Abstract
Background:
The influence of the supplementation of pomegranate peel extract containing anthocyanins on atherosclerotic plaque formation induced by hypercholesterolemia was investigated in renal arteries in rabbits.
Materials and Methods:
After the determination of polyphenol and anthocyanin's content of P. granatum peel hydroalcoholic extract, 30 male rabbits were randomly divided into three groups. They were fed basic diet, hypercholesterolemic diet and hypercholesterolemic diet along with P. granatum peel extract (polyphenolic content for each rabbit 1 g/kg diet) for 2 month. Blood samples were collected at the begging, middle and end of the study in order to measure lipid concentration and oxidative and antioxidative status variables, and renal arteries were taken for the assessment of atherosclerotic plaques at the end of the study.
Results:
The results reveal that P. granatum peel extract significantly increases serum antioxidant capacity in the extract recipient group in comparison with hypercholesterolemic control (P < 0.05). No significant differences are observed in total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, very low-density lipoprotein and in mean size of accumulated fatty streaks in renal arteries in the extract treatment group in comparison with hypercholesterolemic control (P > 0.05).
Conclusion:
The results of this study indicate that consumption of pomegranate peel extract containing anthocyanins (polyphenol content 1 g/kg diet) despite of a significant increase in serum antioxidant capacity cannot protect the kidneys from hypercholesterolemia-induced damages during the treatment period.
Key Words: Anthocyanin, atherosclerosis, pomegranate peel, renal artery stenosis
INTRODUCTION
Renal artery stenosis (RAS) is defined as a 50% or greater occlusion of a renal artery (unilateral or bilateral).[1] RAS may create by atherosclerosis in elderly people or fibro-muscular dysplasia in young women.[2,3] It affects blood circulation in kidney and then it can cause renovascular hypertension and renal insufficiency.[4,5] Atherosclerotic renal artery stenosis is a common disease in patients older than 50 years particularly those with diffuse atherosclerotic vascular disease.[6] This subject is the most usual cause of increasing blood pressure.[7] Atherosclerosis is an inflammatory disease that is caused by accumulation of lipids in intima and media of large and medium-sized artery and after decades thicken the inner layer of artery.[8] In fact, the earliest type of lesion called fatty streak starts in the first decades of life but symptom of this disease doesn’t appear until middle age and after that.[9] Risk factors of atherosclerotic renal artery stenosis include long-term hypertension, diabetes mellitus, smoking and dislipidemia. These factors are considered as risk factors of suddenly coronary disease.[3] Epidemiologic studies confirm associations between renovascular diseases and mortality of cardiovascular.[10]
Using of complementary nutrition supplements rich in antioxidants is effective for inhibition atherogenic changes of low-density lipoprotein (LDL), formation of foam cells and finally inhibition of atherosclerosis.
Pomegranate with scientific name Punica granatum L. from Punicaceae family is an important source of polyphenols and other antioxidants.[11,12] Pomegranate can be divided into three parts: Seeds, peel, and juice, all of which seem to have medicinal benefits. Pomegranate extracts have been used since ancient times to treat several conditions including parasitic and microbial infections, diarrhea, ulcers, aphthae, hemorrhage, and respiratory complications.[13,14] Moreover, other therapeutic properties such as antitumor, anti-inflammatory, antiviral, antibacterial, antidiarrheal, and antiobesity are currently under investigation.[15] Many beneficial effects are related to the presence of ellagic acid, ellagitannins (including punicalagins), punicic acid and other fatty acids, flavonoids, anthocyanidins, anthocyanins, estrogenic flavonols, and flavones, which seem to be its most therapeutically beneficial components.[14] The pomegranate peels make up about 60% of the fruit, and they are rich in many compounds such as phenolics, flavonoids, ellagitannins (including punicalagins), proanthocyanidin compounds, complex polysaccharides, and many minerals.[16] The peel is the part of the fruit with the highest antioxidant activity, which is in line with its high content of polyphenols. The results of investigation and comparison of antioxidants of the peel, pulp and seed in 28 different spices show that pomegranate peel has the highest antioxidant activity.[17,18] Anthocyanins, an antioxidant in peel, are the most important and abundant natural pigments belonging flavonoid family.[19,20]
This study was designed to evaluate the effects of extract containing anthocyanins of pomegranate peel on formation and progress of fatty streaks in renal arteries as well as biochemical factors involving this process in male hypercholesterolemic rabbits.
MATERIALS AND METHODS
Punica granatum L. (post siah Saveh) was supplied and authenticated at the Research Centre of Isfahan Province Natural Resources. Then required parts of the plant (peel) were dried at room temperature. The dried peels were ground and extracted with soaked method. Plant powder (50 g) was soaked in 100 ml ethanol 70% and 1% acetic acid and was stirred for 24 hours. The hydroalcoholic extract was centrifuged for 10 min (11000 g) and supernatant was used for determination of total polyphenols and total anthocyanin content.[21] The extracts were concentrated under vacuum at 40°C and the resulted powder was stored at −80°C until used.
Determination of total polyphenol content
Total polyphenol content in the extract was determined using the Folin-Ciocalteau assay.[22] One milliliter of extract solution or standard solution of garlic acid (20, 40, 60, 80 and 100 mg/l) was added in a test tube containing 9 ml dieionized-distilled water (dd H2O). A reagent blank using dd H2O was prepared. One milliliter of Folin-Ciocalteau's phenol reagent was added to the mixture and shaken. After 30 sec to 8 min, 10 ml of 7% Na2CO3 was added, then total volume was reached to 20 ml with dd H2O. After incubation for 90 min at room temperature the absorbance against prepared reagent blank was determined at 750 nanometers. Total phenolic content of extract was expressed as mg gallic acid equivalents (GAE)/100 g dried peel powder. All samples were analyzed in triplicates.
Determination of total anthocyanin content
We used the method described by Lapornik et al. for determination of total anthocyanins.[21] The principle of this method is pH decreasing of extracts to the values between 0.5 and 0.8, what causes all anthocyanins to transform to flavilium cation, which is colored red.
One milliliter of extract was pipetted into two tubes. 1 ml of 0.01% HCl solution in 95% ethanol was added into each tube. After that into the first tube (A1) 10 ml of 2% aqueous HCl solution was added, into another tube (A2) 10 ml of solution with pH = 3.5 (prepared from 0.2 M Na2HPO4 and 0.1 M citric acid). The absorbances of both samples were measured at 520 nm against blank sample (water instead of extract). All samples were analyzed in triplicates, and the mean content of total anthocyanins was calculated with this equation.
Content of total anthocyanins (mg/l) = (A1–A2) × f
f = 396/598
Animal treatment
Thirty male New Zealand white rabbits with the average weight of 1.5 kg were purchased from Pastor Institute, Teheran, Iran. The rabbits were acclimated in an air conditioned room for 2 weeks and daily provided with 100 g basic diet and free access for water. Then they were randomly divided into three groups of 10 animals each. Each group of animals had its specific diet. Rabbits fed a basic diet were used as normal controls, hypercholesterolemic control group (fed 1% high-cholesterol diet),[23] treatment group (fed 1% high cholesterol diet supplemented with hydroalcoholic extract of pomegranate peel). The extract powder was dissolved in water and was fed to the treatment group via gavage route at 1 gr (in terms of polyphenolic content)/kg diet.[24] The experiment was conducted for 60 days.
Hypercholesterolemic was induced in animals with gavage 1 gr cholesterol powder in olive oil (without E and C vitamins and other antioxidants) daily.
Animals were weighted at the beginning, middle and end of the study to clarify the effects of dietary intake of anthocyanins.
Assay for serum lipids
Fasting blood samples were taken in beginning, middle and end of the study to measure total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). Lipid levels were determined using an automated enzymatic assay by an auto-analyzer Hitachi 902-model and Zist-Chem kits from Germany. Very low-density lipoprotein (VLDL) was calculated with the Friedwal formula.[25]
Determination of antioxidant capacity (AC)
In this study, antioxidant capacity was measured according to inhibition percentage of globular lysis. After preparing 20% globular suspension, this suspension along with serum of animals (normal control, hypercholesterolemic control group, treatment group) was incubated at 37°C for 10 minutes. Then 1 ml 2–2’-azo-bis- (2-amidinopropane) dihydrochloride (AAPH) (40 mM) was added to each tube and tubes were incubated for 24 h at room temperature. After that tubes were centrifuged (3000 rpm for 10 min) and absorption of the supernatant solution at 540 nm was measured using a spectrophotometer.[26]
Percentage of inhibition cell lysis was defined as follow:
(1-ODT/ODS) × 100
ODT = Optical Density in Test tube
ODS = Optical Density in Standard tube
Histological evaluation of atherosclerosis
At the end of the treatment period, the rabbits were killed and then left and right renal arteries from ostium to 2-cm proximal segment were excised and kept in 10% formalin solution to be used for pathologic evaluation with respect to the presence of fatty streaks.[2] After slicing and staining with hematoxylin, eozine, tissue specimens were used for determination of relative size plaque.
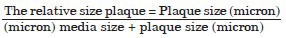
The mean of the relative size plaques in renal arteries of each rabbit were considered as the mean of relative size plaque in that rabbit and the mean of relative size of each group was compared separately with the other experimental groups.
Statistical analysis
Results were expressed as the Mean ± SD. All analyses were performed using SPSS 10 statistical software. Data were analyzed by univariate ANOVA. If a resultant fraction was found to be significant, i.e., established at P < 0.05, a post-ANOVA Tukey's test was used to specify pair-wise differences.
RESULTS
Quantitative results of the isolated extract from the analyzed herb are shown in Table 1.
Table 1.
Mean of yield (ml/100gr pomegranate peel dry weight), mean of total polyphenols (mg gallic acid/100 gr pomegranate peel dry weight) and mean of total anthocyanins (mg/100 gr pomegranate peel dry weight)

Biochemical factors
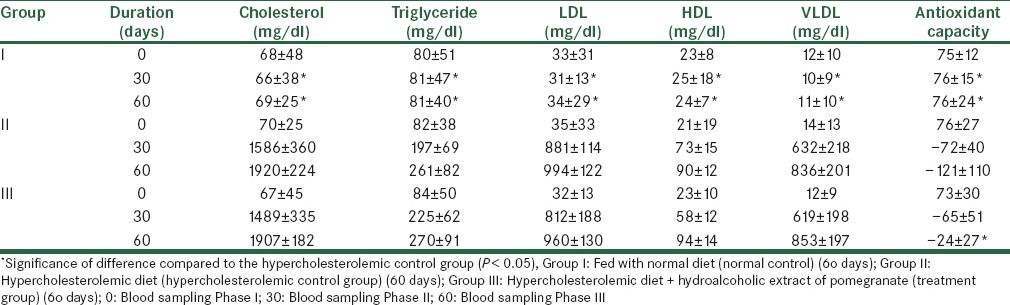
The results showed that at the beginning of the study there was no significant difference between studied groups regarding biochemical factors (TC, TG, HDL, LDL, VLDL) and serum antioxidant capacity (P > 0.05). In the middle and end of the study, the hypercholesterolemic diet increased the level of biochemical factors (TC, TG, HDL, LDL, VLDL) and decreased serum antioxidant capacity in the hypercholesterolemic control group as compared to the normal control group and as compared to the beginning of the study (P < 0.05). In the middle and end of the study, in the treatment group there was no significant difference in biochemical factors as compared with the hypercholesterolemic control group (P > 0.05) but about serum antioxidant capacity there was significant difference only at the end of the study (P < 0.05) [Table 2].
Table 2.
The effect of extract containing anthocyanins on serum biochemical factors: Results are shown as mean ± SD
Weight evaluation
At the beginning, middle and end of the study animals were weighted and there was no significant difference between the weight in the treatment group and the hypercholesterolemic control group (P > 0.05).
Pathological results
Pathological assessments showed that in normal control, atherosclerotic changes were absent and inner surfaces of two renal arteries were normal and no injury was seen in intima and media. By contrast, in the hypercholesterolemic control group, protruding atherosclerotic plaques were recognizable. In these plaques, macrophages filled with lipid droplets constituted the foam cells [Figure 1].
Figure 1.
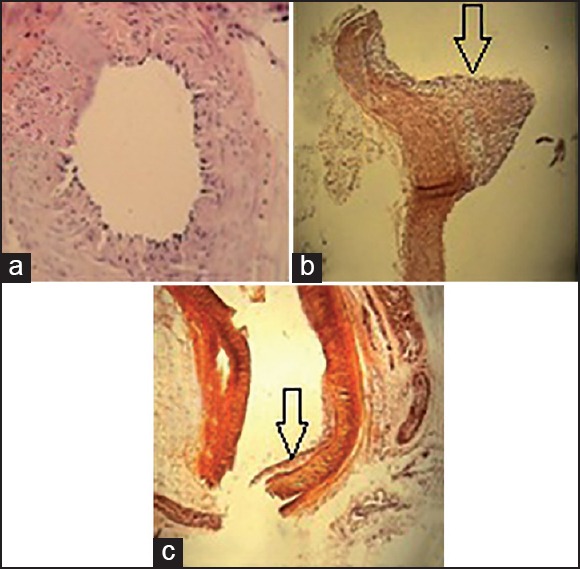
Pathological evaluation of renal arteries in all groups: (a) normal control group, (×40) normal arterial wall, (b) hypercholesterolemic control group (×10) highly protruding atherosclerotic plaque, (c) treatment group (×10) foam cells in intima. Arrows: Atherosclerotic plaques
The mean relative sizes of plaques in renal arteries were 0.12 ± 0.10 and 0.11 ± 0.09 μm in the hypercholesterolemic control group and treatment group, respectively. However, this size showed a reduction in the treatment group as compared to the hypercholesterolemic control group, but this difference wasn’t significant (P > 0.05) [Figure 2].
Figure 2.
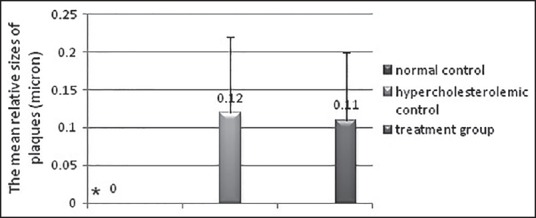
The relative mean of atherosclerotic plaque thickness in the groups under study; Results are shown as mean ± SD; *Significance of difference compared to the hypercholesterolemic control group (P < 0.05)
DISCUSSION
The results of polyphenol content show that the polyphenol content of extract is 0/070%. This result is comparable to results reported with Sultana et al.[27] In this study, method of extraction causes that extraction of anthocyanins are done more than of other polyphenols, so that more than 98% of polyphenol content are related to anthocyanins. The results of serum lipid measurements in hypercholesterolemic animals administrated with extract show that they have no significant difference as compared with the hypercholesterolemic control group. In the study performed by Fatma Labib et al. it was shown that in hypercholesterolemic rats fed with 1, 2 and 3% ethanolic extract of peel pomegranate levels of tested parameters (TC, TG, LDL and VLDL) except HDL decreased significantly as compared with hypercholesterolemic control.[11] In the same study, the methanolic extract of peel pomegranate significantly decreased TC, TG, LDL and VLDL and increased HDL in hypercholesterolemic rats as compared with hypercholesterolemic control.[28] Beside, studies performed with some groups of researchers show that rats are not a suitable model for induction hypercholesterolemia.[29,30] In a study Uchid et al. showed that diet supplemented with 2% cholesterol makes a temporary increase in plasma cholesterol. As well as, this diet strikingly increases the level of cholesterol in liver, biliary excretion of bile acids and excretion of bile acids and sterols in feces.
As a result, more studies are necessary for determination antihyperlipidemic effects of different doses of pomegranate peel extract that they are consumed during different times in admissible model animal to induce hypercholesterolemia.
The results of biochemical factors in this study show that using cholesterol in the hypercholesterolemic control group decreases serum antioxidant capacity (227%). Using extract containing anthocyanins of peel pomegranate in hypercholesterolemic animals increases 80% serum antioxidant capacity as compared with the hypercholesterolemic control group.
However, amount of antioxidant capacity in the end of the study in the treatment group is decreased 131% as compared with the normal control group. According to oxidative stress caused in hypercholesterolemic animals and decline in antioxidant capacity of serum in the hypercholesterolemic control group; it seems that using extract in the treatment group increases antioxidant capacity.
Toxicity mechanisms induced with AAPH as a producer of soluble free radicals in water are created by inducing cell membrane peroxidation that destroys aminophospholipid arrangement in a bilayer membrane of cell and causes leakage of material out of the cell.[26] In the study by Parmer et al. it was reported that the alcoholic extract of peel pomegranate is able to scavenge free radicals such as β-carotene, DPPH, NO and prevents lipid peroxidation induced with H2O2 in red blood cells and liver.[31]
In a similar study, Li et al. showed that the peel extract of pomegranate has more antioxidant properties as compared with the nutritional part of pomegranate. The results of this study show that the peel extract of pomegranate notably increases antioxidant capacity for inhibition of free radicals such as peroxyl, hydroxyl and superoxide.[17] Hence, it seems that in this study in the treatment group, an increase in serum concentration of polyphenols specially anthocyanins can protect human erythrocytes versus injury of free radicals resulting from AAPH.
The pathology results demonstrate that high-cholesterol diet significantly increases formation of fatty streaks in renal arteries of the hypercholesterolemic control group as compared normal control. Comparing the hypercholesterolemic control group with normal control illustrates that hypercholesterolemic diet develops hyperlipidemia, reduction in total antioxidant capacity and subsequence formation of fatty streaks as a first visible lesion of atherosclerosis.
Yong-hui et al. in their study indicate that hypercholesterolemia condition in rabbits not only increases serum lipid parameters but also decreases renal GFR. Also, this situation increases formation of lipidemic plaque in renal arteries and enhances expression of the LOX1 gene as a receptor of OX-LDL.[32]
In this study plaque's relative size in renal arteries of hypercholesterolemic animals treated with extract aren’t significantly different as compared the hypercholeterolemic control group. The results of this study and other similar studies express that favorable effects of antioxidants on kidneys depend on dose of extract, time of consumption and extract composition. A study by Reckelhoff et al. proves this claim. In this study, they used E vitamin to inhibit oxidative stress and improve kidney function in old rats for 9 months. The results showed that high doses of this vitamin are more effective than low doses.[33]
Usage of vitamin E for 4 weeks in hypercholesterolemic rabbits doesn’t have vital effects on improvement of endothelial of renal kidney function,[34] while usage for 12 and 32 weeks of this vitamin decreases kidney injuries.[35,36]
Therefore, more studies with higher doses and longer duration of using of pomegranate peel extract are necessary to clarify the effects of this extract on the renal arteries.
The results show that in this study using 1 g/kg diet of extract containing anthocyanins of peel pomegranate for 8 weeks doesn’t have antihyperlipidemic effects and despite of a significant increase in serum antioxidant capacity can’t protect kidneys from hypercholesterolemia-induced damages and development of lipidemic plaques in their arteries.
Footnotes
Source of Support: Physiology Research Center of Medical Science, University of Isfahan
Conflicts of Interest: None declared.
REFERENCES
- 1.Rai P, Gigliotti OS, Mathews B, Kurian KC. Renal artery stenosis: An update on diagnosis and management. Indian Heart J. 2006;58:393–400. [PubMed] [Google Scholar]
- 2.Olin JW. Atherosclerotic renal artery disease. Cardiol Clin. 2002;20:547–62. doi: 10.1016/s0733-8651(02)00091-7. [DOI] [PubMed] [Google Scholar]
- 3.Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol. 2006;22:623–8. doi: 10.1016/s0828-282x(06)70286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431–42. doi: 10.1056/NEJM200102083440607. [DOI] [PubMed] [Google Scholar]
- 5.Textor SC. Creager M, Beckman J, Loscalzo J, editors. Pathophysiology of Renal Artery Disease. Vascular Medicine: A Companion to Braunwald's Heart Disease. 2013:285–95. [Google Scholar]
- 6.Kuroda S, Nishida N, Uzu T, Takeji M, Nishimura M, Fujii T, et al. Prevalence of renal artery stenosis in autopsy patients with stroke. Stroke. 2000;31:61–5. doi: 10.1161/01.str.31.1.61. [DOI] [PubMed] [Google Scholar]
- 7.Cheung CM, Hegarty J, Kalra PA. Dilemmas in the management of renal artery stenosis. Br Med Bull. 2005;73:35–55. doi: 10.1093/bmb/ldh049. [DOI] [PubMed] [Google Scholar]
- 8.Tan C, Liu Y, Li W, Deng F, Liu X, Wang X, et al. Associations of matrix metalloproteinase-9 and monocyte chemoattractant protein-1 concentrations with carotid atherosclerosis, based on measurements of plaque and intima-media thickness. Atherosclerosis. 2014;232:199–203. doi: 10.1016/j.atherosclerosis.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 10.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: A prospective, population-based study. Arch Intern Med. 2005;165:207–13. doi: 10.1001/archinte.165.2.207. [DOI] [PubMed] [Google Scholar]
- 11.Fatma Labib AH. Effect of pomegranate (Punica granatum) peels and it's extract on obese hypercholesterolemic rats. Pakistan J Nutr. 2009;8:1251–7. [Google Scholar]
- 12.Mathew AS, Capel-Williams GM, Berry SE, Hall WL. Acute effects of pomegranate extract on postprandial lipaemia, vascular function and blood pressure. Plant Foods Hum Nutr. 2012;67:351–7. doi: 10.1007/s11130-012-0318-9. [DOI] [PubMed] [Google Scholar]
- 13.Naqvi SA, Khan MS, Vohara SB. Antibacterial, antifungal and anthelmintic investigations on Indian medicinal plants. Fitoterapia. 1991;62:221–8. [Google Scholar]
- 14.Viladomiu M, Hontecillas R, Lu P, Bassaganya-Riera J. Preventive and prophylactic mechanisms of action of pomegranate bioactive constituents. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/789764. 789764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johanningsmeier SD, Harris GK. Pomegranate as a functional food and nutraceutical source. Annu Rev Food Sci Technol. 2011;2:181–201. doi: 10.1146/annurev-food-030810-153709. [DOI] [PubMed] [Google Scholar]
- 16.Viuda-Martos M, Fernández-Lóaez J, Pérez-Álvarez JA. Pomegranate and its many functional components as related to human health: A review. Compr Rev Food Sci F. 2010;9:635–54. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–60. [Google Scholar]
- 18.Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res. 2003;23:1719–26. [Google Scholar]
- 19.Negi PS, Jayaparkasha GK, Jena BS. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003;80:393–7. [Google Scholar]
- 20.Williams CA, Grayer RJ. Anthocyanins and other flavonoids. Nat Prod Rep. 2004;21:539–73. doi: 10.1039/b311404j. [DOI] [PubMed] [Google Scholar]
- 21.Lapornik B, Pros¡ek M, Wondra AG. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J Food Eng. 2005;71:214–22. [Google Scholar]
- 22.Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in bulgarian fruits and vegtables. JUCTM. 2005;40:255–60. [Google Scholar]
- 23.Cooke JP, Singer AH, Tsao P, Zera P, Rowan RA, Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–72. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez-Tortosa C, Andesen ØM, Cabrita L, Gardner PT, Morrice PC, Wood SG, et al. Anthocyanin - rich extract decreases indices of lipid peroxidation and DNA damage in vitamin E-depleted rats. Free Radic Biol Med. 2001;31:1033–7. doi: 10.1016/s0891-5849(01)00618-9. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Botta A, Martinez V, Mitjans M, Balboa E, Conde E, Vinardell MP. Erythrocytes and cell line-based assays to evaluate the cytoprotective activity of antioxidant components obtained from natural sources. Toxicol In Vitro. 2014;28:120–4. doi: 10.1016/j.tiv.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Sultana B, Anwar F, Rafique Asi M, Shahid Chatha SA. Antioxidant potential of extracts from different agro wastes:Stabilization of corn oil. Grasas Y Aceites. 2008;59:205–17. [Google Scholar]
- 28.Parmar HS, Kar A. Protective role of Citrus sinensis, Musa paradisiaca, and Punica granatum peels against diet-induced atherosclerosis and thyroid dysfunctions in rats. Nutr Res. 2007;27:710–8. [Google Scholar]
- 29.Uchida K, Nomura Y, Kadowaki M, Takeuchi N, Yamamura Y. Effect of dietary cholesterol on cholesterol and bile acid metabolism in rats. Jpn J Pharmacol. 1977;27:193–204. doi: 10.1254/jjp.27.193. [DOI] [PubMed] [Google Scholar]
- 30.Wilson JD. Relation between dietary cholesterol and bile acid excretion in the rat. Am J Physiol. 1962;203:1029–32. doi: 10.1152/ajplegacy.1962.203.6.1029. [DOI] [PubMed] [Google Scholar]
- 31.Parmar HS, Kar A. Comparative analysis of free radical scavenging potential of several fruit peel extracts by in vitro methods. Drug Discov Ther. 2009;3:49–55. [PubMed] [Google Scholar]
- 32.YuYH, Wang Y, Dong B, Shu SZ, Chen Y, Meng XH, et al. Fluvastatin prevents renal injery and expression of lactin-like oxidized low-density lipoprotein receptor-1 in rabbits with hypercholesterolemia. Chin Med J(Engl) 2005;118:621–6. [PubMed] [Google Scholar]
- 33.Reckelhoff JF, Kanji V, Racusen LC, Schmidt AM, Yan SD, Marrow J, et al. Vitamin E ameliorates enhanced renal lipid peroxidation and accumulation of F2-isoprostanes in aging kidneys. Am J Physiol. 1998;274:R767–74. doi: 10.1152/ajpregu.1998.274.3.R767. [DOI] [PubMed] [Google Scholar]
- 34.Stewart-Lee AL, Ferns GA, Anggård EE. Differences in onset of impaired endothelial responses and in effects of vitamin E in the hypercholesterolemic rabbit carotid and renal arteries. J Cardiovasc Pharmacol. 1995;25:906–13. doi: 10.1097/00005344-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Eddy AA. Interstitial fibrosis in hypercholesterolemic rats: Role of oxidation, matrix synthesis, and proteolytic cascades. Kidney Int. 1998;53:1182–9. doi: 10.1046/j.1523-1755.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee HS, Jeong JY, Kim BC, Kim YS, Zhang YZ, Chung HK. Dietary antioxidant inhibits lipoprotein oxidation and renal injury in experimental focal segmental glomerulosclerosis. KidneyInt. 1997;51:1151–9. doi: 10.1038/ki.1997.158. [DOI] [PubMed] [Google Scholar]


