Abstract
Background:
The function of renin angiotensin system (RAS) is gender-related, and this system affects cisplatin (CP)-induced nephrotoxicity. In this study, we compared the effect of enalapril as an angiotensin-converting enzyme (ACE) inhibitor on CP-induced nephrotoxicity between male and female rats.
Materials and Methods:
Sixty-two adult male and female Wistar rats were divided into eight groups. Both genders received CP (2.5 mg/kg, i.p.) and enalapril (30 mg/kg, i.p.) for 7 days in compared with CP alone or enalapril alone or vehicle alone treated groups. At the end of the experiment, blood samples were obtained, and the kidney tissue was investigated for histopathological changes.
Results:
CP increased the serum levels of blood urea nitrogen and creatinine as well as kidney weight and kidney tissue damage score in both genders (P < 0.05). However, not only enalapril failed to ameliorate the aforementioned parameters in both genders, but also it intensified nephrotoxicity in females (P < 0.05). In addition, enalapril enhanced body weight loss induced by CP in females (P < 0.05). CP alone decreased kidney level of nitrite in both genders (P < 0.05) and enalapril could not reverse this decreasing. The combination of enalapril and CP significantly increased serum level of nitrite in females, but this was not observed in males (P < 0.05).
Conclusion:
Enalapril as an ACE inhibitor failed to ameliorate nephrotoxicity induced by CP in both male and female rats. In addition, enalapril aggravated CP-induced nephrotoxicity in female possibly due to gender-dependent RAS response.
Key Words: Cisplatin, enalapril, gender, nephrotoxicity, rat
INTRODUCTION
Cisplatin (CP), as an antitumor drug, is used to treat solid tumors.[1,2] However, it is accompanied with side-effects such as nephrotoxicity.[3] Nephrotoxicity is characterized by decrease in glomerular filtration rate and renal blood flow[4] and increase in the serum levels of creatinine (Cr) and blood urea nitrogen (BUN).[5] Recently, the role of gender in acute renal failure induced by nephrotoxins[6,7,8,9,10,11] and our previous reports have shown that gender affected CP-induced nephrotoxicity in animals treated by different supplementations such as L-arginine,[12] L-NAME,[13] losartan,[14] erythropoietin,[15] and Vitamin E.[16] In renin angiotensin system (RAS), angiotensin-converting enzyme (ACE) inhibition decreases cardiovascular and renal diseases and prevents the progression of diabetes,[17,18] and scavenges free radicals and oxidants.[19] The protective effects of captopril as an ACE inhibitor on nephrotoxicity induced by CP is documented;[20,21] however, the role of gender was not reported yet. Previously, we reported that the effect of angiotensin II receptor blocker; losartan on CP-induced nephrotoxicity was gender-related.[14] Enalapril, as an ACE inhibitor, affects kidney function in renal cystic disease in the animal model.[22] In addition, RAS function is gender-related,[23,24,25,26,27] and ACE does not equally act in males and females.[28] Accordingly, this study was designed to investigate the role of gender in CP-induced nephrotoxicity in response to enalapril in rats.
MATERIALS AND METHODS
Animals
Thirty-one adult female (175.25 ± 3.05 g) and thirty one adult male (224.31 ± 6.38 g) Wistar rats (Animal Center, Isfahan University of Medical Sciences, Isfahan, Iran) were housed at the temperature of 23°C–25°C. The rats had free access to water and rat chow. The experimental procedures were approved by the Isfahan University of Medical Sciences Ethics Committee in advance.
Experimental protocol
The animals were randomly divided into eight groups as follows;
Groups 1 (male, n = 6) and 2 (female, n = 6); named as saline that received vehicle for 7 days. Groups 3 (male, n = 7) and 4 (female, n = 7); named as Ena that received enalapril (30 mg/kg/day, i.p.) and saline for 7 days.
Groups 5 (male, n = 9) and 6 (female, n = 9); named as CP that received CP (2.5 mg/kg/day, i.p.) and saline for 7 days.
Groups 7 (male, n = 9) and 8 (female, n = 9); named as CP + Ena that received CP (2.5 mg/kg/day, i.p.) and enalapril (30 mg/kg/day, i.p.) for 7 days. Doses of CP was selected based on previous study.[8,13]
All animals were weighed daily. At the end of the study, all animals were anesthetized by ketamine (75 mg/kg) and xylazine (10 mg/kg), and blood samples were obtained by heart puncture. Finally, all animals were killed, and the kidneys were removed and immediately weighed. The left kidney was fixed in 10% neutral formalin solution for histopathology procedures, and the right kidney was homogenized for biochemical measurements.
Measurements
The levels of serum Cr and BUN were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum and kidney levels of nitrite (stable nitric oxide [NO] metabolite) were measured using a colorimetric assay kit that involves the Griess reaction.
Histopathological procedures
After fixing, the left kidney was embedded in paraffin and prepared for histopathological staining. The slices were stained by hematoxylin and eosin. The kidney tissue damage score (KTDS) was determined by the presence of tubular atrophy, hyaline cast, ischemic necrosis, vacuolization, and debris, and graded from 1 to 4 based on the damage intensity. The zero score was assigned to the normal tissue.
Statistical analysis
Data are expressed as mean ± standard error of the mean. One-way ANOVA followed by LSD was applied to compare the groups with regard to the body weight loss (ΔW) and kidney weight (KW), serum levels of BUN and Cr, kidney and serum levels of nitrite. KTDS was compared using the Mann–Whitney and Kruskal–Wallis tests. P < 0.05 were considered as statistically significant.
RESULTS
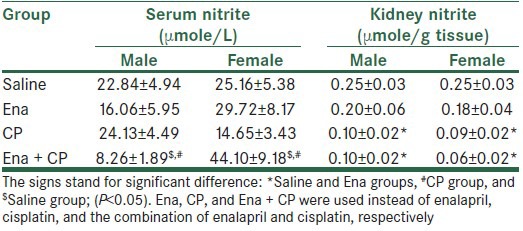
CP alone induced body weight loss significantly in both genders (P < 0.05). Enalapril did not ameliorate body weight loss in male gender while increased body weight loss in the female gender (P < 0.05). CP increased the serum levels of BUN and Cr, KW, and KTDS in both genders (P < 0.05) and enalapril not only failed to ameliorate these parameters, but also intensified nephrotoxicity in female gender (P < 0.05) [Figure 1]. Images of KTDS are provided in Figure 2. Kidney level of nitrite was decreased by CP alone in both genders (P < 0.05) and enalapril could not reverse this trend. The combination of enalapril and CP increased serum level of nitrite in females significantly, but this was not observed in males (P < 0.05) [Table 1].
Figure 1.
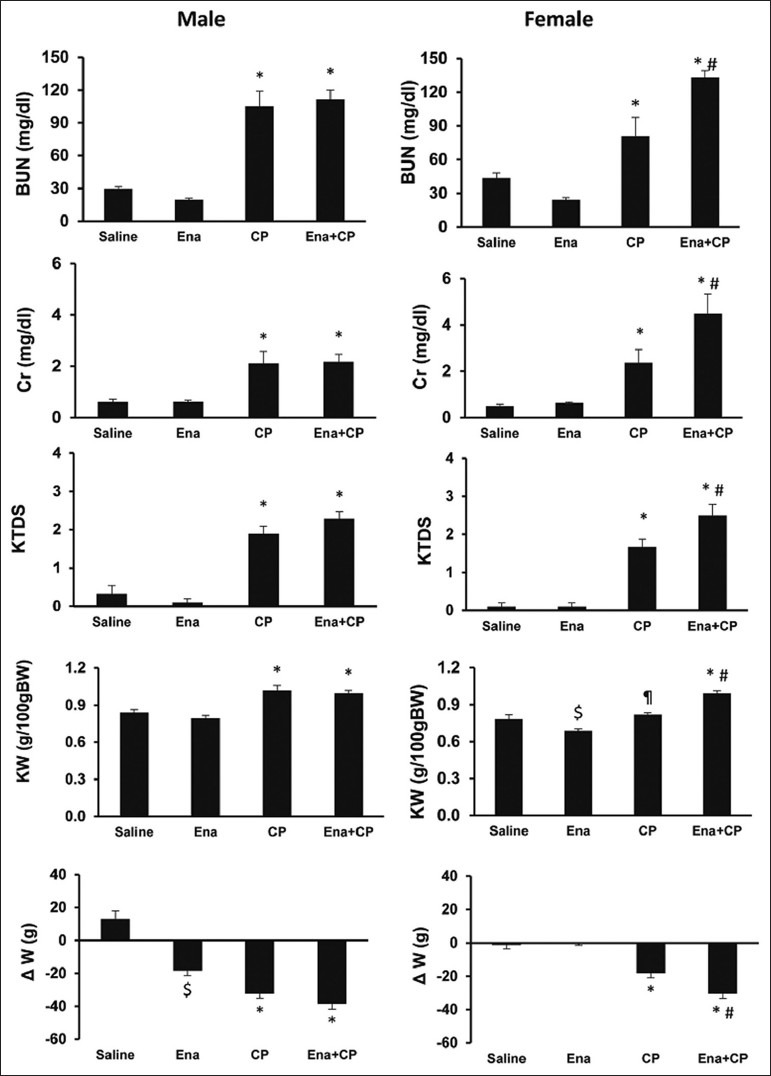
Serum levels of blood urea nitrogen and creatinine, kidney tissue damage score, kidney weight g/100 g body weight, and Δbody weight (ΔW) in experimental groups. Abbreviations of Ena, cisplatin (CP), and Ena + CP stand for enalapril, CP, and the combination of enalapril and CP, respectively. The signs stand for significant difference (∗) from Saline and Ena groups; (#) from CP group; ($) from saline group; and (¶) from Ena group; (P < 0.05)
Figure 2.
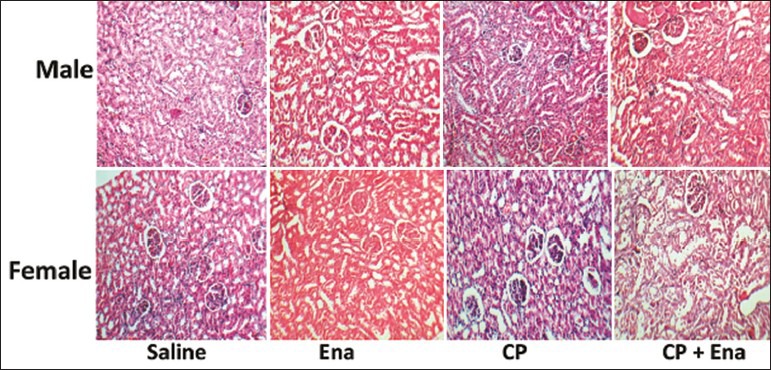
Images of kidney tissue damage score in experimental groups (magnification, ×100). Abbreviations of Ena, cisplatin (CP), and Ena + CP were used instead of enalapril, CP, and the combination of enalapril and CP, respectively
Table 1.
Serum and kidney levels of nitrite
DISCUSSION
The main objective of this study was to determine the role of gender in CP-induced nephrotoxicity in rats treated with enalapril. CP itself induced nephrotoxicity in both genders, characterized by increasing values of BUN and Cr as well as KTDS and KW. These results were in agreement with the previous reports.[8,9,13,29] CP induces apoptosis,[30] lipid peroxidation, and generation of free radicals.[31] Administration of enalapril had no positive effects on nephrotoxicity induced by CP in males. This is while the protective effects of captopril, as another inhibitor of ACE, on nephrotoxicity induced by CP have been shown.[20,21] Herlitz et al. found that both captopril and enalapril reduce blood pressure, but only captopril ameliorates proteinuria and hematuria in the animal model of murine systemic lupus erythematosus.[32] Furthermore, it is reported that captopril is more efficient than enalapril in inhibition of spontaneous hypertension.[33] Ohishi et al. stated that captopril can reduce the progression of chronic renal failure in hypertensive rats. This renoprotective effect of captopril is due to the presence of the thiol group rather than its ACE inhibitor property.[34] Moreover, the preventive effect of enalapril against hypertension and nephrotoxicity induced by cyclosporine A in hypertensive male rat has been demonstrated.[35] One study showed that administration of cyclosporine A accompany with enalapril increases urea and creatinine concentrations in uremic rats.[36] In addition, male offsprings treated with chronic administration of enalapril exhibited abnormalities in kidney function and histology.[37] It seems that these different reports probably result from drug interactions and different experimental protocols. In the present study, administration of enalapril aggravated nephrotoxicity induced by CP in the female gender. Previous findings have demonstrated that administration of some supplementations not only did not ameliorate renal toxicity induced by CP in females, but also intensified it.[12,13,14,15,16] It seems that the different responses observed in female, and male genders are associated with sex hormones. One evidence showed that estrogen alone could progress renal failure induced by CP.[38] In addition, administration of estrogen affects protective effects of some supplementations on nephrotoxicity induced by CP.[39,40] Moreover, it is documented that RAS acts differently in males and females.[23,24,25,26,27] Also, ACE activity is lower in women than that in men[28] and administration of estradiol reduces ACE activity in ovariectomized female rats.[41] Therefore, it is thought that opposite effects of enalapril in the genders is due to the gender-dependent RAS response. In the agreement with the previous results,[13,29,39,42,43] this study demonstrated that CP alone induced body weight loss in both genders probably due to diarrhea and decreased appetite.[44] Also, administration of enalapril elevated body weight loss in female. Consistently, the effect of enalapril on reducing body weight gain, food intake, fat accumulation, and serum leptin concentration in normotensive rats has been demonstrated.[45] The present study showed that administration of CP similarly decreased renal nitrite level in both genders. Such observation was reported in previous studies.[29,43] NO as a critical agent is produced by three NO synthase (NOS) isoforms; neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). CP up-regulates iNOS and down-regulates eNOS and nNOS.[46] It seems that CP decreases nitrite renal level in both genders via decreasing eNOS. The combination of enalapril and CP increased serum nitrite level in females and also elevated BUN, Cr, and KTDS. It has been shown that enalapril induces apoptosis in neointima by up-regulation of iNOS in the animal model.[47] In this study, CP increased serum level of nitrite in males probably via increasing iNOS. However, it was not significant, and administration of enalapril attenuated this enhancement and enalapril did not intensify renal failure. This observation was in agreement with our previous studies.[11,48] Finally, observations of this study showed that administration of enalapril aggravates nephrotoxicity induced by CP in the female gender. Moreover, it is documented that women experience adverse drug reactive (ADR) more than men, which is related to several parameters such as therapeutic agent class, type of ADR, and age and physiological condition of women.[49]
CONCLUSION
It is concluded that enalapril, as an ACE inhibitor, not only failed to ameliorate nephrotoxicity induced by CP in both male and female genders, but also aggravated nephrotoxicity in the female gender.
ACKNOWLEDGMENT
We appreciate Isfahan University of Medical Science for supporting this project.
Footnotes
Source of Support: This research was supported by Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Bagatell R, Beliakoff J, David CL, Marron MT, Whitesell L. Hsp90 inhibitors deplete key anti-apoptotic proteins in pediatric solid tumor cells and demonstrate synergistic anticancer activity with cisplatin. Int J Cancer. 2005;113:179–88. doi: 10.1002/ijc.20611. [DOI] [PubMed] [Google Scholar]
- 2.Bergamo A, Gagliardi R, Scarcia V, Furlani A, Alessio E, Mestroni G, et al. In vitro cell cycle arrest, in vivo action on solid metastasizing tumors, and host toxicity of the antimetastatic drug NAMI-A and cisplatin. J Pharmacol Exp Ther. 1999;289:559–64. [PubMed] [Google Scholar]
- 3.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–79. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 4.Winston JA, Sa firstein R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am J Physiol. 1985;249:F490–6. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafi F, Haghshenas S, Nematbakhsh M, Nasri H, Talebi A, Eshraghi-Jazi F, et al. The role of magnesium supplementation in cisplatin-induced nephrotoxicity in a rat model: No nephroprotectant effect. Int J Prev Med. 2012;3:637–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Ali B, Ismail TB, Bashir A. Sex difference in the susceptibility of rats to gentamicin nephrotoxicity: Influence of gonadectomy and hormonal replacement therapy. Indian J Pharmacol. 2001;33:369–73. [Google Scholar]
- 7.Bennett WM, Parker RA, Elliott WC, Gilbert DN, Houghton DC. Sex-related differences in the susceptibility of rats to gentamicin nephrotoxicity. J Infect Dis. 1982;145:370–3. doi: 10.1093/infdis/145.3.370. [DOI] [PubMed] [Google Scholar]
- 8.Nematbakhsh M, Ebrahimian S, Tooyserkani M, Eshraghi-Jazi F, Talebi A, Ashrafi F. Gender difference in cisplatin-induced nephrotoxicity in a rat model: Greater intensity of damage in male than female. Nephrourol Mon. 2013;5:818–21. doi: 10.5812/numonthly.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nematbakhsh M, Talebi A, Nasri H, Safari T, Dolatkhah S, Ashrafi F. Some evidence for sex-based differences in cisplatin-induced nephrotoxicity in rats. Clin Exp Med Lett. 2012;53:29–32. [Google Scholar]
- 10.Nematbakhsh M, Nasri H. Cisplatin nephrotoxicity may be sex related. Kidney Int. 2013;83:1201. doi: 10.1038/ki.2013.37. [DOI] [PubMed] [Google Scholar]
- 11.Nematbakhsh M, Pezeshki Z. Sex-related difference in nitric oxide metabolites levels after nephroprotectant supplementation administration against cisplatin-induced nephrotoxicity in wistar rat model: The role of Vitamin E, erythropoietin, or n-acetylcysteine. ISRN Nephrol 2013. 2013 doi: 10.5402/2013/612675. 612675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eshraghi-Jazi F, Nematbakhsh M, Nasri H, Talebi A, Haghighi M, Pezeshki Z, et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389–96. [PMC free article] [PubMed] [Google Scholar]
- 13.Moslemi F, Nematbakhsh M, Eshraghi-Jazi F, Talebi A, Nasri H, Ashrafi F, et al. Inhibition of Nitric Oxide Synthase by L-NAME Promotes Cisplatin-Induced Nephrotoxicity in Male Rats. ISRN Toxicol 2013. 2013 doi: 10.1155/2013/242345. 242345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: Gender-related differences. Ren Fail. 2012;34:1046–51. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 15.Eshraghi-Jazi F, Nematbakhsh M, Pezeshki Z, Nasri H, Talebi A, Safari T, et al. Sex differences in protective effect of recombinant human erythropoietin against cisplatin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2013;7:383–9. [PubMed] [Google Scholar]
- 16.Jilanchi S, Nematbakhsh M, Bahadorani M, Talebi A, Eshraghi-Jazi F, Mansouri A, et al. Vitamin E is a nephroprotectant agent in male but not in female in a model of Cisplatin-induced nephrotoxicity. ISRN Nephrol 2013. 2013 doi: 10.5402/2013/280395. 280395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol. 2003;91:30H–7. doi: 10.1016/s0002-9149(03)00432-6. [DOI] [PubMed] [Google Scholar]
- 18.Omata K, Kanazawa M, Sato T, Abe F, Saito T, Abe K. Therapeutic advantages of angiotensin converting enzyme inhibitors in chronic renal disease. Kidney Int Suppl. 1996;55:S57–62. [PubMed] [Google Scholar]
- 19.Chopra M, Beswick H, Clapperton M, Dargie HJ, Smith WE, McMurray J. Antioxidant effects of angiotensin-converting enzyme (ACE) inhibitors: Free radical and oxidant scavenging are sulfhydryl dependent, but lipid peroxidation is inhibited by both sulfhydryl- and nonsulfhydryl-containing ACE inhibitors. J Cardiovasc Pharmacol. 1992;19:330–40. doi: 10.1097/00005344-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Vidhya R, Thi MJ, Agavalli U. Protective effect of captopril against cisplatin induced nephrotoxicity in rats. Pharmacol Online. 2010;1:12–5. [Google Scholar]
- 21.El-Sayed el-SM, Abd-Ellah MF, Attia SM. Protective effect of captopril against cisplatin-induced nephrotoxicity in rats. Pak J Pharm Sci. 2008;21:255–61. [PubMed] [Google Scholar]
- 22.Keith DS, Torres VE, Johnson CM, Holley KE. Effect of sodium chloride, enalapril, and losartan on the development of polycystic kidney disease in Han: SPRD rats. Am J Kidney Dis. 1994;24:491–8. doi: 10.1016/s0272-6386(12)80907-3. [DOI] [PubMed] [Google Scholar]
- 23.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, et al. Gender differences in pressure-natriuresis and renal autoregulation: Role of the angiotensin type 2 receptor. Hypertension. 2011;57:275–82. doi: 10.1161/HYPERTENSIONAHA.110.166827. [DOI] [PubMed] [Google Scholar]
- 24.Pezeshki Z, Eshraghi-Jazi F, Nematbakhsh M. Vascular response to graded angiotensin II infusion in offspring subjected to high-salt drinking water during pregnancy: The effect of blood pressure, heart rate, urine output, endothelial permeability, and gender. Int J Vasc Med 2014. 2014 doi: 10.1155/2014/876527. 876527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safari T, Nematbakhsh M. Angiotensin 1-7 receptor and angiotensin II receptor 2 blockades prevent the increased serum and kidney nitric oxide levels in response to angiotensin II administration: Gender-related difference. Int J Prev Med. 2013;4:311–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Nematbakhsh M, Safari T. Role of Mas receptor in renal blood flow response to angiotensin (1-7) in male and female rats. Gen Physiol Biophys. 2014;33:365–72. doi: 10.4149/gpb_2014008. [DOI] [PubMed] [Google Scholar]
- 27.Safari T, Nematbakhsh M, Hilliard LM, Evans RG, Denton KM. Sex differences in the renal vascular response to angiotensin II involves the Mas receptor. Acta Physiol (Oxf) 2012;206:150–6. doi: 10.1111/j.1748-1716.2012.02468.x. [DOI] [PubMed] [Google Scholar]
- 28.Zapater P, Novalbos J, Gallego-Sandín S, Hernández FT, Abad-Santos F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J Cardiovasc Pharmacol. 2004;43:737–44. doi: 10.1097/00005344-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Rastghalam R, Nematbakhsh M, Bahadorani M, Eshraghi-Jazi F, Talebi A, Moeini M, et al. Angiotensin type-1 receptor blockade may not protect kidney against cisplatin-induced nephrotoxicity in rats. ISRN Nephrol 2014. 2014 doi: 10.1155/2014/479645. 479645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamate J, Sato K, Machida Y, Ide M, Sato S, Nakatsuji S, et al. Cisplatin-induced rat renal interstitial fibrasis; a possible pathogenesis based on the data. J Toxicol Pathol. 2000;13:237–47. [Google Scholar]
- 31.Hannemann J, Baumann K. Cisplatin-induced lipid peroxidation and decrease of gluconeogenesis in rat kidney cortex: Different effects of antioxidants and radical scavengers. Toxicology. 1988;51:119–32. doi: 10.1016/0300-483x(88)90143-6. [DOI] [PubMed] [Google Scholar]
- 32.Herlitz H, Svalander C, Tarkowski A, Westberg G. Effect of captopril on murine systemic lupus erythematosus disease. J Hypertens Suppl. 1988;6:S684–6. doi: 10.1097/00004872-198812040-00215. [DOI] [PubMed] [Google Scholar]
- 33.Pechanova O. Contribution of captopril Thiol group to the prevention of spontaneous hypertension. Physiol Res Acad Sci Bohemoslov. 2007;56(Suppl 2):S41–8. doi: 10.33549/physiolres.931396. [DOI] [PubMed] [Google Scholar]
- 34.Ohishi A, Suzuki H, Nakamoto H, Katsumata H, Sakaguchi H, Saruta T. Differences in the effects of angiotensin converting enzyme inhibitors with or without a thiol group in chronic renal failure in rats. Clin Sci (Lond) 1989;76:353–6. doi: 10.1042/cs0760353. [DOI] [PubMed] [Google Scholar]
- 35.Lassila M, Finckenberg P, Pere AK, Krogerus L, Ahonen J, Vapaatalo H, et al. Comparison of enalapril and valsartan in cyclosporine A-induced hypertension and nephrotoxicity in spontaneously hypertensive rats on high-sodium diet. Br J Pharmacol. 2000;130:1339–47. doi: 10.1038/sj.bjp.0703422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzadin A, Malyszko J, Malyszko JS, Tankiewicz A, Mysliwiec M, Buczko W. Effects of combination of cyclosporine with losartan or enalapril on kidney function in uremic rats. Pol J Pharmacol. 2002;54:469–73. [PubMed] [Google Scholar]
- 37.Guron G. Renal haemodynamics and function in weanling rats treated with enalapril from birth. Clin Exp Pharmacol Physiol. 2005;32:865–70. doi: 10.1111/j.1440-1681.2010.04278.x. [DOI] [PubMed] [Google Scholar]
- 38.Pezeshki Z, Nematbakhsh M, Nasri H, Talebi A, Pilehvarian AA, Safari T, et al. Evidence against protective role of sex hormone estrogen in Cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20:43–7. doi: 10.4103/0971-6580.111568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nematbakhsh M, Pezeshki Z, Eshraghi-Jazi F, Ashrafi F, Nasri H, Talebi A, et al. Vitamin E, Vitamin C, or losartan is not nephroprotectant against cisplatin-induced nephrotoxicity in presence of estrogen in ovariectomized rat model. Int J Nephrol 2012. 2012 doi: 10.1155/2012/284896. 284896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pezeshki Z, Nematbakhsh M, Mazaheri S, Eshraghi-Jazi F, Talebi A, Nasri H, et al. Estrogen abolishes protective effect of erythropoietin against cisplatin-induced nephrotoxicity in ovariectomized rats. ISRN Oncol 2012. 2012 doi: 10.5402/2012/890310. 890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean SA, Tan J, O’Brien ER, Leenen FH. 17beta-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R759–66. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- 42.Soltani N, Nematbakhsh M, Eshraghi-Jazi F, Talebi A, Ashrafi F. Effect of oral administration of magnesium on Cisplatin-induced nephrotoxicity in normal and streptozocin-induced diabetic rats. Nephrourol Mon. 2013;5:884–90. doi: 10.5812/numonthly.11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazaheri S, Nematbakhsh M, Bahadorani M, Pezeshki Z, Talebi A, Ghannadi AR, et al. Effects of fennel essential oil on cisplatin-induced nephrotoxicity in ovariectomized rats. Toxicol Int. 2013;20:138–45. doi: 10.4103/0971-6580.117256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohno T, Kato S, Wakatsuki M, Noda SE, Murakami C, Nakamura M, et al. Incidence and temporal pattern of anorexia, diarrhea, weight loss, and leukopenia in patients with cervical cancer treated with concurrent radiation therapy and weekly cisplatin: Comparison with radiation therapy alone. Gynecol Oncol. 2006;103:94–9. doi: 10.1016/j.ygyno.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 45.Santos EL, de Picoli Souza K, Guimarães PB, Reis FC, Silva SM, Costa-Neto CM, et al. Effect of angiotensin converting enzyme inhibitor enalapril on body weight and composition in young rats. Int Immunopharmacol. 2008;8:247–53. doi: 10.1016/j.intimp.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Leung EL, Fraser M, Fiscus RR, Tsang BK. Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells: Involvement in p53 regulation and cisplatin resistance. Br J Cancer. 2008;98:1803–9. doi: 10.1038/sj.bjc.6604375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohwada T, Ishibashi T, Yaoita H, Shindo J, Noji H, Ohkawara H, et al. Different contribution of apoptosis to the antiproliferative effects of L-arginine, enalapril and losartan on neointimal growth inhibition after balloon arterial injury. Circ J. 2002;66:965–71. doi: 10.1253/circj.66.965. [DOI] [PubMed] [Google Scholar]
- 48.Pezeshki Z, Nematbakhsh M. Nitric oxide metabolites change in cisplatin-induced nephrotoxicity: The effect of L-arginine and losartan. J Anal Oncol. 2013;2:117–9. [Google Scholar]
- 49.Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55:81–95. doi: 10.1016/j.phrs.2006.11.001. [DOI] [PubMed] [Google Scholar]


