Abstract
Background
To define how the incidence of peripheral arterial disease (PAD) in chronic kidney disease (CKD) differs according to sex and age.
Methods and Results
The Chronic Renal Insufficiency Cohort (CRIC) is a multi-center, prospective cohort study of CKD participants. Fine and Gray methods were used to determine the cumulative incidence of PAD, defined by an ankle brachial index (ABI) < 0.90 or a confirmed PAD event, with death as a competing event. Adjusted subdistribution hazard ratios from the Fine and Gray model determined the risk of PAD according to sex. A priori, we hypothesized that the relationship between sex and cumulative incidence of PAD differed according to age. The mean age of the 3,174 participants in this study was 56.6 years and consisted of 55% males. Over a median follow-up of 5.9 years, 17.8% developed PAD, 13.0% were lost to followup and 11.1% died. Females had a 1.53-fold greater adjusted PAD risk compared to men (95% CI 1.27-1.84, p<0.001). These sex-related differences in PAD risk also differed by age (p=0.013). Women, compared to men, were at a markedly increased risk for PAD at younger ages; however, at ages greater than 70 years, the risk was similar across both sexes. Older men had a substantially greater PAD risk compared to younger men. In women, PAD risk did not vary with age.
Conclusions
Females with CKD have a higher PAD risk compared to males at younger ages. There is an important need to improve our understanding of the biological and clinical basis for these differences.
Keywords: peripheral artery disease, chronic kidney disease, epidemiology, sex
The American Heart Association estimates that peripheral artery disease (PAD) affects 8 million people over 40 years of age in the United States, and 12-20% of Americans above the age of 65.1,2 PAD is a major source of morbidity and mortality resulting in functional impairment, limb loss, as well as death.3 Despite epidemiologic studies which have contributed to our understanding of PAD prevalence and its association with traditional atherosclerotic risk factors, there have been conflicting studies published on the incidence of PAD and differences in treatment outcomes in women versus men.4-7 Much of the uncertainty surrounding this topic stems from the fact that because PAD surveillance is not conducted in any state or nation, the prevalence of PAD by sex remains incompletely evaluated.8 In addition, the majority of clinical trials published in PAD have enrolled primarily men9-11 and did not evaluate for effect modification by sex, thus widening this knowledge gap.
There is a growing concern, however, that PAD outcomes are worse in women. In a recent study of the Nationwide Inpatient Sample between 1998 and 2009 of patients undergoing a revascularization procedure, women were likely to have advanced disease at presentation and have a higher in-hospital mortality.7 In addition, studies have reported that women with PAD have greater functional impairment,12 a worse quality of life, 13 and more depressive symptoms compared to men.14 US census data from 2010 also suggest that more women than men ≥ 40 years of age suffer from PAD.8
And yet, how risk factors for PAD may impact this possible sex-based difference is unknown. For example, age is an established PAD risk factor,15 but the incidence of PAD according to sex across a broad spectrum of ages remains incompletely defined. Patients who are at particularly high risk for PAD also include those with chronic kidney disease (CKD). Data from the National Health and Nutrition Examination Survey (NHANES) demonstrated that 24% of persons with CKD stage 3 or greater had PAD as defined by an ankle brachial index (ABI) < 0.9, which was significantly greater than the 4% prevalence in the group with normal renal function.16 Indeed, the National Kidney Foundation Task Force issued a statement suggesting that patients with CKD be considered in the highest risk category for subsequent cardiovascular events such as myocardial infarction, stable and unstable angina, and cardiac death.17
The CRIC (Chronic Renal Insufficiency Cohort) is well-balanced with regard to sex, has participants throughout a wide age range, and has detailed vascular function data obtained at standardized intervals. As such, it is an ideal population to study sex-based differences in PAD incidence. The objective of this study is to leverage the strengths of a uniquely phenotyped cohort of individuals with CKD to determine the differences in PAD incidence between men and women, and to define how this relationship differs by age.
Methods
Study Design
The CRIC study design and methods have been described in detail previously.18 Briefly, CRIC is a prospective cohort study of 3,939 participants enrolled from June 2003 to August 2008 through 7 clinical centers in the United States (Ann Arbor and Detroit, Michigan; Baltimore, Maryland; Chicago, Illinois; Cleveland, Ohio; New Orleans, Louisiana; Philadelphia, Pennsylvania; and Oakland, California) who were followed for up to 8.7 years. Participants included men and women 21 to 74 years of age at study entry with mild to moderate CKD as defined by glomerular filtration rate (GFR). Age-based GFR was used as an inclusion criterion for the study and included the following: for Age 21-44: GFR 20-70 ml/min/1.73 m2, Age 45-64: GFR 20-60 ml/min/1.73 m2, Age 65-74: GFR 20-50 ml/min/1.73 m2.18 Exclusion criteria included New York Heart Association class 3 and 4 heart failure, cancer and immunosuppressive therapies during the previous 6 months. The initial protocol called for each of the seven clinical centers to enroll 450 participants during a 33-month period (May 2003 through March 2006). In August 2005 after Hurricane Katrina, enrollment at Tulane was halted after enrollment of 405 patients and recruitment targets at the other six clinical centers were increased. Recruitment strategies varied from center to center and consisted of computerized searches of laboratory databases as well as medical record searches and referrals from healthcare providers.19 Participants underwent detailed baseline examinations and annual in-person follow-up exams. The study was approved by the institutional review board of each participating clinical center. Written informed consent was obtained from all participants.
For the purposes of this study, participants were excluded from this analysis if they had an ABI<0.90 at their baseline visit or had a prior history of an amputation or revascularization procedure for PAD. Therefore, we focused on a subset of participants (N=3,174) in this analysis.
Ascertainment of PAD
The ankle brachial index (ABI) is the standard test utilized to diagnose PAD.20 After lying supine for 5 minutes, systolic blood pressure was measured in both arms using appropriately sized cuffs. The systolic blood pressure for the dorsalis pedis artery and posterior tibial artery was measured for each leg using a Doppler probe. The leg-specific ABI was determined by dividing the higher systolic blood pressure for the dorsalis pedis artery or posterior tibial artery by the higher systolic blood pressure of the brachial artery. In participants with functioning fistulae or arteriovenous grafts, the available contralateral brachial artery blood pressure was used. The patient-specific ABI was defined as the lower leg-specific ABI. In CRIC, the ABI is measured at the baseline visit and annually. Clinical PAD events defined as revascularization (angioplasty or surgical bypass) or major amputation (below the knee or above the knee) were captured by hospital chart review at the annual clinic visit or 6 month telephone visit when specific ICD-9 codes suggested that an amputation or revascularization procedure was performed. These events were then adjudicated by a specially trained research nurse. Aortic aneurysm treatment was not considered an event in this study. Incident PAD was thus defined by the first-occurring event amongst the following: 1) ABI <0.90, 2) revascularization procedure or 3) major amputation . Subjects were censored at the time of withdrawal or at completion of the study.
Death was treated as a competing event. Deaths were captured from medical records, death certificates, next of kin, and linkage to the Social Security Death Master File.18 Dates of death were determined from the death certificate and or medical records whenever possible. Participants were contacted at least every 6 months and next of kin were questioned regarding ascertainment of death.
Statistical Analysis
Summary statistics stratified by sex were calculated for basic demographic and clinical characteristics. The chi-square test was used to compare categorical variables and t-tests were used for continuous variables. Poisson regression was first used to describe annualized incidence rates (number of cases/100 person years) for PAD as defined by an ABI <0.90, annualized incidence rates for death, as well as annualized incidence rates for the composite endpoint of PAD and death in men and women. Survival analysis endpoints included PAD-free survival and overall survival. Both outcomes were analyzed by sex using the Kaplan Meier method, and statistical comparison was performed via the log-rank test.
Because of the high prevalence of mortality in this cohort of participants, competing risks regression analyses were implemented. We used Fine and Gray methods to determine cumulative incidence of PAD over time and explore the relationship between sex and PAD, accounting for death as a competing event. 21 Gray's test was used to compare the cumulative incidence curves of PAD between men and women in the presence of death as the competing risk.21, 22 Univariable analysis using the Fine and Gray proportional hazard model for the subdistribution of competing risks was performed and from these models, the subdistribution hazard ratio (SHR) was derived. All variables from the univariate model which had a p-value <0.05 were considered for inclusion into the multivariable model, which was built using forward stepwise-selection. Because of the increased risk of incident PAD and death with age, the effect of age on the relationship between sex and PAD cumulative incidence was also explored. Based on the pooled prevalence data derived from a meta-analysis of seven US population studies23, we hypothesized a priori that age would affect the cumulative incidence of PAD in women differently than in men. All analyses were performed using STATA version 12 (College Station, TX).
Results
Study Population
The overall mean age of the cohort of 3,174 participants at time of study entry was 56.6 years and consisted of 55.0% males and 44.0% with diabetes. There were twelve individuals that were excluded after their baseline visit and were omitted from time to event analysis. A total of 61 participants either had no follow-up or had missing covariates, leaving 3,113 for complete case analysis. The mean ABI in males was 1.13 ±0.15 and that in females was 1.08 ±0.13. The mean GFR was 46.7 mL/min per 1.73 m2 and the mean BMI was 31.9 kg/m2. Males had more hypertension (86.1% vs 82.2%, p=0.003), coronary artery disease (20.6% vs 13.0%, p<0.001), history of tobacco use (58.1% vs 41.9%, p<0.001) as well as hypercholesterolemia (85.2% vs 73.5%, p<0.001) compared to females. However, the prevalence of diabetes, congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD) was no different between men and women. All demographic data and clinical characteristics are summarized in Table 1.
Table 1. Summary statistics of demographics and clinical characteristics by sex (N=3174).
| Variable | Males N=1747 | Females N=1427 | P-value |
|---|---|---|---|
| Age mean ± SD | 56.8±11.1 | 56.5±11.4 | 0.474 |
|
| |||
| ABI mean ± SD | 1.13 ±0.15 | 1.08 ±0.13 | <0.001 |
| Race | <0.001 | ||
| White | 50.9% | 43.9% | ----- |
| Black | 35.7% | 45.1% | ----- |
| Other | 13.4% | 11.1% | ----- |
| Diabetes | 44.5% | 43.4% | 0.535 |
| eGFR | 47.3±16.6 | 46.0± 17.8 | 0.040 |
| BMI | 31.1±6.3 | 32.9±9.1 | <0.001 |
| CHF | 8.9% | 7.2% | 0.076 |
| Hypertension | 86.1% | 82.2% | 0.003 |
| CAD | 20.6% | 13.0% | <0.001 |
| Smoking | <0.001 | ||
| Never | 41.9% | 58.1% | ----- |
| Former | 45.7% | 31.0% | ----- |
| Current | 12.4% | 10.9% | ----- |
| Hypercholesterolemia | 85.2% | 73.5% | <0.001 |
| COPD | 2.2% | 3.0% | 0.147 |
| ACE inhibitor | 53.7% | 40.6% | <0.001 |
| ARB | 26.1% | 24.4% | 0.277 |
| Beta blocker | 47.7% | 44.4% | 0.066 |
| Statin | 53.1% | 49.2% | 0.027 |
| Aspirin | 41.6% | 36.6% | 0.004 |
eGFR-estimated glomerular filtration rate, BMI-body mass index, CHF-congestive heart failure, CAD-coronary artery disease, COPD-chronic obstructive pulmonary disease, ACE-angiotensin-converting-enzyme, ARB-Angiotensin receptor blockers
p-value represents the comparison of continuous and categorical variables by sex
With a median follow up of 5.9 years (range, 12.0 days-8.7 years), 17.8% of the cohort developed PAD,11.1% died, 13.0% were censored at the time of withdrawal, and 58.1% censored at the end of the study. Overall, females had a higher PAD incidence (PAD combined) compared to men (22.6% versus 13.8%, p<0.001) (Table 2). This translated to an incidence of 13.85/100 person years for women and 8.48/100 person years for men. When PAD incidence as defined by ABI<0.90 was considered separately, women had a higher incidence compared to men (21.7% versus 12.6%, p<0.001, which translated to an incidence of 12.59/100 person years for women and 7.30/100 person years for men. In contrast, when PAD incidence as defined by revascularization or amputation was considered, the incidence rates for women were similar to that for men (0.9% versus 1.3%, p=0.355). However, the overall death rate was lower (8.6% versus 13.1%, p<0.001) compared to men with an incidence of 13.10/100 person years for women and 19.93/100 person years for men. For the combined endpoint of PAD or death, females had an overall higher rate as compared to men (31.2% versus 27.0%, p=0.025).
Table 2. Number of events, percentage and incidence rates (95% CI) for PAD, death and composite outcome of PAD or death.
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| No. of cases | % | Incidence/100 Person-Years | No. of cases | % | Incidence/100 Person-Years | P-value | |
| PAD combined | 242 | 13.8 | 8.48(6.44, 11.16) | 323 | 22.6 | 13.85 (12.21, 15.71) | <0.001 |
|
| |||||||
| PAD ABI<0.90 | 220 | 12.6 | 7.30(5.48, 9.72) | 310 | 21.7 | 12.59(11.03, 14.37) | <0.001 |
|
| |||||||
| PAD Adjudicated event | 22 | 1.3 | 1.74(0.64, 4.72) | 13 | 0.9 | 1.26(0.83, 1.91) | 0.355 |
| Death | 229 | 13.1 | 20.19 (14.84, 27.47) | 123 | 8.6 | 13.51 (11.89, 15.35) | <0.001 |
| PAD or Death | 471 | 27.0 | 23.26 (18.98, 28.49) | 446 | 31.2 | 26.96 (24.63, 29.51) | 0.025 |
Incidence rates are from the unadjusted Poisson regression model.
PAD and Survival in Females and Males
Kaplan-Meier curves for PAD-free survival showed a steady decline during the period of observation for both sexes as shown in Figure 1A. Initially, the curves approximated each other initially, but they separated by 2 years (88.0% for females versus 90.1% males, p=0.057). Overall, men had greater PAD-free survival compared to women (log rank test p=0.0034). Kaplan-Meier curves for overall survival separated at approximately 4 years (93.8% for females versus 91.9% males, p=0.056). Females experienced greater overall survival compared to males (log rank test, p=0.0010, Figure 1B).
Figure 1.
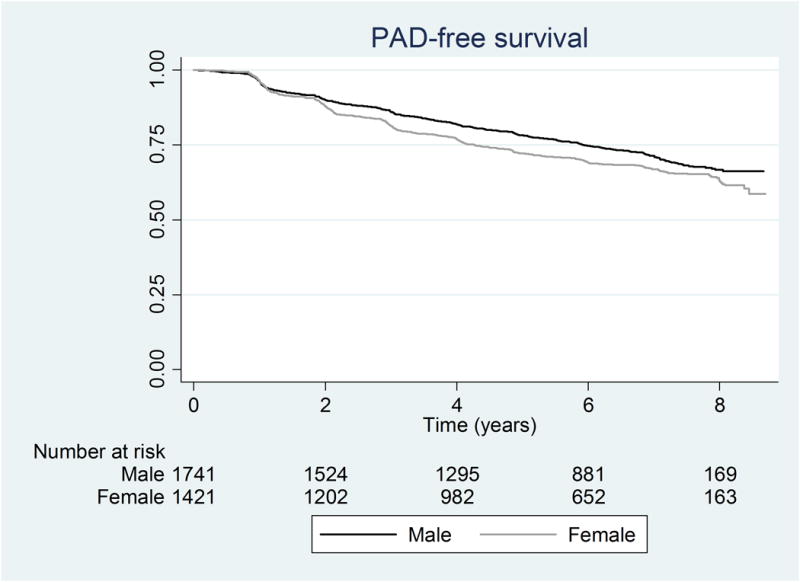
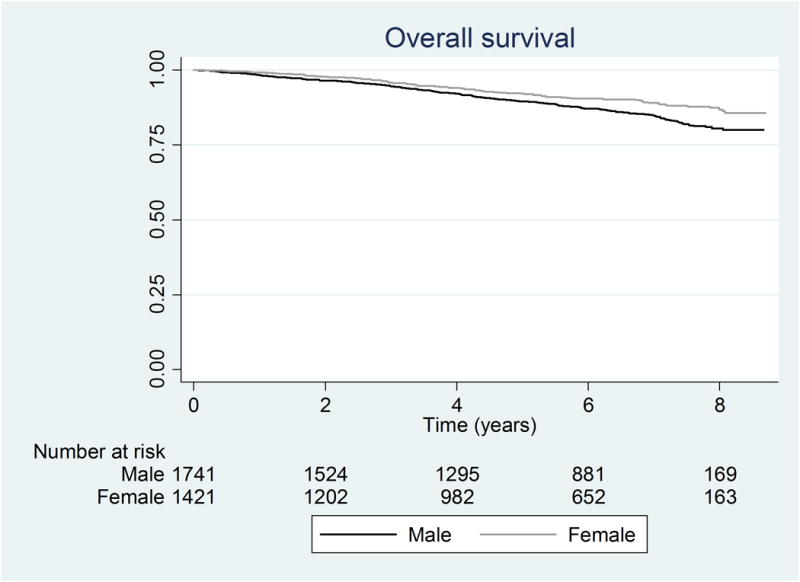
Kaplan Meier curves for a) PAD-free survival (log rank p-value= 0.0034) and b) Overall survival (log rank p-value= 0.0010)
PAD Risk in Females and Males
Females had a statistically significantly higher risk of PAD in the unadjusted Fine and Gray model, with subdistribution hazard ratio (SHR 1.73; 95% CI, 1.47-2.05, p<0.001). This risk was relatively unchanged and remained significant after adjusting for age, baseline ABI, diabetes, coronary artery disease, smoking, hypertension, body mass index (BMI), race and estimated glomerular filtration rate (eGFR) (SHR=1.53, 95% CI 1.27-1.84, p<0.001). Figure 2 demonstrates the predicted risk of PAD in females, assuming a mean age of 57 years, eGFR of 46.7 mL/min/m2, baseline ABI of 1.10 and BMI of 31.9 kg/m2.
Figure 2. Cumulative Incidence of PAD by sex.
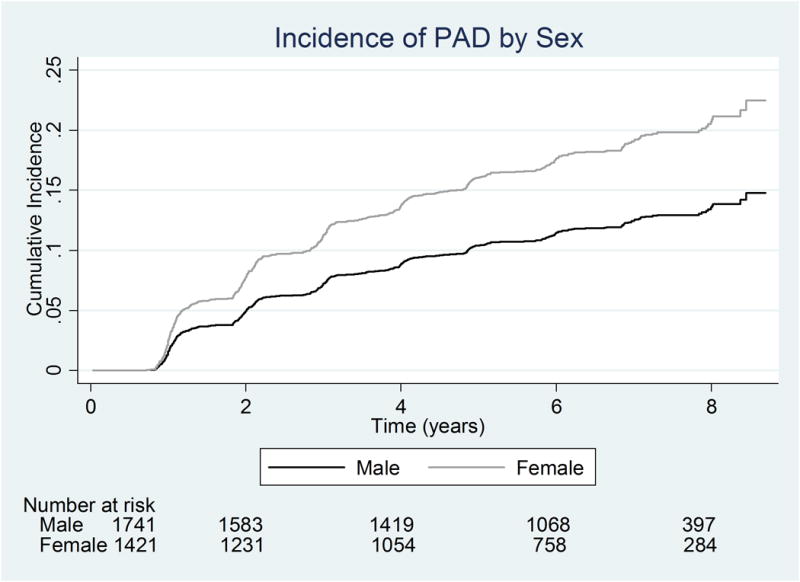
*Using Fine and Gray model at the mean age of the cohort (57 years) as well as the mean for other variables in the model
Ɨadjusts for diabetes, smoking, BMI, GFR, CAD, race, hypertension, baseline ABI and allows for effect modification of age by sex
However, the increased risk of PAD in females compared to males was modified by age (p<0.001). Specifically, women had a 2.57-fold increased risk compared to men in those younger than 40 years of age (SHR 2.57; 95% CI, 1.27-5.20, p=0.009, Table 3). The magnitude of this increased risk gradually decreased with age, such that the risk was similar in women and men past the 7th decade (SHR 1.05; 95% CI, 0.66-1.67, p=0.821).
Table 3. The effect of age on the subdistribution hazard ratio (SHR) of PAD comparing Females to Males.
| Age, years (n) | SHR (95% CI) Females vs Males | P-value |
|---|---|---|
| <40 (n=302) | 2.57 (1.27, 5.20) | 0.009 |
| 40-49 (n=439) | 2.12 (1.22, 3.68) | 0.008 |
| 50-59 (n=972) | 1.54 (1.12, 2.11) | 0.007 |
| 60-69 (n=1,098) | 1.49 (1.12, 1.98) | 0.006 |
| >70 (n=363) | 1.05 (0.66, 1.67) | 0.821 |
estimated by the multivariable Fine and Gray competing risk regression adjusted for diabetes, smoking, BMI, GFR, CAD, race, hypertension, baseline ABI
However, amongst men alone, PAD risk clearly worsened with age, such that for a 10-year increase in age, the SHR was significantly greater in men (SHR 1.32; 95% CI, 1.14-1.52, p<0.001). In women alone, this relationship was not significant (SHR 1.06; 95% CI, 0.94-1.19, p=0.310). This effect of age on the subdistribution hazard ratio for PAD for males and females is reflected in Table 4 and Figures 3A and 3B. In Figure 3A, the age-stratified cumulative incidence for males are clearly separated according to age. In contrast, in Figure 3B, the age-specific curves for females are largely overlapping. Similarly, the SHR for males increased with each age group, whereas the SHR for females did not change with increasing in age.
Table 4. The effect of age on the subdistribution hazard ratio of PAD, stratified by sex.
| Males | Females | ||||
|---|---|---|---|---|---|
| Age | SHR (95%CI) vs baseline age category | P-value | SHR (95%CI) vs baseline age category | P-value | |
| <40 (n=169) | (Reference) | ------ | <40 (n=133) | (Reference) | ------ |
| 40-49 (n=236) | 1.00 (0.49, 2.04) | 0.994 | 40-49 (n=203) | 0.82 (0.48, 1.41) | 0.474 |
| 50-59 (n=532) | 1.53 (0.84, 2.80) | 0.168 | 50-59 (n=440) | 0.92 (0.57, 1.48) | 0.726 |
| 60-69 (n=605) | 1.75 (0.96, 3.20) | 0.068 | 60-69 (n=493) | 1.02 (0.63, 1.63) | 0.947 |
| >70 (n=205) | 2.47 (1.29, 4.71) | 0.006 | >70 (n=158) | 1.01 (0.58, 1.76) | 0.964 |
estimated by the multivariable Fine and Gray competing risk model, adjusted for diabetes, smoking, BMI, GFR, CAD, race, hypertension, baseline ABI
Figure 3.
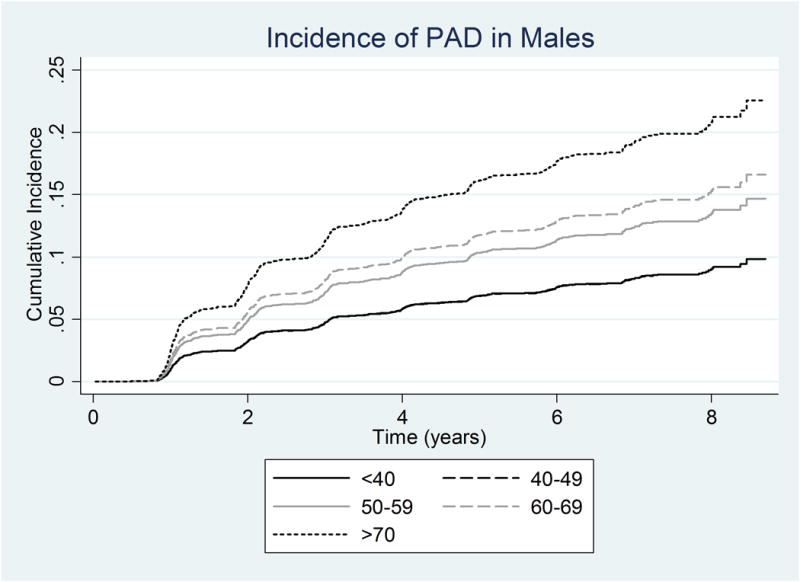
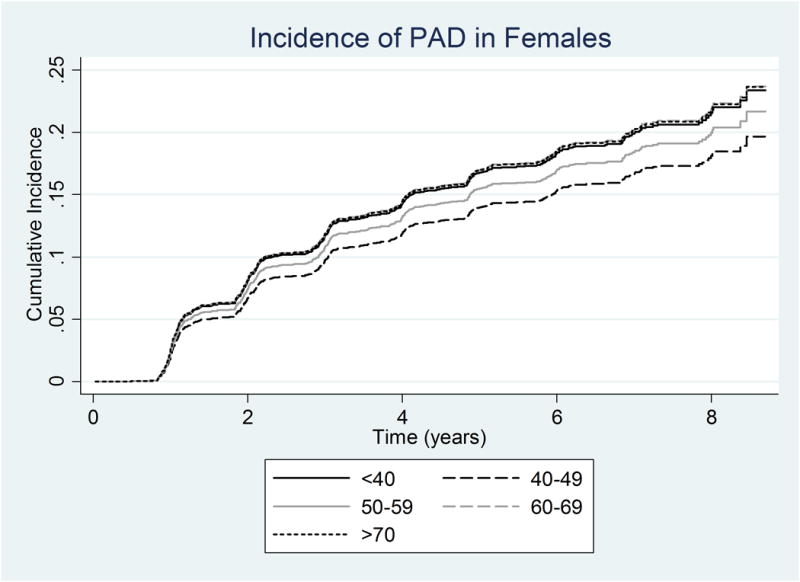
Adjusted Cumulative Incidence of PAD using the multivariable Fine and Gray model by age category adjusted for the mean of other variables in the model in a) Males b) Females
Discussion
In this study, we demonstrate that the cumulative incidence of PAD is higher in women compared to men in this cohort of participants with chronic kidney disease, with an important interaction by age. Using the Fine and Gray model, the overall risk of PAD in this cohort was 1.53-fold greater in women compared to men. However, by age 70, these risks were similar between the two sexes. Furthermore, the risk of PAD increased with age in men but not in women. Women in this cohort developed PAD at an earlier age, and this risk remained relatively constant over time. Women did not experience an increase in the cumulative incidence of PAD later in life, as observed in men.
While epidemiologic studies have reported PAD prevalence in defined populations and its association with traditional atherosclerotic risk factors, incomplete and contradictory data have been published on the prevalence and differences in outcome of PAD in women versus men. Population based prevalence is difficult to discern because PAD screening is not currently uniformly conducted at a national level. Furthermore, the few population-based studies that do exist do not report prevalence by gender.8 Guidelines from the American College of Cardiology (ACC)/American Heart Association (AHA) 2005 refer to male gender as a risk factor for PAD24 but this was later refuted as the rates of PAD in women have been suggested to be as high as those in men.8 More contemporary data from cohort studies25, 26 suggest that women are older, present with more severe disease, and have inferior rates of limb salvage compared to men.8, 27, 28 Other studies suggest comparable outcomes between men and women.25 However, our results shed insight into an important interaction by age in these sex-related differences in the cumulative incidence of PAD, which may, in part, explain these conflicting findings. Women are at a particularly increased risk for PAD at younger ages; but at ages greater than 70 years, the PAD risk becomes more similar between sexes. Moreover, older men compared to younger men have a substantially greater predilection for developing PAD. In women, this relationship is relatively constant over a broad spectrum of ages.
As these data suggest that incident PAD is more common in females with CKD at younger ages, current screening methods may not be adequate for targeting women for primary prevention of cardiovascular outcomes. From the 2011 ACCF/AHA American College of Cardiology Foundation/American Heart Association Task Force Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease29, the ABI should be used as a diagnostic study in individuals with 1 or more of the following: exertional leg symptoms, nonhealing wounds, age 65 years and older, or 50 years and older with a history of smoking or diabetes. Our data indicates that this age-related screening window is likely “too late” for women. While it has previously been suggested that women are “protected” from cardiovascular events at younger ages due to the effect of estrogens and other sex hormones, data regarding hormone replacement therapy from the Women's Health Initiative (WHI)30 and the Heart and Estrogen/progestin Replacement Study (HERS)31 did not support this notion. Indeed, the data from our study indicates that women with CKD are not protected at a younger age, but rather, suffer from an increased risk of PAD compared to men. This finding, compounded with the fact that women with PAD have been shown to exhibit a more rapid decline in function compared to men32 suggests that PAD has a more virulent course in women, justifying earlier and more aggressive screening measures.
These findings do raise the question of why the incidence of PAD would be higher in women compared to men at younger ages. There may be potential anatomic and biologic reasons for our findings. Females are known to have smaller diameter vessels compared to men, and this anatomic feature may lead to earlier hemodynamically important stenoses even with lesser plaque burden.8, 33 The severity and distribution of arterial disease in the lower extremity may also differ between women and men. In a study comparing outcomes following stenting of the femoropopliteal segment, women were more likely to have distal extension of their occlusive disease to the popliteal artery.6 This difference in pattern of disease could lead to earlier development of PAD. The difference in risk of PAD could also be related to differences in the histopathologic characteristics of the vessel wall. In a study examining breast arterial calcification (BAC) in women with CKD, BAC was found exclusively in the media, was associated with peripheral arterial calcification, and was found to be a marker of generalized medial arterial calcification (MAC), a histologic finding common in amputated limbs.34 In a follow-up study, BAC was independently associated with new PAD events in women with ESRD.35 As these analyses did not include men, it is unknown if these histologic correlates indicate increased PAD risk in women, but they show that women with CKD have more MAC, and that MAC may be associated with a more accelerated form of PAD. While prior studies have shown that overall, women have lower ABI values compared to men36-38, which was reflected in the baseline ABI values of this cohort, adjusting for ABI did not affect the gender by age effect with regard to PAD incidence.
There are limitations to our study. The first is that patients with CKD can have calcific, noncompressible vessels, which can lead to an overestimation of the ABI and misclassification. This has previously been shown in the diabetic population, who are also commonly affected with “stiff” arteries.39 However, there was a low prevalence of ABI>1.3 (9.27% in males and 6.38% in females) which may have included some participants with calcific, noncompressible vessels, suggesting that our main findings would not be different. Moreover, sensitivity analysis excluding those with noncompressible vessels yielded the same relationship (SHR=1.49; 95% CI, 1.23-1.80, p<0.001). The exact date at which the actual drop in ABI occurred was not known; rather, the date was assigned to the clinic visit associated with the drop in ABI<0.9, making this event interval-censored. Nonetheless, annual ABI measurement has been recommended as the frequency with which to surveill patients with known PAD.40 Loss to follow-up is a limitation of all cohort studies, but this is estimated to be only 2% per year in this cohort. Lastly, the cohort is designed to study participants with chronic kidney disease, and thus may not generalizable to the population at large. However, this is a rich dataset which lends important insight into a highly relevant population commonly afflicted by PAD.
We also note important strengths of this study. These include the rigorous exploration of competing risks, including the use of Fine and Gray modeling approaches which comprehensively accounts for death as a competing risk. The near equal representation of the sexes, and the clear definition of incident PAD given the thorough baseline and follow-up assessment of the cohort via ABI also represent key strengths. These circumvent the pitfalls of earlier studies which likely led to under-recognition of PAD in women, and thus, uniquely leverage a rich database to address this important knowledge gap.
In summary, in our cohort of 3,174 participants with CKD, women suffered from a 1.53-fold higher risk of PAD compared to men prior to the age of 70. Future studies should be devoted to understanding the impact of earlier detection of PAD in women and improving our understanding of the biological and clinical basis for these sex-based differences.
What is Known.
Peripheral artery disease (PAD) is a national and worldwide health burden and is a major source of morbidity and mortality.
Sex differences in PAD incidence is not well understood due in part to underrepresentation of females in prior clinical trials.
Prior studies suggest that PAD outcomes may be worse in women compared to men.
PAD is common in patients with chronic kidney disease.
What the Study Adds.
This study utilized the Chronic Renal Insufficiency Cohort (CRIC), which is well-balanced with regard to sex, includes participants of various ages, and has detailed vascular function data obtained at standardized intervals to study sex differences in PAD incidence.
Females had a significantly higher incidence of PAD as defined by an ABI<0.90 or revascularization or amputation procedure, compared to men.
The sex effect on PAD incidence was modified by age. There was a markedly increased risk of PAD in women compared to men at younger ages. By age 70, however, the incidence was similar between males and females.
There is an important need to improve our understanding of the biological and clinical basis for these differences.
Acknowledgments
We would like to thank Sally Thompson for her administrative assistance.
Sources of Funding: Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this study was supported in part by the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003; Johns Hopkins University UL1 TR-000424; University of Maryland GCRC M01 RR-16500; Clinical and Translational Science Collaborative of Cleveland UL1TR000439; Michigan Institute for Clinical and Health Research UL1TR000433; University of Illinois at Chicago CTSA UL1RR029879; Tulane University Translational Research in Hypertension and Renal Biology P30GM103337; Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, and K01DK092353.
Appendix
The CRIC Study Investigators include Lawrence J. Appel1, MD, MPH, Alan S. Go2, MD, Jiang He3, MD, PhD, John W. Kusek4, PhD, James P. Lash5, MD, and Mahboob Rahman6, MD.
1Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, MD
2Departments of Epidemiology, Biostatistics, and Medicine, University of California, San Francisco, CA
3Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, LA
4National Institutes of Health, Bethesda, MD
5Department of Medicine, University of Illinois College of Medicine, Chicago, IL
6Department of Medicine, Case Western Reserve University, Cleveland, OH
Footnotes
Disclosures: None.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH. Peripheral arterial disease--epidemiological aspects. Vasc Med. 2001;6:3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JP, Higgins JA. Epidemiology of peripheral arterial disease in women. J Epidemiol. 2003;13:1–14. doi: 10.2188/jea.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vavra AK, Kibbe MR. Women and peripheral arterial disease. Womens Health (Lond Engl) 2009;5:669–683. doi: 10.2217/whe.09.60. [DOI] [PubMed] [Google Scholar]
- 6.Stavroulakis K, Donas KP, Torsello G, Osada N, Schonefeld E. Gender-related long-term outcome of primary femoropopliteal stent placement for peripheral artery disease. J Endovasc Ther. 2015;22:31–37. doi: 10.1177/1526602814564382. [DOI] [PubMed] [Google Scholar]
- 7.Lo RC, Bensley RP, Dahlberg SE, Matyal R, Hamdan AD, Wyers M, Chaikof EL, Schermerhorn ML. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409–418. doi: 10.1016/j.jvs.2013.07.114. e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, Hiatt WR, Karas RH, Lovell MB, McDermott MM, Mendes DM, Nussmeier NA, Treat-Jacobson D. A call to action: Women and peripheral artery disease: A scientific statement from the american heart association. Circulation. 2012;125:1449–1472. doi: 10.1161/CIR.0b013e31824c39ba. [DOI] [PubMed] [Google Scholar]
- 9.Hoel AW, Kayssi A, Brahmanandam S, Belkin M, Conte MS, Nguyen LL. Under-representation of women and ethnic minorities in vascular surgery randomized controlled trials. J Vasc Surg. 2009;50:349–354. doi: 10.1016/j.jvs.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim ES, Menon V. Status of women in cardiovascular clinical trials. Arterioscler Thromb Vasc Biol. 2009;29:279–283. doi: 10.1161/ATVBAHA.108.179796. [DOI] [PubMed] [Google Scholar]
- 11.Mosenifar Z. Population issues in clinical trials. Proc Am Thorac Soc. 2007;4:185–187. doi: 10.1513/pats.200701-009GC. discussion 187-188. [DOI] [PubMed] [Google Scholar]
- 12.McDermott MM, Greenland P, Liu K, Criqui MH, Guralnik JM, Celic L, Chan C. Sex differences in peripheral arterial disease: Leg symptoms and physical functioning. J Am Geriatr Soc. 2003;51:222–228. doi: 10.1046/j.1532-5415.2003.51061.x. [DOI] [PubMed] [Google Scholar]
- 13.Collins TC, Suarez-Almazor M, Bush RL, Petersen NJ. Gender and peripheral arterial disease. J Am Board Fam Med. 2006;19:132–140. doi: 10.3122/jabfm.19.2.132. [DOI] [PubMed] [Google Scholar]
- 14.Smolderen KG, Spertus JA, Vriens PW, Kranendonk S, Nooren M, Denollet J. Younger women with symptomatic peripheral arterial disease are at increased risk of depressive symptoms. J Vasc Surg. 2010;52:637–644. doi: 10.1016/j.jvs.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. Cardiovascular heart study (chs) collaborative research group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 16.O'Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: Results from the national health and nutrition examination survey 1999-2000. Circulation. 2004;109:320–323. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT., Jr Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National kidney foundation task force on cardiovascular disease. Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 18.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The chronic renal insufficiency cohort (cric) study: Design and methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 19.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic renal insufficiency cohort (cric) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med. 2012;79:651–661. doi: 10.3949/ccjm.79a.11154. [DOI] [PubMed] [Google Scholar]
- 21.Jason PF, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 22.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 23.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the united states. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. Acc/aha 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the american association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the acc/aha task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): Endorsed by the american association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; transatlantic inter-society consensus; and vascular disease foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 25.Ballotta E, Gruppo M, Lorenzetti R, Piatto G, DaGiau G, Toniato A. The impact of gender on outcome after infrainguinal arterial reconstructions for peripheral occlusive disease. J Vasc Surg. 2012;56:343–352. doi: 10.1016/j.jvs.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Ortmann J, Nuesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. 2012;55:98–104. doi: 10.1016/j.jvs.2011.07.074. [DOI] [PubMed] [Google Scholar]
- 27.Ballard JL, Bergan JJ, Singh P, Yonemoto H, Killeen JD. Aortoiliac stent deployment versus surgical reconstruction: Analysis of outcome and cost. J Vasc Surg. 1998;28:94–101. doi: 10.1016/s0741-5214(98)70204-6. discussion 101-103. [DOI] [PubMed] [Google Scholar]
- 28.Hertzer NR, Bena JF, Karafa MT. A personal experience with direct reconstruction and extra-anatomic bypass for aortoiliofemoral occlusive disease. J Vasc Surg. 2007;45:527–535. doi: 10.1016/j.jvs.2006.09.065. discussion 535. [DOI] [PubMed] [Google Scholar]
- 29.2011 WRITING GROUP MEMBERS; 2005 WRITING COMMITTEE MEMBERS; ACCF/AHA TASK FORCE MEMBERS. 2011 accf/aha focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;124:2020–2045. doi: 10.1161/CIR.0b013e31822e80c3. [DOI] [PubMed] [Google Scholar]
- 30.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and estrogen/progestin replacement study follow-up (hers ii) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 32.McDermott MM, Ferrucci L, Liu K, Guralnik JM, Tian L, Kibbe M, Liao Y, Tao H, Criqui MH. Women with peripheral arterial disease experience faster functional decline than men with peripheral arterial disease. J Am Coll Cardiol. 2011;57:707–714. doi: 10.1016/j.jacc.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AhChong AK, Chiu KM, Wong M, Yip AW. The influence of gender difference on the outcomes of infrainguinal bypass for critical limb ischaemia in chinese patients. Eur J Vasc Endovasc Surg. 2002;23:134–139. doi: 10.1053/ejvs.2001.1564. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill WC, Han KH, Schneider TM, Hennigar RA. Prevalence of nonatheromatous lesions in peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2015;35:439–447. doi: 10.1161/ATVBAHA.114.304764. [DOI] [PubMed] [Google Scholar]
- 35.Abou-Hassan N, Tantisattamo E, D'Orsi ET, O'Neill WC. The clinical significance of medial arterial calcification in end-stage renal disease in women. Kidney Int. 2015;87:195–199. doi: 10.1038/ki.2014.187. [DOI] [PubMed] [Google Scholar]
- 36.Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC, Jr, Manolio TA. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: The multi-ethnic study of atherosclerosis (mesa) J Vasc Surg. 2007;45:319–327. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 37.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The san luis valley diabetes study. Circulation. 1995;91:1472–1479. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 38.Richey Sharrett A, Coady SA, Folsom AR, Couper DJ, Heiss G. Smoking and diabetes differ in their associations with subclinical atherosclerosis and coronary heart disease-the aric study. Atherosclerosis. 2004;172:143–149. doi: 10.1016/j.atherosclerosis.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D. Measurement and interpretation of the ankle-brachial index: A scientific statement from the american heart association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 40.Conte MS, Pomposelli FB. Society for vascular surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities management of asymptomatic disease and claudication. Introduction. J Vasc Surg. 2015;61:1S. doi: 10.1016/j.jvs.2014.12.006. [DOI] [PubMed] [Google Scholar]


