Abstract
Pancreas divisum (PD) is the most common developmental anatomic variant of pancreatic duct. Endoscopic ultrasound (EUS) is often performed to evaluate idiopathic pancreatitis and has been shown to have high accuracy in diagnosis of PD. The different techniques to identify PD by linear EUS have been described differently by different authors. If EUS is done with a proper technique it can be a valuable tool in the diagnosis of PD. The anatomical and technical background of different signs has not been described so far. This article summarizes the different techniques of imaging of pancreatic duct in a suspected case of PD and gives a technical explanation of various signs. The common signs seen during evaluation of pancreatic duct in PD are stack sign of linear EUS, crossed duct sign on linear EUS, the dominant duct and ventral dorsal duct (VD) transition. Few other signs are described which include duct above duct, short ventral duct /absent ventral duct, separate opening of ducts with no communication, separate opening of ducts with filamentous communication, stacking of duct of Santorini and indirect signs like santorinecele. The principles of the sign have been explained on an anatomical basis and the techniques and the principles described in the review will be helpful in technical evaluation of PD during EUS.
Keywords: Endoscopic ultrasound (EUS), pancreas divisum (PD), pancreatitis
INTRODUCTION
Pancreas divisum (PD) is the most common developmental anatomic variation of the pancreatic duct. PD has a reported incidence of 4%-14% in the population at autopsy series, 3%-8% at endoscopic retrograde cholangiopancreatography (ERCP), and 9% at magnetic resonance cholangiopancreatography (MRCP).[1,2,3,4,5] PD is characterized by the failure of fusion of the ducts of Santorini and Wirsung. The abnormal fusion causes abnormal drainage of the majority of pancreatic juice into the minor papilla and the minority (about 10%) through the major papilla.[1,2] PD is of three types. Type 1 (classic PD) is the complete failure of fusion of the ducts of Santorini and Wirsung, type 2 is the absence of the duct of Wirsung, and type 3 (incomplete PD) is the presence of a filamentous or tiny caliber communication between the dominant dorsal duct of Santorini and the duct of Wirsung [Figure 1a–d]. PD is often asymptomatic, but is thought to be an unrecognized cause of many cases of recurrent acute pancreatitis.[6] Timely and appropriate therapeutic interventions such as minor papillotomy or stent placement in the dorsal pancreatic duct or surgical procedures can significantly benefit patients with symptomatic PD.[7]
Figure 1.
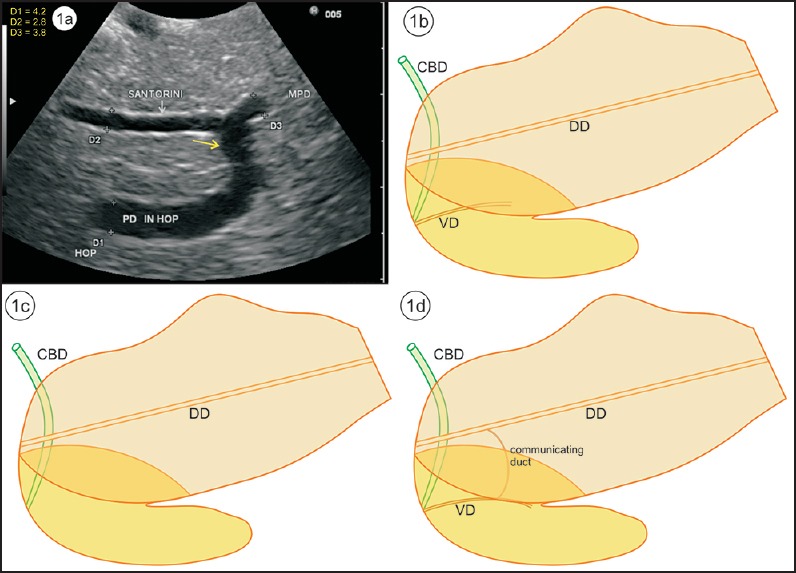
(a) An image showing the communication of the ventral and dorsal duct from stomach. (b) Type 1 total nonunion of dorsal duct (DD) and ventral duct (VD). DD opens at minor papilla. (c) Type 2 absence of ventral duct. (d) Type 3 incomplete PD with rudimentary communication between the ventral and dorsal duct
ERCP with a pancreatogram obtained after cannulating both the major and minor papilla is the gold standard in the diagnosis of PD.[2,8,9] ERCP is, however, seldom used for diagnosis, as minor papilla cannulation is associated with a high failure rate, significant risk of pancreatitis, and risk of radiation.[10,11] ERCP is superior to multidetector-row computed tomography (MDCT) in assessing the presence of a ductal anomaly of PD.[12] Studies have shown that there is a great correlation between MRCP and ERCP in detecting PD.[10,13] A retrospective study has shown the sensitivities of MDCT (15.5%), MRCP (60%), and endoscopic ultrasound (EUS) (86.7%); the sensitivity of MRCP went up to 83.3% if the main pancreatic duct could be visualized and the study was being reviewed by an expert radiologist.[14,15] MRCP with secretin stimulation can provide even better visualization of the pancreatic duct, resulting in higher sensitivity and specificity for diagnosis of the pancreatic abnormalities.[16,17] MRCP can be performed together with magnetic resonance imaging (MRI), and MRCP with MRI have been referred to as primary diagnostic tools for pancreatitis with PD, whereas ERCP is reserved for those who require therapeutic interventions.[18]
EUS has been shown to have high accuracy in diagnosis of PD and is often performed to evaluate idiopathic pancreatitis. There are limited data comparing EUS to other imaging modalities for the detection of PD. In experienced hands, linear EUS has been shown to have a sensitivity of 95% and an overall accuracy of 97% for the diagnosis of PD, when the main pancreatic duct can be well visualized.[19] Although EUS has not been directly compared with MRCP for the diagnosis of PD, studies have evaluated the diagnostic yield of these two modalities in patients with idiopathic acute pancreatitis. In a prospective comparison of EUS and MRCP for etiological diagnosis of idiopathic acute pancreatitis in 49 patients, MRCP could identify PD in 4 patients, whereas EUS could diagnose PD in only 1 patient.[20] However, the low detection rate of PD by EUS may be due to operator-dependent imaging. In a large series of patients with PD, secretin-stimulated EUS (S-EUS) showed dilation of the dorsal duct by 1 mm or more following intravenous secretin.[21] A comparison of S-EUS with secretin MRCP has shown that S-EUS had a higher diagnostic yield than secretin MRCP.[22]
This review explains the basic imaging techniques for the imaging of pancreatic duct in PD by linear EUS.
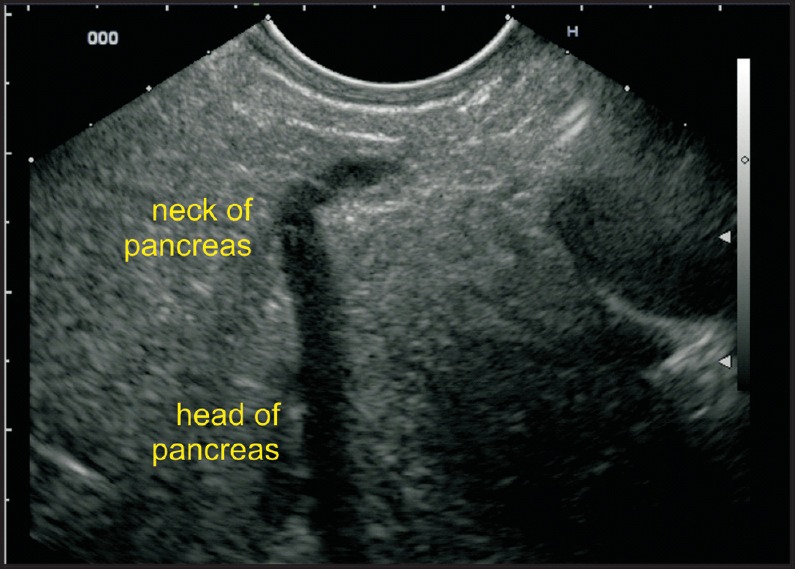
The absence of “stack sign”: Earlier gastroenterologists, more trained for cross-sectional imaging and radial endosonography, used the absence of the “stack sign” as a criterion for diagnosing PD [Figure 2a and b]. A stack is a pile of objects, typically one that is neatly arranged in a parallel manner, and “stack sign” is conventionally described for radial EUS from the duodenal bulb from where the distal common bile duct (CBD), ventral pancreatic duct, and portal vein can be seen to run on a parallel axis [Figure 3a and b]. Although “stack sign” is conventionally described for radial EUS, the imaging from linear EUS also shows remarkable similarity in the duodenum, from where CBD and pancreatic duct (two out of three structures of the stack) are usually seen on a parallel axis [Video 1]. This can be termed as “stack sign of linear EUS” [Figure 3d, Video 2]. During linear EUS the superior mesenteric vein (SMV) — and sometimes the superior mesenteric artery (SMA) — rather than the portal vein is seen as the third pile of the “stack sign of linear EUS” on a different axis after clockwise rotation [Figure 3c]. The anatomical background of “stack sign” is helpful in linear imaging from the duodenal bulb, but the pancreatic duct in the head of pancreas and neck is more often seen in cross section [Figure 4a and b].
The “trace back”: Expert sonographers can follow the course of the duct of Wirsung, from the major and the duct of Santorini from the minor papilla. The duct of Wirsung is followed up from the papilla by gently withdrawing the scope with a clockwise rotation to the pancreatic body [Figure 5].[23] The duct of Santorini is identified from the minor papilla. The minor papilla is seen as a triangular hypoechoic area in the groove between the hyperechoic part of the dorsal pancreas and the duodenal wall without the presence of any mucosal fold covering the minor papilla [Figure 6]. The minor papilla is easily visualized in cases of PD where the duct of Santorini is more prominent and often dilated because of physiological overload. Real-time tracing of the course of the duct is also possible from different positions, namely the stomach, bulb, and descending duodenum [Figures 7a–d, 8a–b, 9a–b].
The “crossed duct sign”: On radial EUS, the “crossed duct sign” for PD is conventionally described for radial EUS from the duodenal bulb where the duct of Santorini will appear to cross over the bile duct if one withdraws the scope toward the minor papilla. Generally the ventral pancreatic duct lies beyond and parallel to CBD and lies away from the probe in comparison to CBD during imaging from the descending duodenum and duodenal bulb. In case of PD, the ventral pancreatic duct is less prominent and the prominent dorsal pancreatic duct is identified closer to the probe. In the sense of distance from the transducer, the CBD and the dorsal pancreatic duct are equally close as they are both openings in the duodenal wall, though at separate locations. The “crossed duct sign” can be described in an analogous manner from the duodenal bulb where the bile duct and the dorsal pancreatic duct are seen entering the second part of the duodenum at separate locations. The dorsal pancreatic duct traverses to the duodenal wall proximal and anterior to the bile duct, and on following the course the dorsal pancreatic duct crosses the CBD [Figure 10].[24]
The dominant Santorini duct: The diameter of the Santorini duct is greater than the diameter of the ventral pancreatic duct in cases of PD, and S-EUS prior to an ERCP has shown an abnormal response with dilation of the dorsal duct (more than 10 min, for 1 mm, or more) following intravenous secretin.
Ventral and dorsal transition (VD transition): A discrete endosonographic border between the hypoechoic ventral anlage and a brighter dorsal anlage can be seen in up to 75% of normal patients, and the diagnosis of PD is excluded if the pancreatic duct shows VD transition [Figure 11a–c, Video 3].[19,25] Elastography shows similar characteristics of hypoechoic ventral anlage and a brighter dorsal anlage [Figure 11d]. The tracing of the course of the duct is commonly used from the duodenal bulb or from the descending duodenum from where the pancreatic duct is followed from the main papilla back around the genu.[23] Compound imaging, if available, and increased depth of penetration can allow the tracing of the pancreatic duct from the stomach, too [Figure 11c].
Figure 2.
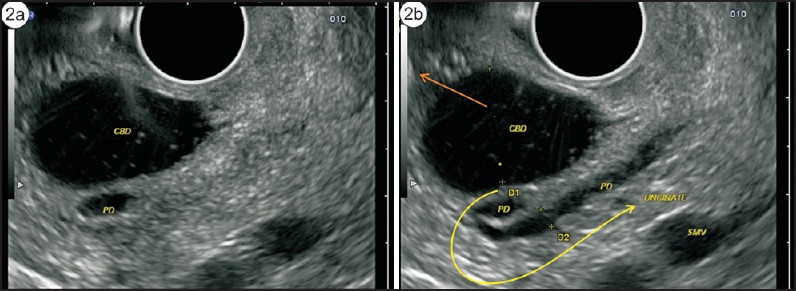
(a) On radial endosonography, the pancreatic duct and the bile duct are seen coming from the ampulla and lying parallel to each other. (b) The bile duct continues up into the hepatoduodenal ligament, whereas the pancreatic duct turns in the neck of pancreas and finally goes toward the body of pancreas
Figure 3.
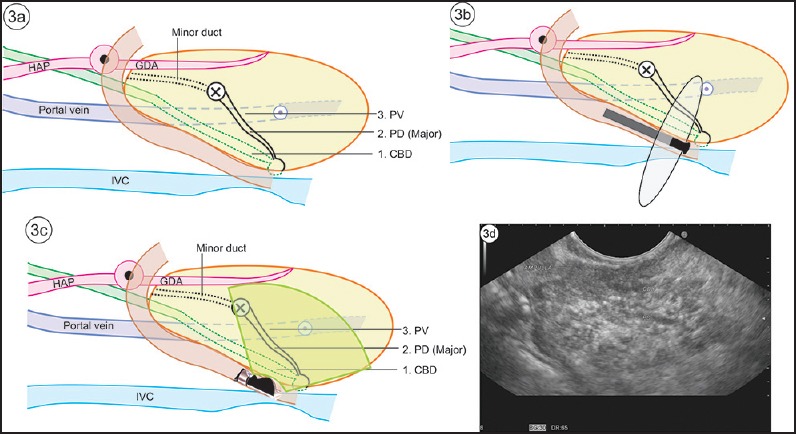
(a) The course of CBD, major pancreatic duct and portal vein (PV), is shown in this figure. (b) The “stack sign” is a demonstration of the three structures in a stack by radial echoendoscope. (c) The stack is more often demonstrated by radial echoendoscope, but it is also possible to demonstrate the same stack with a linear echoendoscope. This has been termed “reverse stack sign” with a linear scope. It is easier to see the SMV continuing as portal vein. However, the axis oflinear imaging of the portal vein and SMV does not lie in the axis of CBD and pancreatic duct. (d) “Stack sign” of linear EUS
Figure 4.
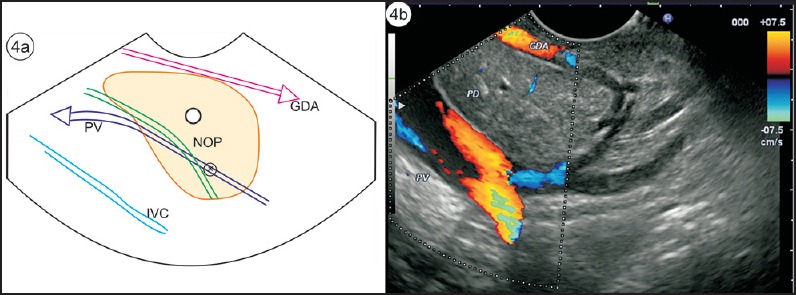
(a) The pancreatic duct is identified in cross section in the neck of pancreas. The gastroduodenal artery lies anterior to the neck of pancreas, and the portal vein and CBD lie posterior to the neck of pancreas. (b) EUS image of 4a
Figure 5.
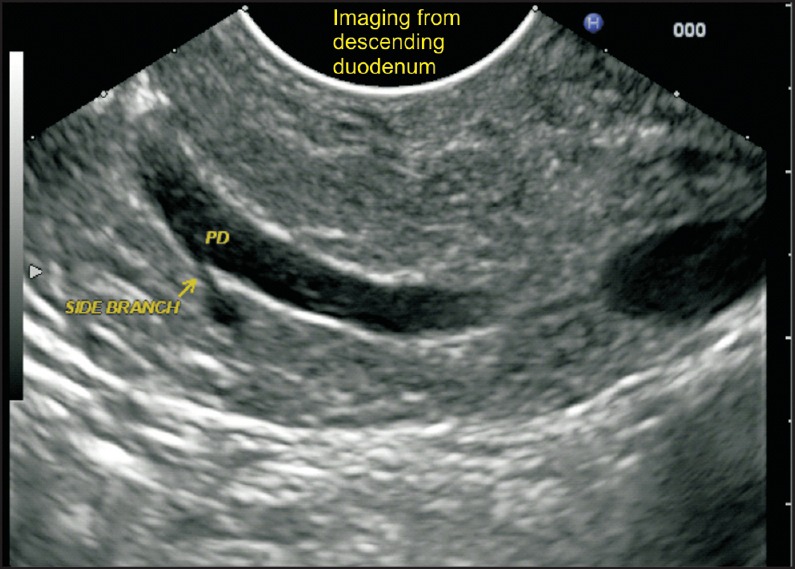
The duct of Wirsung is followed up from the major papilla by gently withdrawing with a clockwise rotation of the echoendoscope. The side branch joining the pancreatic duct comes from the ventral part of pancreas
Figure 6.
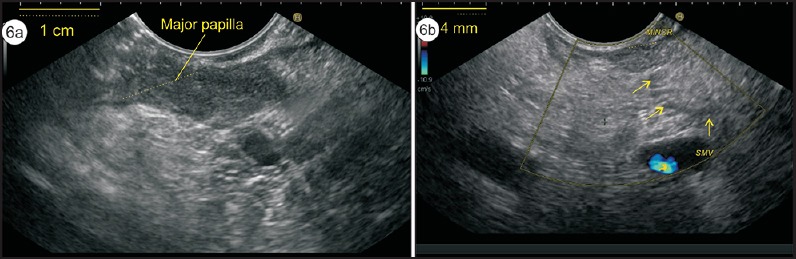
(a) The imaging of minor papilla is done about 1-2 cm above the major papilla. It is seen as a 2-4-mm hypoechoic area along the muscularis propria of the duodenal wall. In this case, the major papilla (size 1 cm) and minor papilla (size 4 mm) is seen. (b) The duct of Santorini is draining the minor papilla
Figure 7.
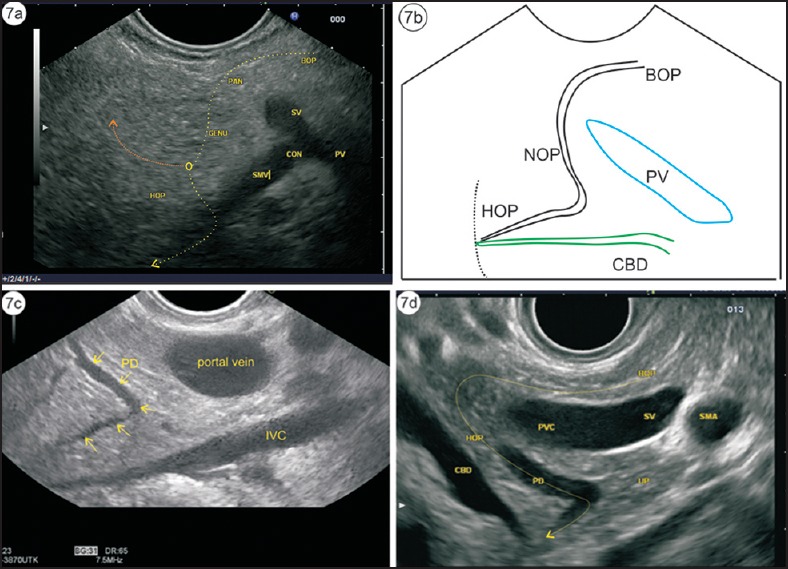
(a) The course of the pancreatic duct is shown by the dotted arrow. (b) This course is taken during demonstration of the pancreatic duct while following it from the body of pancreas. The bile duct is seen coming toward the papilla. (c) The pancreatic duct is seen coming from the body of pancreas, turning in the neck of pancreas, and finally running toward the papilla in the head of pancreas. (d) A radial EUS image is given for the sake of clarification of the course of the duct
Figure 8.
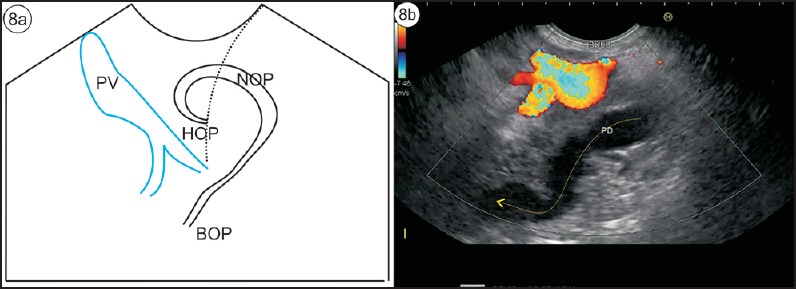
(a) The pancreatic duct is seen coming from the papilla and turning around in the neck of pancreas before it falls away from the probe toward the body of pancreas. (b) On following up with anticlockwise rotation, the pancreatic duct can be followed into the body of pancreas
Figure 9.
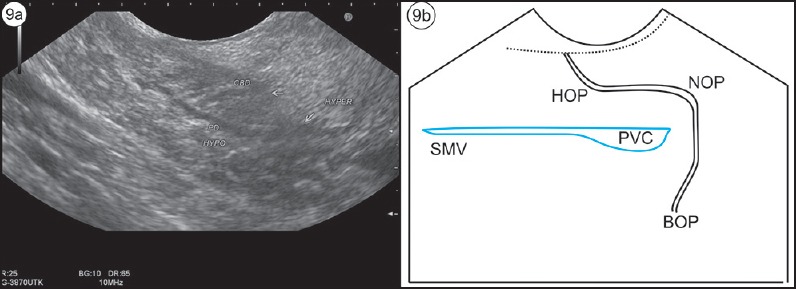
(a) The VP duct travels in part of hypoecheic pancreas. (b) On following the duct, it can be traced to the muscularis layer and the papilla on anticlockwise rotation, and it turns around the portal venous confluence toward the body of pancreas on clockwise rotation
Figure 10.
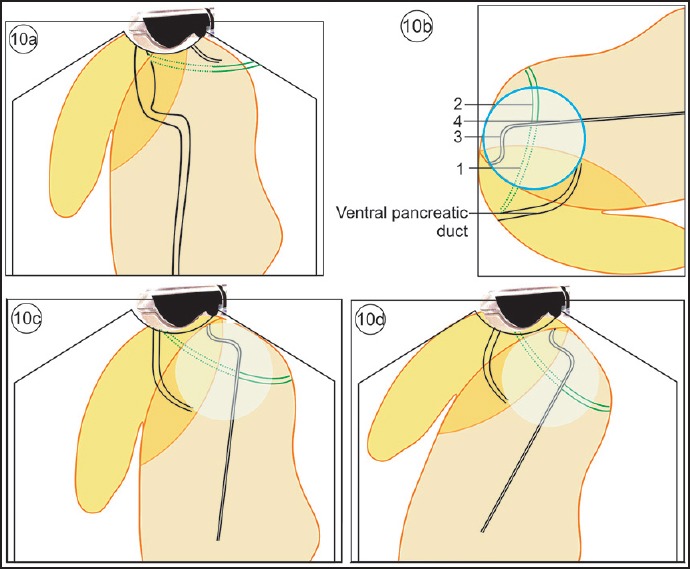
(a) The VP duct lies beyond the bile duct and remains parallel to the CBD. The DP duct is smaller in diameter and tapers within the pancreatic parenchyma. (b) The circle within which the crossing over of the duct occurs is seen. This crossing over is also visualized in MRCP. The four parts related to the crossing are the intrapancreatic or retropancreatic CBD, CBD within hepatoduodenal ligament, the dorsal pancreatic duct before crossing, and the dorsal pancreatic duct after crossing. (c) The imaging of “cross duct sign” from the second part of duodenum shows the crossing over of pancreatic duct. (d) The imaging of “cross duct sign” from the duodenum bulb shows the crossing over of pancreatic duct with the bile duct
Figure 11.
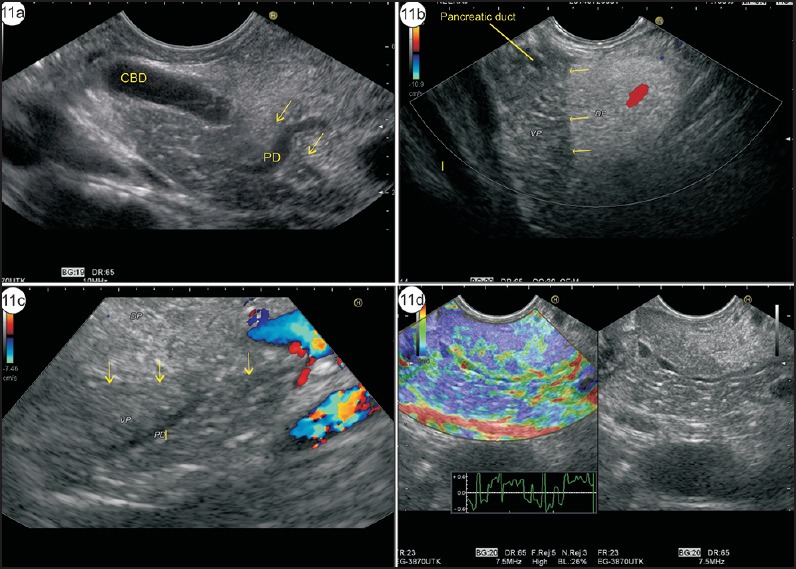
(a) Imaging from stomach shows hyperechoic DP close to the probe and hypoechoic VP close to the portal vein. The CBD lies closer than the pancreatic duct. The main pancreatic duct is seen moving from DP to VP. The yellow arrows show the transition (DP–VP) zone. (b) Imaging from duodenal bulb shows hypoechoic VP and hyperechoic DP. The main pancreatic duct is seen within hypoechoic VP. The yellow arrows show the transition zone. (c) The imaging of DP–VP zone can be done from descending duodenum. In these two different cases, the transition zone of hyper- and hypoechoeic pancreas is seen from the duodenum and the Wirsung duct is seen traversing from hypoechoic VP to hyperechoic DP. (d) The imaging of DP–VP zone is seen traversing from hypoechoic VP to hyperechoic DP. The elastography image shows no difference in the characteristics of VP and DP
The different techniques to identify PD by linear EUS can be separately described from three positions: imaging from the stomach, imaging from the duodenal bulb, and imaging from the duodenum.
TECHNIQUE OF PANCREATIC DUCT IMAGING FROM STOMACH
Positioning and movements
Clockwise rotation of the shaft with up angulation presses the scope against the posterior side of the stomach and allows the examination of the left portion of the pancreatic body and tail. Anticlockwise rotation of the shaft with up angulation usually places the echoendoscope along the vertical part of the lesser curve and allows the examination of the right portion of the pancreatic body up to its junction with the neck. Further rotation, up angulation, and positioning of the scope in the distal part of the body of the stomach can usually allow the examination of the entire head of pancreas [Figure 12].
Figure 12.
From the stomach, the pancreatic duct is initially visualized in the body of pancreas and can be followed by anticlockwise rotation toward the head of the pancreas, where it falls away from the transducer. This can be similar to a waterfall and can be called pancreatic duct fall in the head of pancreas
The course of normal pancreatic duct
The examination of the pancreatic duct from the stomach may be started in the body of pancreas where it lies closest to the transducer. Anticlockwise rotation traces the course of the pancreatic duct toward the head of pancreas, where it tends to move away from the dorsal part of the head of pancreas toward the ventral part of the head of pancreas, which lies in the right paravertebral gutter [Figure 13].
Figure 13.
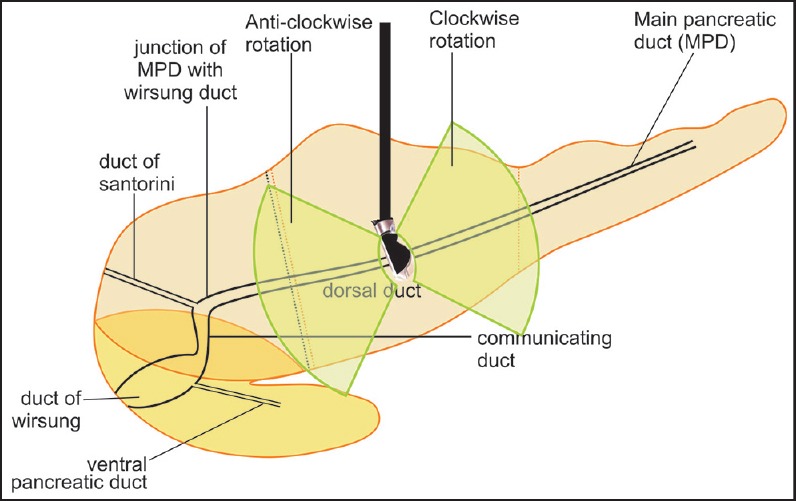
Examination of pancreatic duct can be done from the stomach. Clockwise rotation shows the pancreatic duct in the tail of pancreas and anticlockwise rotation shows the pancreatic duct in the head of pancreas
Technical demonstration of PD from stomach, DP to VP transition
The echotexture of pancreatic parenchyma and the border showing dorsal pancreas–ventral pancreas (DP-VP) transition can be appreciated from the stomach [Figure 11c]. In a person with normal pancreas, the movement of the pancreatic duct from the DP to VP is seen, whereas it is absent in PD. Sometimes in case of PD the entire course of the dorsal pancreatic duct can be followed all the way to the minor papilla within the hyperechoic part of DP without a fall toward the ventral part of the head of pancreas [Video 4].
TECHNIQUE OF PANCREATIC DUCT IMAGING FROM DUODENAL BULB
Positioning and movements
The positioning of a scope in the duodenal bulb can be done in a long loop while advancing toward the duodenum or after reinsertion into the duodenal bulb once the scope comes out of D2 [Figure 14].
Figure 14.
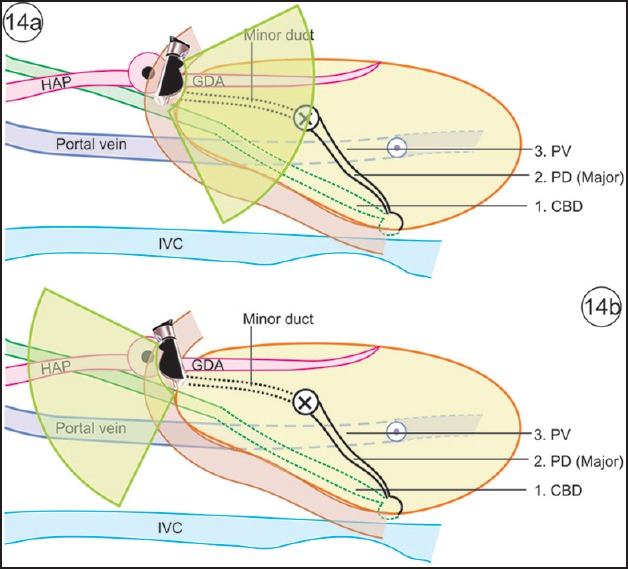
(a) Once the scope is in the duodenal bulb, clockwise and anticlockwise rotation can demonstrate the course of bile duct and pancreatic duct. This image shows the structures that can be seen in the infraduodenal area on clockwise rotation. (b) This image shows the structures that can be seen in the supraduodenal area on anticlockwise rotation
The course of normal pancreatic duct
The examination of pancreatic duct from the bulb may be started after locating the gastroduodenal artery (running close to the duodenal wall) anterior to the neck of pancreas and the portal vein (running away from the duodenal wall) posterior to the neck of pancreas [Figure 14]. The main pancreatic duct in the neck is almost always visible between the duodenal wall (gastroduodenal artery) and the portal vein. An anticlockwise rotation follows the pancreatic duct toward the body of pancreas, and clockwise rotation follows it toward the papilla, where a part of the duct may be seen on long axis and an image similar to a “stack sign” may be produced [Figure 14a]. On anticlockwise rotation, the pancreatic duct moves around the confluence of the portal vein and is seen to fall away from the transducer toward the body of pancreas. On clockwise rotation, an important change in view is the fall of the muscularis propria layer of the duodenal wall in which the ampulla is located, and the pancreatic duct proceeds toward the muscularis propria layer of the duodenal wall to open at the ampulla/papilla.
Technical demonstration of PD from duodenal bulb
In a case of PD, the dorsal pancreatic duct is seen above the CBD and closer to the duodenal wall. The dorsal pancreatic duct emerges from the minor duct in the hyperechoic part and continues into the body while remaining within the more echogenic dorsal pancreas. During following, the dorsal pancreatic duct crosses the path of the bile duct [Figure 15].
Figure 15.
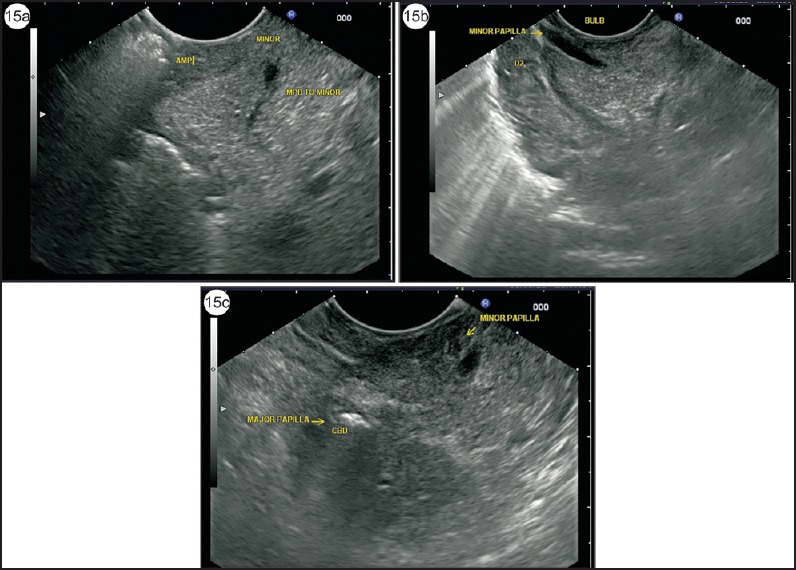
(a) Application of color Doppler can identify the small anechoic structures when the scope is wedged into the duodenal bulb in a long loop position or during withdrawal from the second part of the duodenum. The duct of minor papilla usually ends in a triangular hypoechoic area near the duodenal wall. The duct is seen in a transverse axis. (b) In such cases, an anechoic duct going toward the duodenal wall within the parenchyma of hyperechoic DP is the duct of Santorini. The duct is seen on a longitudinal axis. (c) This image shows both major and minor papilla, which are placed at a distance of about 1.5 cm. The air is seen in CBD
TECHNIQUE OF PANCREATIC DUCT IMAGING FROM DUODENUM
Positioning and movements
Duodenal imaging from the horizontal duodenum (D3), descending duodenum (D2), and ascending of duodenum (D1) is the mainstay of pancreatic duct imaging from the duodenum [Figure 16]. A combination of movements of withdrawal and rotation is useful. As experience is gained, the movements of withdrawal and rotation get combined into a single smooth movement.
Figure 16.
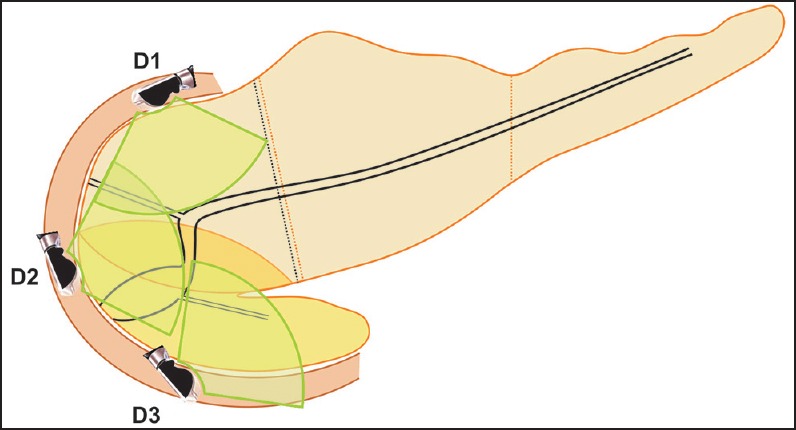
Duodenal imaging of pancreatic duct can be done from the third part (D3), second part (D2), and first part of the duodenum (D1)
The course of normal pancreatic duct
Repeated pushing of the scope two or three times should be done. While pushing in the scope goes to about 80 cm distance from incisors and while pulling out it comes to around 55 cm. This repeated pushing and pulling out positions the scope deeper into the third part of the duodenum. In D3 and D2, clockwise rotation shows the anterior segment of the head of pancreas and the mesenteric vessels, and anticlockwise rotation shows the posterior segment of head of pancreas and the uncinate process. On anticlockwise rotation, the uncinate process is generally seen between the transducer and aorta, and the ventral pancreatic duct is identified within the pancreatic parenchyma as the first branch joining the aspect of the main pancreatic duct away from the transducer [Figure 1]. The distal CBD is closer to the duodenal wall than the pancreatic duct [Figure 3d, 11a].
TECHNICAL DEMONSTRATION OF PD FROM DESCENDING DUODENUM
Short duct/absent ventral duct
The imaging from duodenum should alert the operator about PD when the main pancreatic duct is not identified or a short pancreatic duct is identified, which ends within the parenchyma of hypoechoic VP [Figure 17].
Figure 17.
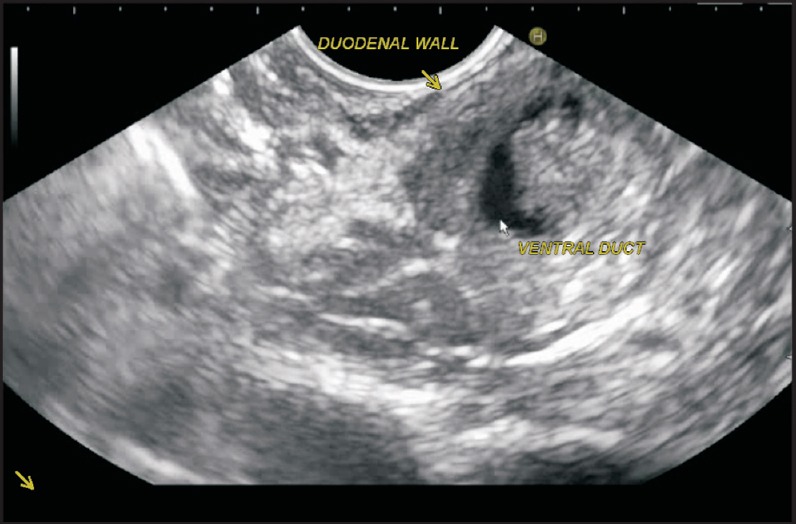
A short pancreatic duct is seen in a case of PD, which ends within the parenchyma of VP
Separate ducts with no communication
The imaging from D2 should alert the operator about PD when a duct above a duct view is seen and the duct of Santorini joins approximately 1-2 cm above the duct of Wirsung (or CBD) [Figure 18, Video 5]. When such a view is seen, the relative diameter of the ducts suggests PD, and on following the course of the duct of Santorini and the CBD, a “crossed duct sign” is seen [Figure 19, Video 6].
Figure 18.
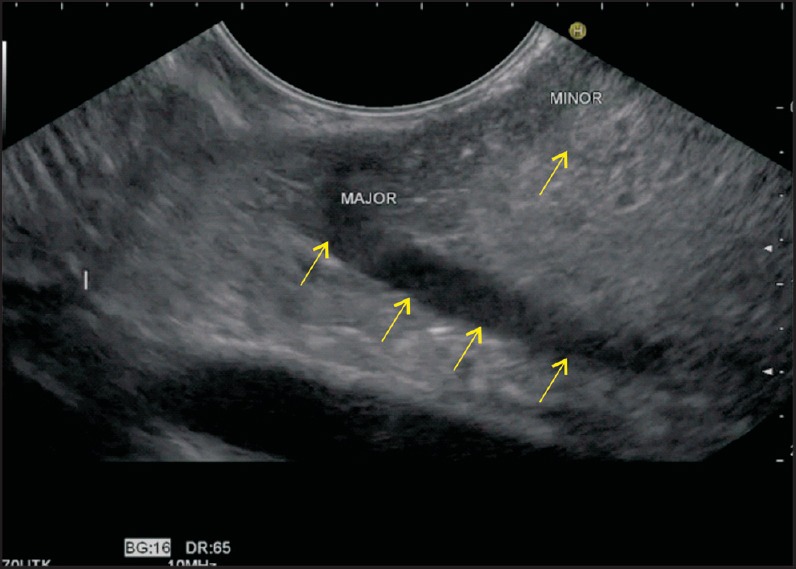
The imaging from the duodenum shows both the major and minor papilla in a normal person. The Santorini duct lies cranial to the Wirsung duct. The diameter of the duct of Wirsung is larger than that of the duct of Santorini
Figure 19.
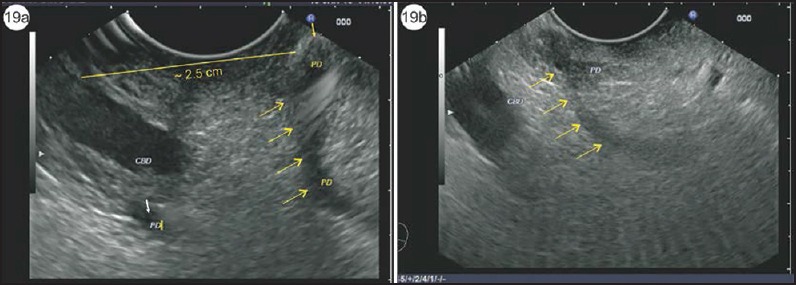
(a) In this case, the opening of the Santorini duct is seen in the wall of the duodenum approximately 2.5 cm above the opening of bile duct in the major papilla. The small yellow arrow shows the opening of the duct of Santorini, which in this case is the major duct draining the pancreas. The VP duct in this case is seen beyond the bile duct and drains only the VP (small white arrow). The diameter of the duct of Santorini is greater than the diameter of the duct of Wirsung. (b) The arrows show the course of the duct of Santorini
Separate ducts with communication (filamentous or more defined communication)
A pancreatic duct is seen with a filamentous transition across the border [Figure 20, Video 7]. If both the ducts are identified and seen to be communicating, the diagnosis of type 3 PD can be established by comparison of the diameters of both the ducts: The dominant duct will have the larger diameter.
Figure 20.
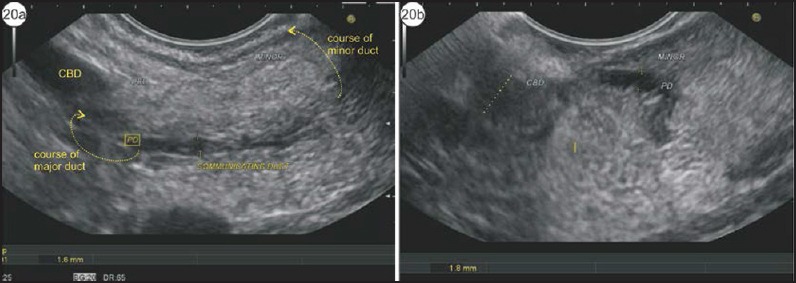
(a) The dilated duct of Santorini is identified above the bile duct. (b) On following the course of the bile duct it is seen to have filamentous communication with the duct of Wirsung, which is smaller in diameter
INDIRECT SIGN
Other sign that has not been specifically mentioned for PD but which is easily explained by the anatomical relationship of the dominant dorsal pancreatic duct, the SMV and SMA is stacking of Santorini against the SMV and SMA. A stacking of duct of Santorini against the SMV and SMA is seen from the 2nd part of duodenum (Figure 21 and Video 8). Once the scope is positioned/wedged/fixed in D2, clockwise rotation shows the anterior segment of pancreas where the duct of Santorini can be identified. In this plane, once the scope is withdrawn to the point where the SMV joins the splenic vein and the SMA joins the aorta, the dominant duct of the Santorini duct is identified near the duodenal wall. The other indirect signs helping in identification of PD are abnormalities at the minor papilla such as santorinicele, stricture, and stone [Figure 22, Video 9].
Figure 21.
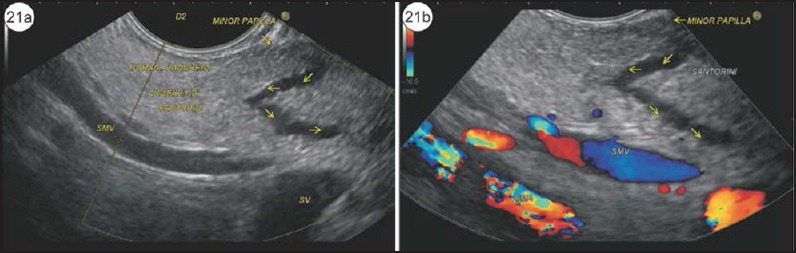
(a) The SMV and SMA are seen lying parallel to each other commonly in the second part of the duodenum. In a case of PD, the dilated duct of Santorini can be seen on the same plane. Although this is not conventionally described, one of the techniques of imaging the minor duct of Santorini is to scan the dorsal part of pancreas while simultaneously visualizing the SMV and SMA. (b) The application of color Doppler shows the presence of flow within SMV and SMA
Figure 22.
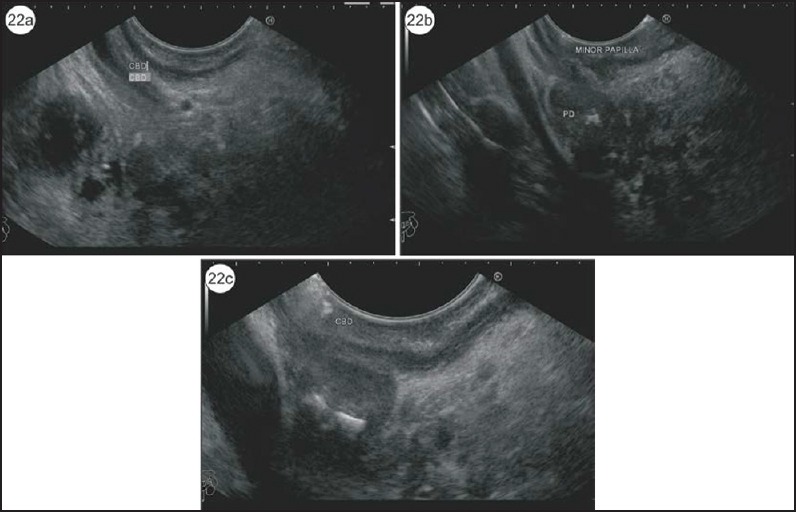
(a) In this case of PD, initially the CBD is identified in the duodenal bulb. (b) The pancreatic duct with calculi is identified in the same position a little cranial to the CBD. (c) This is a case of PD with santorinicele and a stone lying just above the santorinicele
DISCUSSION
In Western countries, incomplete PD is uncommon, with a reported incidence of 0.13%-0.9%. However, there is a much higher prevalence of incomplete PD in the recent reports from Japan and Korea, indicating 48% and 52% of PD, respectively.[26,27] Partially, the fluctuation of the frequency of incomplete PD could result from the different techniques employed for the detection of PD, namely the ERCP or MRCP, and even for the same imaging modality, the techniques may be different with time due to intrinsic advances resulting in improved resolution.[26] A prospective study with comprehensive comparison of the imaging of MRCP by an expert radiologist after secretin injection and EUS by expert sonographers after secretin injection may offer the best comparison.
CONCLUSION
EUS is a valuable tool in the diagnosis of PD. Here, the anatomical and technical background is highlighted with explanations of the “stack sign” of linear EUS and the “crossed duct sign,” the dominant duct, VD transition, duct above a duct, short ventral duct/absent ventral duct, separate openings of ducts with no communication, separate openings of ducts with filamentous communication, stacking of Santorini and indirect signs such as santorinicele are highlighted. The techniques and the principles described in the review will be helpful in technical evaluation of PD during EUS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on: www.eusjournal.com
REFERENCES
- 1.Kozu T, Suda K, Toki F. Pancreatic development and anatomical variation. Gastrointest Endoscopy Clin N Amer. 1995;5:1–30. [PubMed] [Google Scholar]
- 2.Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointest Endosc Clin N Am. 1998;8:55–77. [PubMed] [Google Scholar]
- 3.Bret PM, Reinhold C, Taourel P, et al. Pancreas divisum: Evaluation with MR cholangiopancreatography. Radiology. 1996;199:99–103. doi: 10.1148/radiology.199.1.8633179. [DOI] [PubMed] [Google Scholar]
- 4.Morgan DE, Logan K, Baron TH, et al. Pancreasdivisum: Implications for diagnostic and therapeutic pancreatography. AJR Am J Roentgenol. 1999;173:193–8. doi: 10.2214/ajr.173.1.10397125. [DOI] [PubMed] [Google Scholar]
- 5.Agha FP, Williams KD. Pancreasdivisum: Incidence, detection, and clinical significance. Am J Gastroenterol. 1987;82:315–20. [PubMed] [Google Scholar]
- 6.Ng WK, Tarabain O. Pancreas divisum: A cause of idiopathic acute pancreatitis. CMAJ. 2009;180:949–51. doi: 10.1503/cmaj.080446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerke H, Byrne MF, Stiffler HL, et al. Outcome of endoscopic minor papillotomy in patients with symptomatic pancreas divisum. JOP. 2004;5:122–31. [PubMed] [Google Scholar]
- 8.Klein SD, Affronti JP. Pancreasdivisum, an evidence-based review: Part I, pathophysiology. Gastrointest Endosc. 2004;60:419–25. doi: 10.1016/s0016-5107(04)01815-2. [DOI] [PubMed] [Google Scholar]
- 9.Saltzman JR. Endoscopic treatment of pancreas divisum: Why, when and how? Gastrointest Endosc. 2006;64:712–5. doi: 10.1016/j.gie.2006.03.924. [DOI] [PubMed] [Google Scholar]
- 10.Sica GT, Braver J, Cooney MJ, et al. Comparison of endoscopic retrograde cholangiopan-creatography with MR cholangiopancreatography in patients with pancreatitis. Radiology. 1999;210:605–10. doi: 10.1148/radiology.210.3.r99fe55605. [DOI] [PubMed] [Google Scholar]
- 11.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest Endosc. 2001;54:425–34. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 12.Asayama Y, Fang W, Stolpen A, et al. Detectability of pancreas divisum in patients with acute pancreatitis on multi-detector row computed tomography. Emerg Radiol. 2012;19:121–5. doi: 10.1007/s10140-011-1008-x. [DOI] [PubMed] [Google Scholar]
- 13.Mortelé KJ, Rocha TC, Streeter JL, et al. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics. 2006;26:715–31. doi: 10.1148/rg.263055164. [DOI] [PubMed] [Google Scholar]
- 14.Kushnir VM, Wani SB, Fowler K, et al. Sensitivity of endoscopic ultrasound, multidetector computed tomography, and magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: A tertiary center experience. Pancreas. 2013;42:436–41. doi: 10.1097/MPA.0b013e31826c711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnes ML, Romagnuolo J, Cotton PB. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas. 2008;37:151–3. doi: 10.1097/MPA.0b013e318164cbaf. [DOI] [PubMed] [Google Scholar]
- 16.Matos C, Metens T, Devière J. Pancreas divisum: Evaluation with secretin-enhanced magnetic resonance cholangio pancreatography. Gastrointest Endosc. 2001;53:728–33. doi: 10.1067/mge.2001.114784. [DOI] [PubMed] [Google Scholar]
- 17.Manfredi R, Costamagna G, Brizi MG, et al. Pancreas divisum and “santorinicele”: Diagnosis with dynamic MR cholangiopancreatography with secretin stimulation. Radiology. 2000;217:403–8. doi: 10.1148/radiology.217.2.r00nv29403. [DOI] [PubMed] [Google Scholar]
- 18.Wang DB, Yu J, Fulcher AS, et al. Pancreatitis in patients with pancreas divisum: Imaging features at MRI and MRCP. World J Gastroenterol. 2013;19:4907–16. doi: 10.3748/wjg.v19.i30.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai R, Freeman ML, Cass OW, et al. Accurate diagnosis of pancreas divisum by linear-array endoscopic ultrasonography. Endoscopy. 2004;36:705–9. doi: 10.1055/s-2004-825663. [DOI] [PubMed] [Google Scholar]
- 20.Ortega AR, Gómez-Rodríguez R, Romero M, et al. Prospective comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the etiological diagnosis of “idiopathic” acute pancreatitis. Pancreas. 2011;40:289–94. doi: 10.1097/MPA.0b013e318201654a. [DOI] [PubMed] [Google Scholar]
- 21.Catalano MF, Rosenblatt ML, Geenen JE, et al. Pancreatic endotherapy of pancreas divisum (PDIV): Response based on clinical presentation and results of Secretin stimulated endoscopic ultrasound (S:EUS) Gastrointest Endosc. 2001;53:AB133. [Google Scholar]
- 22.Mariani A, Arcidiacono PG, Curioni S, et al. Diagnostic yield of ERC Pandsecretin-enhanced MRCP and EUS in patients with acute recurrent pancreatitis of unknown aetiology. Dig Liver Dis. 2009;41:753–8. doi: 10.1016/j.dld.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Tessier G, Sahai A. A prospective validation of multiple EUS criteria to diagnose or to exclude pancreas divisum: EUS can accurately exclude pancreas divisum, but ERCP Is still required for a definitive diagnosis. Gastrointest Endosc. 2005;61:AB302. [Google Scholar]
- 24.Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol. 2001;96:705–9. doi: 10.1111/j.1572-0241.2001.03609.x. [DOI] [PubMed] [Google Scholar]
- 25.Savides TJ, Gress FG, Zaidi SA, et al. Detection of embryologic ventral pancreatic parenchyma with endoscopic ultrasound. Gastrointest Endosc. 1996;43:14–9. doi: 10.1016/s0016-5107(96)70253-5. [DOI] [PubMed] [Google Scholar]
- 26.Kamisawa T, Tu Y, Egawa N, et al. Clinical implications of incomplete pancreas divisum. JOP. 2006;7:625–30. [PubMed] [Google Scholar]
- 27.Kim MH, Lee SS, Kim CD, et al. Incomplete pancreas divisum: Is it merely a normal anatomic variant without clinical implications? Endoscopy. 2001;33:778–85. doi: 10.1055/s-2001-16521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


