Xuemei Huang: designed the study; reviewed the manuscript.
Jaebum Park: developed apparatus and methods; performed the experiment; and reviewed the manuscript.
Robert Sainburg: designed the study; supervised patient selection; reviewed the manuscript.
Mark L. Latash: conceived the experiment; developed apparatus and methods; designed and reviewed statistical analysis; and reviewed the manuscript.
REFERENCES
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- Bobath B. Adult Hemiplegia: Evaluation and Treatment. William Heinemann; London: 1978. [Google Scholar]
- Brunnström S. Movement Therapy in Hemiplegia: A Neurophysiological Approach. Harper & Row; New York, NY: 1970. [Google Scholar]
- Chen C-M, Tsai C-C, Chung C-Y, Chen C-L, Wu KPH, Chen H-C. Potential predictors for health-related quality of life in stroke patients undergoing inpatient rehabilitation. Health Qual Life Outcomes. 2015;13:118. doi: 10.1186/s12955-015-0314-5. DOI 10.1186/s12955-015-0314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danion F, Latash ML, Li S. Finger interactions studied with transcranial magnetic stimulation during multi-finger force production tasks. Clin Neurophysiol. 2003a;114:1445–1455. doi: 10.1016/s1388-2457(03)00105-6. [DOI] [PubMed] [Google Scholar]
- Danion F, Schöner G, Latash ML, Li S, Scholz JP, Zatsiorsky VM. A force mode hypothesis for finger interaction during multi-finger force production tasks. Biol Cybern. 2003b;88:91–98. doi: 10.1007/s00422-002-0336-z. [DOI] [PubMed] [Google Scholar]
- d'Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- DeWald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Gelfand IM, Latash ML. On the problem of adequate language in movement science. Motor Control. 1998;2:306–313. doi: 10.1123/mcj.2.4.306. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–58. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Hackel ME, Wolfe GA, Bang SM, Canfield JS. Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Phys Ther. 1992;72:373–377. doi: 10.1093/ptj/72.5.373. [DOI] [PubMed] [Google Scholar]
- Heaton R, Miller S, Taylor M, Grant I. Demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources Inc. Revised comprehensive norms for an expanded Halstead-Reitan Battery; Lutz, FL: 2004. [Google Scholar]
- Houk JC. Agents of the mind. Biol Cybern. 2005;92:427–437. doi: 10.1007/s00422-005-0569-8. [DOI] [PubMed] [Google Scholar]
- Hughes S, Gibbs J, Dunlop D, Edelman P, Singer R, Chang RW. Predictors of decline in manual performance of older adults. J Am Geriatr Soc. 1997;45:905–910. doi: 10.1111/j.1532-5415.1997.tb02957.x. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004;556:267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Park J, Lewis MM, Huang X, Latash ML. Prehension synergies and hand function in early-stage Parkinson's disease. Exp Brain Res. 2015;233:425–440. doi: 10.1007/s00221-014-4130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle synergies during shifts of the center of pressure by standing persons. Exp Brain Res. 2003;152:281–292. doi: 10.1007/s00221-003-1574-6. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J Neurophysiol. 2003;90:1160–1170. doi: 10.1152/jn.00130.2003. [DOI] [PubMed] [Google Scholar]
- Latash ML. Synergy. Oxford University Press; New York: 2008. [Google Scholar]
- Latash ML. The bliss (not the problem) of motor abundance (not redundancy). Exp Brain Res. 2012;217:1–5. doi: 10.1007/s00221-012-3000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Huang X. Neural control of movement stability: Lessons from studies of neurological patients. Neurosci. 2015;301:39–48. doi: 10.1016/j.neuroscience.2015.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Li Z-M, Zatsiorsky VM. A principle of error compensation studied within a task of force production by a redundant set of fingers. Exp Brain Res. 1998;122:131–138. doi: 10.1007/s002210050500. [DOI] [PubMed] [Google Scholar]
- Latash ML, Li S, Danion F, Zatsiorsky VM. Central mechanisms of finger interaction during one- and two-hand force production at distal and proximal phalanges. Brain Res. 2002;924:198–208. doi: 10.1016/s0006-8993(01)03234-6. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JF, Danion F, Schöner G. Structure of motor variability in marginally redundant multi-finger force production tasks. Exp Brain Res. 2001;141:153–165. doi: 10.1007/s002210100861. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schöner G. Toward a new theory of motor synergies. Motor Control. 2007;11:276–308. doi: 10.1123/mcj.11.3.276. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Li ZM, Latash ML, Zatsiorsky VM. Force sharing among fingers as a model of the redundancy problem. Exp Brain Res. 1998;119:276–286. doi: 10.1007/s002210050343. [DOI] [PubMed] [Google Scholar]
- Li S, Latash ML, Yue GH, Siemionow V, Sahgal V. The effects of stroke and age on finger interaction in multi-finger force production tasks. Clin Neurophysiol. 2003;114:1646–1655. doi: 10.1016/s1388-2457(03)00164-0. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium – interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Olafsdottir H, Yoshida N, Zatsiorsky VM, Latash ML. Anticipatory covariation of finger forces during self-paced and reaction time force production. Neurosci Lett. 2005;381:92–96. doi: 10.1016/j.neulet.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsdottir H, Yoshida N, Zatsiorsky VM, Latash ML. Elderly show decreased adjustments of motor synergies in preparation to action. Clin Biomech. 2007;22:44–51. doi: 10.1016/j.clinbiomech.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Jo HJ, Lewis MM, Huang X, Latash ML. Effects of Parkinson's disease on optimization and structure of variance in multi-finger tasks. Exp Brain Res. 2013a;231:51–63. doi: 10.1007/s00221-013-3665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lewis MM, Huang X, Latash ML. Effects of olivo-ponto-cerebellar atrophy (OPCA) on finger interaction and coordination. Clin Neurophysiol. 2013b;124:991–998. doi: 10.1016/j.clinph.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lewis MM, Huang X, Latash ML. Dopaminergic modulation of motor coordination in Parkinson's disease. Parkinsonism Rel Disord. 2014;20:64–68. doi: 10.1016/j.parkreldis.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wu Y-H, Lewis MM, Huang X, Latash ML. Changes in multi-finger interaction and coordination in Parkinson's disease. J Neurophysiol. 2012;108:915–924. doi: 10.1152/jn.00043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prilutsky BI, Zatsiorsky VM. Optimization-based models of muscle coordination. Exer Sport Sci Rev. 2002;30:32–38. doi: 10.1097/00003677-200201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschechtko S, Zatsiorsky VM, Latash ML. Stability of multi-finger action in different spaces. J Neurophysiol. 2014;112:3209–3218. doi: 10.1152/jn.00395.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D, Scholz JP. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain. 2003;126:2510–2527. doi: 10.1093/brain/awg246. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Scholz JP. Workspace location influences joint coordination during reaching in post-stroke hemiparesis. Exp Brain Res. 2006;170:265–276. doi: 10.1007/s00221-005-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart JK, Singleton RD, Adair JC, Sadek JR, Haaland KY. Arm use after left or right hemiparesis is influenced by hand preference. Stroke. 2009;40:545–550. doi: 10.1161/STROKEAHA.108.528497. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. NeuroImage. 2012;61(4):957–65. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: Normative values for the finger tapping and grooved pegboard tests. Percept Mot Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Dissociation of initial trajectory and final position errors during visuomotor adaptation following unilateral stroke. Brain Res. 2009a;1298:78–91. doi: 10.1016/j.brainres.2009.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009b;47:2953–66. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Lang CE, Reilly KT, McNulty P, Sirigu A. Selective activation of human finger muscles after stroke or amputation. Adv Exp Med Biol. 2009;629:559–575. doi: 10.1007/978-0-387-77064-2_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. 2004;96:2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schöner G. The uncontrolled manifold concept: Identifying control variables for a functional task. Exp Brain Res. 1999;126:289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Danion F, Latash ML, Schöner G. Understanding finger coordination through analysis of the structure of force variability. Biol Cybern. 2002;86:29–39. doi: 10.1007/s004220100279. [DOI] [PubMed] [Google Scholar]
- Shim JK, Olafsdottir H, Zatsiorsky VM, Latash ML. The emergence and disappearance of multi-digit synergies during force production tasks. Exp Brain Res. 2005;164:260–270. doi: 10.1007/s00221-005-2248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Li S, Kang N, Zatsiorsky VM, Latash ML. Effects of age and gender on finger coordination in maximal contractions and submaximal force matching tasks. J Appl Physiol. 2003;94:259–270. doi: 10.1152/japplphysiol.00643.2002. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Scholz JP, Zatsiorsky VM, Latash ML. Finger interaction during accurate multi-finger force production tasks in young and elderly persons. Exp Brain Res. 2004;156:282–292. doi: 10.1007/s00221-003-1786-9. [DOI] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005;93:609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehab. 2005;86(4):776–781. doi: 10.1016/j.apmr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Wilhelm L, Zatsiorsky VM, Latash ML. Equifinality and its violations in a redundant system: Multi-finger accurate force production. J Neurophysiol. 2013;110:1965–1973. doi: 10.1152/jn.00461.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Merians A, Sullivan K. Motor learning after unilateral brain damage. Neuropsychologia. 1999;37:975–987. doi: 10.1016/s0028-3932(98)00145-6. [DOI] [PubMed] [Google Scholar]
- Wu Y-H, Pazin N, Zatsiorsky VM, Latash ML. Improving finger coordination in young and elderly persons. Exp Brain Res. 2013;226:273–283. doi: 10.1007/s00221-013-3433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke M. Effect of brain lesions on the rapidity of arm movement. Neurology. 1967;17:1113–1120. doi: 10.1212/wnl.17.11.1113. [DOI] [PubMed] [Google Scholar]
- Yang J-F, Scholz JP, Latash ML. The role of kinematic redundancy in adaptation of reaching. Exp Brain Res. 2007;176:54–69. doi: 10.1007/s00221-006-0602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Exp Brain Res. 2000;131:187–195. doi: 10.1007/s002219900261. [DOI] [PubMed] [Google Scholar]
- Zhou T, Zhang L, Latash ML. Intentional and unintentional multi-joint movements: Their nature and structure of variance. Neurosci. 2015;289:181–193. doi: 10.1016/j.neuroscience.2014.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]