Abstract
Background:
Nonfunctional pancreatic neuroendocrine tumors (NF-pNETs) are increasingly being diagnosed but management, especially of small tumors, remains a clinical dilemma. Endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) is now routinely used for diagnosis of pancreatic neuroendocrine tumors (pNETs) but has not been well studied as a tool for identifying aggressive disease.
Materials and Methods:
A systematic search of the cytology database identified all patients at our center who underwent EUS-FNA from 1999 through 2011 and were diagnosed with NF-pNET.
Results:
A total of 50 patients were identified. Though patients with metastatic disease had a mean tumor size of 40 mm compared to 25 mm in patients without metastatic disease (P = 0.04), we also identified several patients with tumors <20 mm who presented with metastatic disease. Furthermore, we found no statistically significant difference in metastatic disease between tumors <20 mm and >20 mm (P = 0.13). Using receiver operating characteristic (ROC) analysis, we found that using a cutoff point of 20 mm only led to a sensitivity of 85% in screening for metastases, while lowering the cutoff point to 18 mm allowed for a sensitivity of 95%.
Conclusion:
Currently, guidelines suggest that only patients with tumors greater than 20 mm undergo surgical resection, as tumors less than this size are thought to have low risk of metastases. Our analysis suggests that these recommendations could lead to undertreating patients with small tumors. Tumor size alone may be inadequate as a marker for aggressive NF-pNETs. Given this, other risk factors for aggressive pNETs should be studied to help identify the patients most likely to benefit from surgery.
Keywords: Endoscopic ultrasound (EUS), neuroendocrine tumor (NET), nonfunctional, pancreas, pancreas cancer, pancreatic neuroendocrine tumor (pNET)
INTRODUCTION
Pancreatic neuroendocrine tumors (pNETs) are rare but increasingly diagnosed, with an incidence of 0.1-1.5 cases/100,000 in the USA, and account for roughly 5% of all pancreatic tumors.[1,2,3,4,5,6] Though less aggressive than pancreatic adenocarcinomas, pNETs are not as indolent as previously thought, with a 5-year survival rate of 27%-35% for patients in the USA according to the Surveillance, Epidemiology, and End Results (SEER) database and 43% for patients in Norway.[1,2,7] Surgery is the only curative treatment, and the overall 5-year survival for resected pNETs is estimated at 83%.[8] Diagnosis and management of pNETs remains challenging; review of SEER has shown that survival for all neuroendocrine tumors (NETs) has not improved between 1973 and 2002.[7] Endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) is minimally invasive and may be a valuable tool not only for preoperative diagnosis of pNET, but also for identifying tumors most likely to metastasize.
Little information exists about the oncogenesis of NETs, but they can be part of hereditary syndromes such as multiple endocrine neoplasia type 1 (MEN 1) and von Hippel-Lindau.[9] Many NETs are found incidentally; however, even in patients with symptoms, the time to diagnosis averages 7 years.[10] Nonfunctional pNETs (NF-pNETs) frequently present incidentally or with symptoms secondary to mass effect or after liver metastasis. As a result, they tend to present at a later stage, or at a larger size, and with a higher incidence of metastases compared to functional pNETs.[11] The estimated detection rate of cross-sectional imaging studies for pNETs depends greatly on size, and is roughly 50% for tumors less than 1 cm and greater than 70% for tumors larger than 3 cm.[11,12,13,14] Once a pancreatic lesion is identified, the current approach for lesions of unclear etiology is EUS-FNA, which offers the ability to further characterize the lesion and to sample the lesion for cytology.[15,16,17,18,19,20] Early studies of EUS-FNA demonstrated a sensitivity of 79%-100% for the diagnosis of pNET. Current guidelines recommend a combination of cross-sectional imaging and EUS-FNA for evaluation of suspicious pancreatic lesions.[8,21,22]
Tumor size is a crucial factor in the algorithm of management of NF-pNETs, and TNM staging systems have taken the place of older pathology-based classification systems.[23,24] Both the European Neuroendocrine Tumor Society (ENETS) and the International Union for Cancer Control/American Joint Cancer Committee/World Health Organization (UICC/AJCC/WHO) stratify the primary pNET into less than or greater than 2 cm.[25,26,27,28] Guidelines suggest that patients with tumors greater than 2 cm undergo surgical resection, as these have been shown to have higher rates of metastases.[8,29]
The present study investigates risk factors for metastases of NF-pNETs at the time of EUS-FNA in a tertiary care center. Specifically, we evaluated risk factors for metastatic disease on EUS examination, cytopathology, and patient characteristics to help guide decision-making in patients with NF-pNETs and identify the patients most likely to benefit from surgery.
MATERIALS AND METHODS
Before the initiation of the study, approval from the Institutional Review Board of the Hospital of the University of Pennsylvania was obtained. All patients who underwent EUS-FNA from January 1, 1999 to December 31, 2011 at the University of Pennsylvania and were diagnosed with pNET were identified by interrogation of the cytology database. Inpatient and outpatient electronic and paper medical records were reviewed to assess information that was available regarding demographics, clinical presentation, laboratory findings, radiography, EUS findings, and fine-needle aspiration (FNA) cytology. Relevant imaging reports, including ultrasound, computed tomography (CT) scan, and magnetic resonance imaging (MRI), were reviewed for descriptions of metastatic disease. Endosonographers had access to all clinical data prior to EUS exam. For some patients, detailed clinical data were not available.
All EUS examinations were performed by one of four experienced gastroenterologists with at least 500 prior pancreatic examinations. EUS examinations were performed in the left lateral decubitus position with Olympus GF-UM160 radial side-viewing echoendoscopes at ultrasound frequency of 5-20 MHz and Olympus GF-UC140P-AL5 FNA side-viewing echoendoscopes at ultrasound frequency of 5-10 MHz (Olympus, Center Valley, PA, USA). The endosonographic data recorded included echogenicity, size, margins, lymph node status, status of vessels, cytology and, when available, histology. Number of EUS-FNA passes and selection of biopsy site were up to the discretion of the endosonographer. When tumor size was not recorded on EUS reports, size was gathered from surgical pathology or imaging reports as available. FNA was performed with the Cook EUSN-3 Needle (Cook Medical, Bloomington, IN, USA). A cytopathologist was present on site in most cases to assess for specimen adequacy and to provide preliminary cytology diagnosis when possible; a final cytology diagnosis was later reported with confirmatory stains, and the concordance between the preliminary and the final diagnosis was compared.
Statistical analysis was performed using chi-square tests, Fisher's exact tests, t-tests, and receiver operating characteristic (ROC) curve on the Stata (StataCorp, College Station, TX, USA) software program when appropriate.
RESULTS
A total of 50 patients were found to have NF-pNETs. As seen in Table 1, 29 patients were male and 21 were female; the age range was 35-89 years (mean 60 +/– 12.9). Nineteen patients (38%) presented with either abdominal pain or weight loss or both. Eleven patients (22%) had incidentally found masses, 10 patients (20%) presented with jaundice, and 5 patients (10%) did not have adequate records regarding presentation. Five patients (10%) underwent screening EUS, three of whom had a previous diagnosis of carcinoid syndrome and two of whom had previously confirmed MEN syndrome and were sent for evaluation because of symptoms of gastroesophageal reflux disease (GERD).
Table 1.
Baseline characteristics of the 50 patients with pancreatic neuroendocrine tumor and EUS-FNA, 1999-2011

The average tumor size was 30.8 mm (range 9-124 mm). Nineteen patients had metastatic disease: 9 patients had metastases to the liver and lymph nodes, 5 to the liver alone, and 5 to the lymph nodes alone. The most common primary tumor location in the pancreas was the head (64.7%), followed by the body (19.6%) and the tail (15.7%) by EUS. The most common characteristics on EUS examination were solid and hypoechoic. However, only 12 examinations described the endosonographic image as consistent with pNET.
Preliminary cytology from EUS-FNA was consistent with pNET in 21 of 50 cases (42%). One patient had a peripancreatic lymph node biopsied rather than the primary tumor. Four of the tumors were so large (two estimated at greater than 40 mm, two estimated at greater than 60 mm by EUS) that the EUS operator was not able to identify the margins; the size estimate came from either surgical pathology or MRI. One patient did not have size recorded on EUS or available imaging and did not have surgical pathology available, and was excluded from analyses of tumor size.
Twenty-six (52%) patients underwent surgery, 25 at our institution. Twelve patients underwent Whipple, 9 distal pancreatectomy and splenectomy, and 5 enucleation of the tumor. Of the 23 cases that had surgical pathology available, final cytology from EUS-FNA was consistent with pNET in 22 cases (96%), while surgical pathology was consistent with pNET in all cases. Eleven (21.6%) patients went on to have chemotherapy.
As seen in Table 2, patients with any metastatic disease had a mean tumor size of 40 mm by EUS compared to patients without metastatic disease, mean tumor size 25 mm (P = 0.04). Figure 1 presents a boxplot comparing tumor size between the two groups. There was no statistically significant difference in symptoms between the two groups. Table 3 reveals that there was no significant difference in size by EUS between patients with liver metastases compared to patients without liver metastases (P = 0.07). However, patients with liver metastases were significantly less likely to present incidentally (P = 0.02). Figure 2 compares tumor size between the two groups. When NF-pNETs were divided into tumors <20 mm and >20 mm [Table 4], the cutoff point above which guidelines recommend considering surgery, there was no statistically significant difference in metastatic disease (P = 0.13), and three patients with metastatic disease on presentation had primary tumor size less than 20 mm. In addition, there was no difference in symptoms at presentation in patients with size greater than 20 mm and those with size less than 20 mm.
Table 2.
Comparison of patients with and without metastases to liver or lymph nodes

Figure 1.
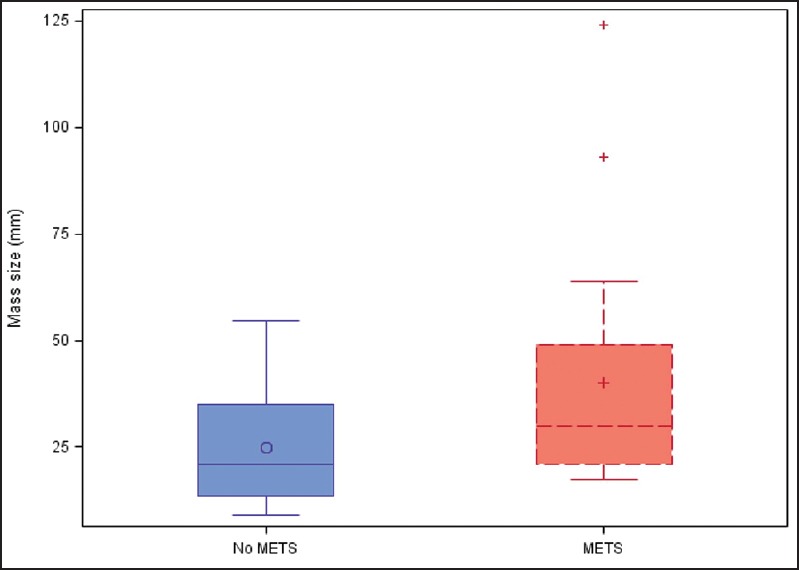
Tumor size in patients with no metastases is compared to tumors size in patients with metastases (liver or lymph node). Quartiles are indicated by the box with the median being the line in the box and the mean being the symbols within the box. The whisker ends are the min and max, excluding the outliers, indicated by a (+)
Table 3.
Comparison of patients with and without liver metastases
Figure 2.
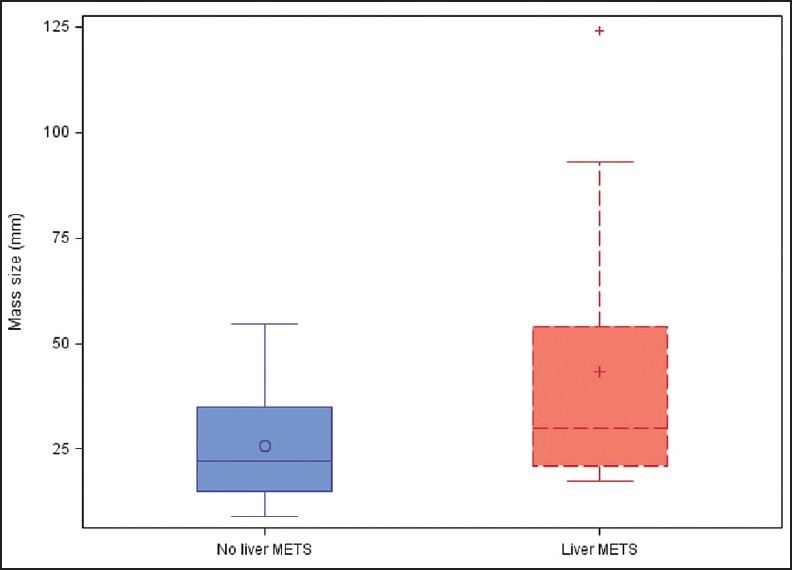
Tumor size in patients with no metastases is compared to tumor size in patients with metastases to liver only. Quartiles are indicated by the box with the median being the line in the box and the mean being the symbols in the box. The whisker ends are the min and max, excluding the outliers, indicated by a (+)
Table 4.
Comparison of tumors <20 mm and >20 mm
An ROC analysis [Figure 3] was performed to determine the sensitivity and specificity of using various tumor size cutoff points above which patients should undergo further evaluation for metastatic disease. We found that using a cutoff point of 18 mm would allow for a sensitivity of 95% and a specificity of 40% for any metastatic disease. At 20 mm, the sensitivity decreased to 85% and the specificity increased to 46.7%. For liver metastases only, an 18-mm cutoff point allowed for a sensitivity of 93% and a specificity of 34%, while the sensitivity decreased to 86% and the specificity increased to 43% at 20 mm.
Figure 3.
ROC curve analysis to determine the sensitivity and specificity of using various tumor size cutoff points above which patients should undergo further evaluation for metastatic disease
DISCUSSION
The average tumor size of 30.2 mm in our study is similar to the tumor sizes seen in recent studies but smaller compared to older studies from the 1980s, where patients with NF-pNETs typically presented with tumors larger than 50 mm.[30,31,32] This may be a result of an increased use of cross-sectional imaging leading to earlier diagnosis of NF-pNETs. Patients in our study had more aggressive disease, with 38% presenting with metastases, compared to 15% of patients in the SEER database.[33] Furthermore, there was no significant decrease in metastatic disease for tumors <20 mm compared to tumors >20 mm; our analysis revealed three patients with metastatic disease on presentation who had primary tumor size less than 20 mm. Two of these patients were not surgical candidates due to the extent of their disease and were treated with systemic chemotherapy.
Our ROC analysis suggests that the current recommendations of using 20 mm as the cutoff point for evaluating for metastatic disease may be relatively insensitive and potentially could lead to undertreating patients with tumors of this size. Though our study is relatively small, it suggests that tumor size alone may be inadequate as a prognostic marker for which patients are more likely to need aggressive management. Given this, other risk factors for aggressive NF-pNETs should be studied to help identify patients most likely to benefit from earlier surgery.
There are some important limitations to our study. The application of this data is unclear in areas where EUS experience is low. Performance of EUS, interpretation of the image to suspect a pNET, and the ability to aspirate sufficient tissue using FNA are all operator-dependent. The reliability of cytology is often dependent on the institution, and bedside interpretation is not available at all centers. Finally, although the goal is to ultimately alter management strategies as appropriate to improve morbidity and mortality, this could not specifically be addressed. The study is a retrospective chart review and it is therefore difficult to make reliable conclusions about outcomes.
EUS-FNA is a valuable diagnostic tool early in the evaluation of a pancreatic mass, especially where the diagnosis is uncertain. In cases where the diagnosis is clear based on endosonographic appearance and other clinical markers and pathology will be available through surgery, EUS-FNA may not be necessary. In some cases, however, differentiating NF-pNET from pancreatic adenocarcinoma is difficult, and surgical and medical management depend on tissue diagnosis. Furthermore, EUS-FNA may play an increasingly valuable role not only in diagnosis but also in disease prognosis as more tumor markers for aggressive disease are identified.[34] Larger studies should continue to analyze the prevalence of metastatic disease at various tumor sizes in order to determine the ideal cutoff point above which tumors can reliably be considered to be aggressive. In addition, other molecular and cytological markers for aggressive disease should be studied.
Financial support and sponsorship
Drs. Ende, Sedarat, Shah, Jhala, Fraker, Drebin, Metz, and Kochman have no financial ties to disclose.
Conflicts of interest
Drs. Ende, Sedarat, Shah, Jhala, Fraker, Drebin, Metz, and Kochman have no conflicts of interest.
REFERENCES
- 1.O’Grady HL, Conlon KC. Pancreatic neuroendocrine tumours. Eur J Surg Oncol. 2008;34:324–32. doi: 10.1016/j.ejso.2007.07.209. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 4.Batcher E, Madaj P, Gianoukakis AG. Pancreatic neuroendocrine tumors. Endocr Res. 2011;36:35–43. doi: 10.3109/07435800.2010.525085. [DOI] [PubMed] [Google Scholar]
- 5.Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumours of the pancreas: Analysis of autopsy cases. Dig Dis Sci. 2004;26:933–42. doi: 10.1007/BF01297144. [DOI] [PubMed] [Google Scholar]
- 6.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–81. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: Contrasting Norway and North America. Cancer. 2008;113:2655–64. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 8.Ramage JK, Davies AH, Ardill J, et al. UKNETwork for Neuroendocrine Tumours. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54(Suppl 4):iv1–16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon E, Pasieka JL. Functioning and nonfunctioning neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2007;19:30–5. doi: 10.1097/CCO.0b013e328011a236. [DOI] [PubMed] [Google Scholar]
- 10.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 11.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–92. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noone TC, Hosey J, Firat Z, et al. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005;19:195–211. doi: 10.1016/j.beem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Rockall AG, Reznek RH. Imaging of neuroendocrine tumours (CT/MR/US) Best Pract Res Clin Endocrinol Metab. 2007;21:43–68. doi: 10.1016/j.beem.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Kulke MH, Lowell AB, Bushnell DL, et al. North American Neuroendocrine Tumor Society (NANETS). NANET Treatment Guidelines: Well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–52. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757–73. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power N, Reznek RH. Imaging pancreatic islet cell tumours. Imaging. 2002;14:147–59. [Google Scholar]
- 17.Anderson MA, Carpenter S, Thompson NW, et al. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271–7. doi: 10.1111/j.1572-0241.2000.02480.x. [DOI] [PubMed] [Google Scholar]
- 18.Ardengh JC, de Paulo A, Ferrari AP. EUS-guided FNA in the diagnosis of pancreatic neuroendocrine tumors before surgery. Gastrointest Endosc. 2004;60:378–84. doi: 10.1016/s0016-5107(04)01807-3. [DOI] [PubMed] [Google Scholar]
- 19.Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–9. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker MS, Knuth JL, DeWitt J, et al. Pancreatic cystic neuroendocrine tumors: Preoperative diagnosis with endoscopic ultrasound and fine-needle immunocytology. J Gastrointest Surg. 2008;12:450–6. doi: 10.1007/s11605-007-0219-7. [DOI] [PubMed] [Google Scholar]
- 21.Rosch T, Braig C, Gain T, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188–99. doi: 10.1016/0016-5085(92)91800-j. [DOI] [PubMed] [Google Scholar]
- 22.Fein J, Gerdes H. Localization of islet cell tumors by endoscopic ultrasonography. Gastroenterology. 1992;103:711–2. doi: 10.1016/0016-5085(92)90877-2. [DOI] [PubMed] [Google Scholar]
- 23.Bosman FT, Carneiro F, Hruban RH, et al. Lyon: International Agency for Research on Cancer (IARC); 2010. WHO Classification of Tumours of the Digestive System. [Google Scholar]
- 24.Hamilton SR, Aaltonen LA. 3rd ed. Lyon: International Agency for Research on Cancer (IARC); 2000. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. [Google Scholar]
- 25.Ekeblad S, Skogseid B, Dunder K, et al. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798–803. doi: 10.1158/1078-0432.CCR-08-0734. [DOI] [PubMed] [Google Scholar]
- 26.Jann H, Roll S, Couvelard A, et al. Neuroendocrine tumors of midgut and hindgut origin: Tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332–41. doi: 10.1002/cncr.25855. [DOI] [PubMed] [Google Scholar]
- 27.Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: Improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–33. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 28.Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: Results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–77. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 29.Sutton R, Doran HE, Williams EM, et al. Surgery for midgut carcinoid. Endocr Relat Cancer. 2003;10:469–81. doi: 10.1677/erc.0.0100469. [DOI] [PubMed] [Google Scholar]
- 30.Atiq M, Bhutani M, Bektas M, et al. EUS-FNA for pancreatic neuroendocrine tumors: A tertiary cancer center experience. Dig Dis Sci. 2012;57:791–800. doi: 10.1007/s10620-011-1912-7. [DOI] [PubMed] [Google Scholar]
- 31.Kent RB, 3rd, van Heerden JA, Wheiland LH. Nonfunctioning islet cell tumors. Ann Surg. 1981;193:185–90. doi: 10.1097/00000658-198102000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson GB, van Heerden JA, Grant CS, et al. Islet cell carcinomas of the pancreas: A twenty-year experience. Surgery. 1988;104:1011–7. [PubMed] [Google Scholar]
- 33.Martin RC, Kooby DA, Weber SM, et al. Analysis of 6,747 pancreatic neuroendocrine tumors for a proposed staging system. J Gastrointest Surg. 2011;15:175–83. doi: 10.1007/s11605-010-1380-y. [DOI] [PubMed] [Google Scholar]
- 34.Wei IH, Harmon CM, Arcerito M, et al. Tumor-associated macrophages are a useful biomarker to predict recurrence after surgical resection of nonfunctional pancreatic neuroendocrine tumors. Ann Surg. 2014;260:1088–94. doi: 10.1097/SLA.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]


