Abstract
Background and Objectives:
Diagnosis of pancreatic lesions remains a clinical challenge. This study aimed to evaluate the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic mass lesions.
Patients and Methods:
Clinical data, laboratory tests, and cytopathological and imaging reports were collected from 185 pancreatic EUS cases performed from March 2010 to January 2014. The final diagnosis was based on surgical findings, EUS-FNA or computed tomography (CT)-guided biopsy.
Results:
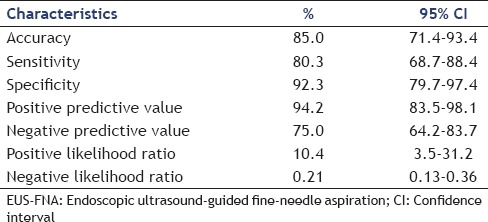
A total of 100 pancreatic FNAs were obtained by EUS. Most positive diagnoses of malignancy were pancreatic adenocarcinomas (n = 61). The site of pancreatic adenocarcinoma was the head in 50 (82.0%), body in seven (11.5%), and tail in four (6.5%). The sensitivity, specificity, and positive and negative predictive values of EUS-FNA for diagnosing adenocarcinoma were 80.3%, 92.3%, 94.2%, and 75.0%, respectively.
Discussion:
We concluded that EUS-FNA of pancreatic lesion accurately diagnoses pancreatic adenocarcinoma and should be considered for the standard management of pancreatic adenocarcinoma.
Keywords: Diagnosis, endoscopic ultrasound-guided fine-needle aspiration, pancreatic neoplasms
INTRODUCTION
Diagnosis of benign and malignant neoplasms of the pancreas is increasing rapidly. Pancreatic cancer is the fourth leading cause of death from cancer.[1,2,3,4,5,6] Imaging modalities have an important role in the diagnosis of pancreatic lesions, especially in pancreatic cancer which is not possible to be reliably diagnosed based on symptoms and signs alone because of the lack of specificity.[7]
Endoscopic ultrasound (EUS) is one of the most sensitive and accurate modality for detecting and evaluating pancreatic mass and staging of pancreatic cancer. EUS gives us high resolution images of the entire pancreas; such as, fine parenchymal details and pancreatic ductal changes.
Usually pancreatic tumors are hypoechoic or inhomogeneous masses or areas with irregular borders within the normal echotexture of the pancreas in EUS views.[8] EUS has been shown to be superior to computed tomography (CT), ultrasound (US), Endoscopic retrograde cholangiopancreatography (ERCP), or angiography in detecting tumors smaller than 3 cm in size.[9]
EUS-guided fine-needle aspiration (EUS-FNA) is the best way for obtaining a histological diagnosis, especially even if the mass is poorly visualized by other imaging modalities.[10]
However, differential diagnosis of pancreatic mass remains a clinical challenge.
The aim of this study was to assess the diagnostic capability of the EUS-FNA in the differentiation of pancreatic mass lesions.
MATERIAL AND METHODS
In this prospective study, a data search was conducted to identify all EUS of pancreatic lesions performed at our hospital from 2010 to 2014. We evaluated diagnostic accuracy of EUS-FNA among all pancreatic mass lesions. Other studies are required to assess diagnostic accuracy of EUS-FNA in particular lesions.
FNA from pancreatic body mass lesion (mixed echoic) has been showed in Figure 1. Those who had undergone FNA of a lesion seen at EUS were evaluated further for the purposes of this study. FNA of peripancreatic lesions, lymph nodes, or bileduct mass lesions were excluded. EUS for guided puncture of the lesion was carried using Olympus equipment (UC 24OP-AL5) and Aloka ProSound color Doppler. The puncture technique was the fanning one (FNA in multiple planes) with an internal stylet, reinserting it before each FNA pass and negative pressure from the beginning till the end of the procedure. The needle size was 22gauge. Seven passes were made for every mass lesion. FNA was done transgastric (body and tail lesions) or transduodenal (head and uncinate process). Onsite cytologist evaluation was not available in these cases.
Figure 1.
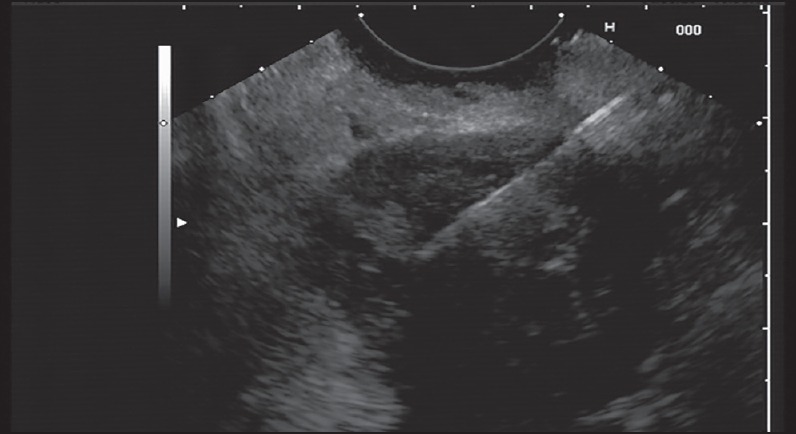
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) from pancreatic body mass lesion (mixed echoic)
After the aspiration needle was withdrawn from the endoscope, the endoscopist immediately washed the aspiration needle in 70% ethanol inside an appropriately labeled screw capped sterile plastic test tube. After transferring to laboratory, the tubes were centrifuged lightly to concentrate the content at the bottom of the tube and processed for cytology evaluation. Then smears and sections of the cell block were evaluated by an expert pathologist for determining the adequacy of specimen (presence of pancreatic cells) and other cytological findings such as the cellularity, necrosis, evidence of fibrosis, and inflammation. EUS-FNA of cystic lesions was done for aspiration of fluid and solid nodules were not seen in these cysts.
For each patient; clinical data, laboratory tests, and cytopathological and imaging reports were collected. Patient characteristics such as age, gender, clinical history, and family history of pancreatic cancer were recorded. Imaging reports including EUS, sonography, CT, and magnetic resonance cholangiopancreatography (MRCP) were reviewed to assess location, size, and characteristics of the pancreatic lesions. All FNAs were performed under EUS guidance or CT. The final diagnosis was based almost on EUS-FNA, but CT-guided biopsy was performed only in five patients. Four patients with a negative EUS-FNA had a positive result for adenocarcinoma on CT-guided biopsy histology. Another case was diagnosed negative for tumor in both EUS-FNA and CT-guided biopsy. Moreover, six patients with a negative EUS-FNA were found to have adenocarcinoma on surgery. All patients provided informed consent before each procedure.
Prism 5 (Graphpad Software, San Diego, CA, USA) software was used for statistical analysis. Intragroup comparison was done using the chi-square test or Fisher's exact test (two-tailed) where appropriate. The results of EUS-guided FNA were compared with the final diagnoses to calculate the accurate sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio of pancreatic adenocarcinoma. Correlation between variables was evaluated by Pearson's correlation analysis. Logistic regression analysis was used to evaluate the correlation between measured variables and the presence of pancreatic adenocarcinoma. Significance was defined at the level of P < 0.05.
RESULTS
Between March 2010 and January 2014, 185 EUS were carried out. Of these patients, 100 underwent FNA of a mass lesion that was seen at the time of EUS. The mean age of these patients was 61.7 ± 13.1 years and 56% of the patients were men. In addition to the EUS-FNA evaluation, some patients were submitted to other diagnostic approaches: 58 sonography, 67 CT, 16 MRCP, and 15 patients underwent surgery.
Seventy-nine (79%) lesions were located in the pancreatic head, 15 (15%) in body, and six (6%) in tail. The general mean size of the lesions was 35.2 ± 5.6 mm. Solid tumors and cystic lesions accounted for 74% and 7% of the cases, respectively. The average size of solid tumors and cystic lesions was 34.4 ± 5.1 and 36.7 ± 3.1 mm, respectively.
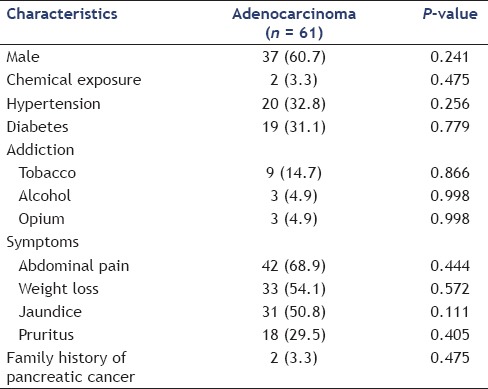
The clinical characteristics of the pancreatic lesions are summarized in Table 1. Most positive diagnoses of malignancy were pancreatic adenocarcinomas (n = 61). The site of pancreatic adenocarcinoma was in the head in 50 (82.0%), body in seven (11.5%), and tail in four (6.5%). The average size of adenocarcinoma was 28.8 ± 10.5 mm and the mean age of patients with pancreatic adenocarcinoma was 62.9 ± 12.9 years. Abdominal pain, weight loss, jaundice, and pruritus were, respectively, most common symptoms of these patients [Table 2]. Positive history of chemical exposure, hypertension (HTN), diabetes, addiction, family history of pancreatic cancer, and gender had no significant association with pancreatic adenocarcinoma (P > 0.05). Using logistic regression only CA-19-9 (P = 0.001, odds ratio (OR; 95% confidence interval (CI)) = 1.003(1.001-1.005)) was an independent predictor of adenocarcinoma in patients with pancreatic lesions. We did not find any other parameter compared against adenocarcinoma to obtain the P-value.
Table 1.
Clinical features of pancreatic lesions (n = 100)
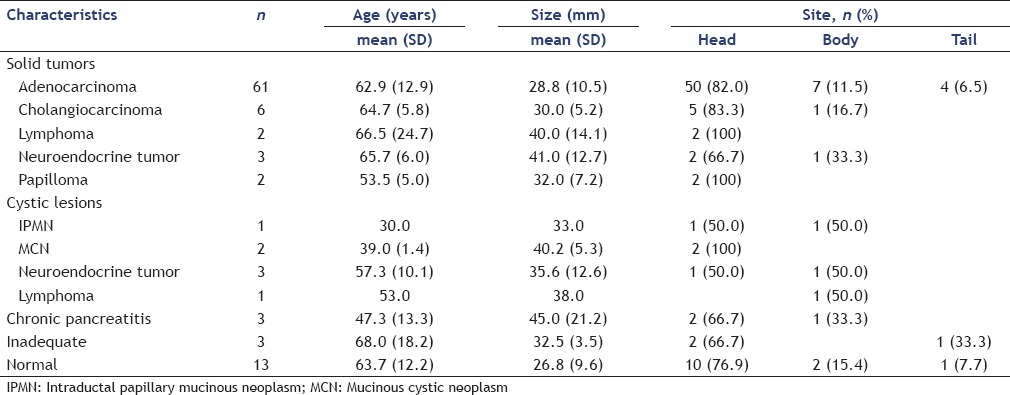
Table 2.
Characterization of patients with pancreatic adenocarcinoma
In an intention-to-treat analysis, EUS-FNA confirmed the final diagnosis in 85 of 100 (85.0%) cases. Overall; the sensitivity, specificity, and positive and negative predictive values of EUS-FNA for diagnosing adenocarcinoma were 80.3%, 92.3%, 94.2%, and 75.0%, respectively [Table 3].
Table 3.
Diagnostic ability of EUS-FNA in pancreatic adenocarcinoma
CT-guided biopsy was performed only in five patients. Four patients with a negative EUS-FNA had a positive result for adenocarcinoma on CT-guided biopsy histology. Another case was diagnosed negative for tumor in both EUS-FNA and CT-guided biopsy. Moreover, six patients with a negative EUS-FNA were found to have adenocarcinoma at surgery.
EUS had significant association with sonography, CT, and MRCP with respect to site (P = 0.045, P < 0.001, and P = 0.037, respectively) and feature (P < 0.001, P = 0.022, and P = 0.031, respectively) of pancreatic tumors. However, the size of lesions in EUS had no significant correlation with sonography (r = 0.237, P = 0.395), CT (r = 0.374, P = 0.127), and MRCP (r = 0.520, P = 0.290).
DISCUSSION
The present study showed that EUS-FNA is a useful diagnostic procedure in the evaluation of pancreatic lesions, especially adenocarcinoma. EUS-FNA was shown to have a high overall accuracy of 85.0% and a high sensitivity and specificity for pancreatic adenocarcinoma.
The diagnosis of pancreatic lesions remains challenging. Histological diagnosis of pancreatic lesions can influence the choice of the best therapeutic approach.[6,7] There is a rapidly accumulating body of literature concerning image-guided FNA of pancreatic lesions. CT or US-guided biopsy of the pancreatic tumors is usually more difficult to undertake due to the retroperitoneal situation of the pancreas.[8,9,10] Moreover, the low sensitivity of these sampling techniques has increased the interest in the use of EUS for pancreatic lesions. EUS-FNA has been shown to be a reliable, low-risk means of providing a tissue diagnosis of pancreatic disease processes with the accuracy of open biopsy. Previous studies have shown the sensitivity, specificity, and positive and negative predictive value as follows: 64%-96%, 80%-100%, 98.4%-100%, 16%-86% for EUS-FNA, respectively.[11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] Our results of pancreas's EUS-FNA are comparable with these findings. In the present study; sensitivity, specificity, and positive and negative predictive values of EUS-FNA for diagnosing adenocarcinoma were 78.4%, 80.3%, 92.3%, 94.2%, and 75.0%, respectively.
False-positive EUS-FNA specimens have been rare.[25,28] Our cases included three cases with an FNA diagnosis of adenocarcinoma in which subsequent resections showed a cholangiocarcinoma, a neuroendocrine tumor and a papilloma. Although EUS-FNA had a high specificity, its negative predictive value was 75.0%, consistent with other studies.[29,30,31] Therefore, a negative result in this situation may be suggestive of benign pathology, but it should not be interpreted as definitely benign disease.
An inadequate specimen was obtained in 3% cases on which FNA was performed. Inadequacy rates for pancreatic FNA have been reported as low as 1.5%-2% for EUS,[13,26] which are in consistent with our study. In cases lacking onsite evaluation, the inadequacy rate was higher (3.5%), but this was not statistically significant. Difficulties in obtaining an adequate specimen can sometimes be because of technical problems in accessing the mass with the FNA needle and this may be exacerbated by the strong inflammatory or fibrotic reaction described in pancreatic tumors.[32] The presence of an adequacy assessment during aspiration minimizes inadequate cellularity of the FNA specimen. However, a negative biopsy, although it strongly suggests benign disease, does not entirely exclude malignancy; and therefore the need for further diagnostic testing or surgical intervention must be considered.
Prior studies reported the usefulness of EUS-FNA to make a diagnosis or to detect malignancy when prior CT-guided biopsies showed negative results.[29,33,34,35] By contrast, in our study four patients with a negative EUS-FNA had a positive result for adenocarcinoma on CT-guided biopsy histology. Horwhat et al.,[36] in a prospective, randomized study demonstrated that EUS-FNA and CT/US-FNA were statistically similar for the diagnosis of pancreatic malignancy. It seems that the difference between performance of EUS-FNA and CT is more likely a partial reflection of available expertise than a reflection of overall test performance.
The major limitation of this study is its inability to provide a direct head-to-head comparison of the two methods (EUS-FNA vs. CT-guided biopsy), as not all tests were performed on all patients. This was not possible for several reasons, not at least the ethical questions that would be raised by performing repetitive biopsies on a patient already diagnosed with malignancy. Another limitation of this study, and indeed of similar studies in this area, is the lack of a clear gold standard with which to compare EUS-FNA. Final diagnosis was assigned from a composite of the EUS-FNA findings themselves, together with surgically obtained histology and CT-guided biopsy. The ideal gold standard would always be a surgical specimen. However, most patients do not undergo surgery (12%), and thus this is impossible in practice.
In conclusion, the present study showed high sensitivity and specificity for EUS-FNA in the diagnosis of pancreatic adenocarcinoma, which may influence the treatment plans of both surgeons and oncologists.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women 1970-2009. J Natl Cancer Inst. 2013;105:1694–700. doi: 10.1093/jnci/djt292. [DOI] [PubMed] [Google Scholar]
- 2.Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: Symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7:189–97. doi: 10.1007/BF02712816. [DOI] [PubMed] [Google Scholar]
- 3.Hunt GC, Faigel DO. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: A review. Gastrointest Endosc. 2002;55:232–7. doi: 10.1067/mge.2002.121342. [DOI] [PubMed] [Google Scholar]
- 4.Powis ME, Chang KJ. Endoscopic ultrasound in the clinical staging and management of pancreatic cancer: Its impact on cost of treatment. Cancer Control. 2000;7:413–20. doi: 10.1177/107327480000700503. [DOI] [PubMed] [Google Scholar]
- 5.Weston BR, Bhutani MS. Optimizing diagnostic yield for EUS-guided sampling of solid pancreatic lesions: A technical review. Gastroenterol Hepatol (N Y) 2013;9:352–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 7.Hunerbein M, Dohmoto M, Haensch W, et al. Endosonography-guided biopsy of mediastinaland pancreatic tumors. Endoscopy. 1998;30:32–6. doi: 10.1055/s-2007-993725. [DOI] [PubMed] [Google Scholar]
- 8.Pinto MM, Avila NA, Criscuolo EM. Fine needle aspiration of the pancreas. A five-year experience. Acta Cytol. 1988;32:39–42. [PubMed] [Google Scholar]
- 9.Rodriguez J, Kasberg C, Nipper M, et al. CT-guided needle biopsy of the pancreas: A retrospective analysis of diagnostic accuracy. Am J Gastroenterol. 1992;87:1610–3. [PubMed] [Google Scholar]
- 10.Welch TJ, Sheedy PF, Jr, Johnson CD, et al. CT-guided biopsy: Prospective analysis of 1,000 procedures. Radiology. 1989;171:493–6. doi: 10.1148/radiology.171.2.2704815. [DOI] [PubMed] [Google Scholar]
- 11.Antillon MR, Chang KJ. Endoscopic and ultrasonography guided fine-needle aspiration. Gastrointest Endosc Clin N Am. 2000;10:619–36. [PubMed] [Google Scholar]
- 12.Baron PL, Aabakken LE, Cole DJ, et al. Differentiation of benign from malignant pancreatic masses by endoscopic ultrasound. Ann Surg Oncol. 1997;4:639–43. doi: 10.1007/BF02303748. [DOI] [PubMed] [Google Scholar]
- 13.Bentz JS, Kochman ML, Faigel DO, et al. Endoscopic ultrasound-guided real-time fine-needle aspiration: Clinicopathologic features of 60 patients. Diagn Cytopathol. 1998;18:98–109. doi: 10.1002/(sici)1097-0339(199802)18:2<98::aid-dc4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Bhutani MS, Hawes RH, Baron PL, et al. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854–8. doi: 10.1055/s-2007-1004321. [DOI] [PubMed] [Google Scholar]
- 15.Chang KJ, Albers CG, Erickson RA, et al. Endoscopic ultrasound-guided fine needle aspiration of pancreatic carcinoma. Am J Gastroenterol. 1994;89:263–6. [PubMed] [Google Scholar]
- 16.Chhieng D, Jhala D, Jhala N, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy: A study of 103 cases. Cancer. 2002;96:233–9. doi: 10.1002/cncr.10714. [DOI] [PubMed] [Google Scholar]
- 17.Di Stasi M, Lencioni R, Solmi L, et al. Ultrasound-guided fine needle biopsy of pancreatic masses: Results of a multi-center study. Am J Gastroenterol. 1998;93:1329–33. doi: 10.1111/j.1572-0241.1998.443_m.x. [DOI] [PubMed] [Google Scholar]
- 18.Eloubeidi MA, Jhala D, Chhieng DC, et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma: Emphasis on atypical, suspicious, and false-negative aspirates. Cancer. 2003;99:285–92. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 19.Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: Results in 141 patients. Endoscopy. 1995;27:171–7. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 20.Palazzo L, Roseau G, Gayet B, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Endoscopy. 1993;25:143–50. doi: 10.1055/s-2007-1010273. [DOI] [PubMed] [Google Scholar]
- 21.Raut CP, Grau AM, Staerkel GA, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118–26. doi: 10.1016/S1091-255X(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 22.Rosch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–52. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 23.Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: The M.D. Anderson Cancer Center experience. Cancer. 2002;96:174–80. doi: 10.1002/cncr.10614. [DOI] [PubMed] [Google Scholar]
- 24.Suits J, Frazee R, Erickson RA. Endoscopic ultrasound and fine needle aspiration for the evaluation of pancreatic masses. Arch Surg. 1999;134:639–43. doi: 10.1001/archsurg.134.6.639. [DOI] [PubMed] [Google Scholar]
- 25.Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–9. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 27.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single-centre experience. Gut. 1999;44:720–6. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell ML, Bittner CA, Wills JS, et al. Fine needle aspiration cytology of the pancreas: A retrospective study of 73 cases. Acta Cytol. 1988;32:447–51. [PubMed] [Google Scholar]
- 29.Eloubeidi MA, Chen VK, Eltoum A, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–8. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 30.Erturk SM, Mortele KJ, Tuncali K, et al. Fine-needle aspiration biopsy of solid pancreatic masses: Comparison of CT and endoscopic sonography guidance. AJR Am J Roentgenol. 2006;187:1531–5. doi: 10.2214/AJR.05.1657. [DOI] [PubMed] [Google Scholar]
- 31.Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: A comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–61. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 32.Kalade A, Desmond PV, Chen RY. Experience of endoscopic ultrasound in an Australian tertiary hospital. ANZ J Surg. 2006;76:1075–80. doi: 10.1111/j.1445-2197.2006.03945.x. [DOI] [PubMed] [Google Scholar]
- 33.Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: A large single-center experience. Gastrointest Endosc. 1999;50:786–91. doi: 10.1016/s0016-5107(99)70159-8. [DOI] [PubMed] [Google Scholar]
- 34.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–91. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 35.Gress F, Gottlieb K, Sherman S, et al. Endoscopic ultrasonography guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459–64. doi: 10.7326/0003-4819-134-6-200103200-00010. [DOI] [PubMed] [Google Scholar]
- 36.Horwhat JD, Paulson EK, McGrath K, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–75. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]


