Abstract
Pancreatic cancer (PC) is a highly lethal cancer. Despite a significant advancement in cancer treatment, the mortality rate of PC is nearly identical to the incidence rates. Early detection of tumor or its precursor lesions with dysplasia may be the most effective approach to improve survival. Screening strategies should include identification of the population at high risk of developing PC, and an intense application of screening tools with adequate sensitivity to detect PC at an early curable stage. Endoscopic ultrasound (EUS) and magnetic resonance imaging (MRI) seem to be the most promising modalities for PC screening based on the data so far. EUS had an additional advantage over MRI by being able to obtain tissue sample during the same examination. Several questions remain unanswered at this time regarding the age to begin screening, frequency of screening, management of asymptomatic pancreatic lesions detected on screening, timing of resection, and extent of surgery and impact of screening on survival. Novel techniques such as needle-based confocal laser endomicroscopy (nCLE), along with biomarkers, may be helpful to identify pancreatic lesions with more aggressive malignant potential. Further studies will hopefully lead to the development of strategies combining EUS with other technological/biological advancements that will be cost-effective and have an impact on survival.
Keywords: BRCA, cancer, endoscopic ultrasound (EUS), familial atypical multiple mole melanoma (FAMMM) syndrome, familial pancreatic, fine-needle aspiration (FNA), intraductal papillary mucinous neoplasm (IPMN), pancreatic cancer (PC), pancreatic intraepithelial neoplasia-3 (PanIN3), Peutz-Jeghers syndrome (PJS), screening
BACKGROUND
Pancreatic cancer (PC) is a highly lethal cancer, which ranks fourth among the cancer-related deaths in the United States.[1] It is estimated that about 48,960 people (24,840 men and 24,120 women) will be diagnosed with PC and about 40,560 people (20,710 men and 19,850 women) will die of PC in 2015.[2] The incidence has been steadily rising over the years and it is projected to become the second leading cause of cancer deaths by 2030.[3] Despite significant advancement in cancer treatment, the mortality rate of PC is nearly identical to the incidence rates. The prognosis remains grim with a 5-year survival rate of about 6%.[4] Main reasons for poor prognosis of PC are delayed diagnosis at late stage and the disease not being cured even with resection. Treatments for metastatic PC are minimally effective, and even with the most advance chemotherapy regimen, the median overall survival is 8.5 months.[5]
Surgical resection is the only curative option for PC but only 15%-20% of the patients are eligible for resection at the time of initial presentation.[6,7] A good proportion of patients (15%-50% depending on the imaging modality used) who were thought to be resectable on imaging are deemed inoperable as they have evidence of metastatic or locally advanced disease.[8,9,10] Even among patients who underwent curative resection, 30% have positive margins.[11] Factors identified as having favorable prognostic significance are negative resection margins, tumor size less than 3 cm, well or moderate tumor differentiation, and postoperative chemoradiation.[11] Surgery is considered palliative in most of the resections as it prolongs median survival by about 14-22 months but 5-year survival remains low at 10%-20%.[7,10] On the other hand, resection of small pancreatic tumors (defined as tumors <2 cm in size or T1 on TNM classification) improves 5-year survival ranging 30%-60%.[12,13,14] Curable PC by definition are small tumors, which are <1 cm in size or well-differentiated stage I cancers for which the 5-year survival rate after resection is as high as 75%.[15,16] All these facts suggest that the early detection of tumor or its precursor lesions with dysplasia may be the most effective approach to improve survival.
PROGRESSION OF PANCREATIC CANCER
PC seems to evolve in a stepwise progressive manner from normal pancreatic ductal epithelium to infiltrative carcinoma.[17] In most cases, patients experience minimal/no symptoms until the tumor grows to a locally unresectable stage. The initial stage in the evolution of PC is called carcinoma in situ [intraductal carcinoma or pancreatic intraepithelial neoplasia-3 (PanIN3)]; patients are asymptomatic and traditional imaging studies are normal. The next stage is called minute PC, defined as tumor <1 cm in size. Patients do not experience any symptoms at this stage also and about half of them may have pancreatic duct dilation. The third stage is called small PC, defined as tumor <2 cm in size. At this stage also, patients generally have no symptoms (unless the tumor is close to the bile duct causing early obstructive jaundice) and many of them already have extra pancreatic spread. Eventually, the tumor evolves into large PC, which is defined as mass >2 cm in size, usually symptomatic, and visible on imaging. Only a proportion of these tumors are resectable at this stage.
There are three known histologically well-defined precursor lesions involved in pancreatic carcinogenesis called pancreatic intraepithelial neoplasms (PanINs), intraductal papillary mucinous neoplasms (IPMNs), and mucinous cystic neoplasms (MCNs).[18] PanINs are microscopic flat or papillary, noninvasive epithelial neoplasms confined to pancreatic ducts and measure <5 mm in diameter. They are further classified into three grades based on cytological and architectural atypia.[19] PanIN-1 are lesions with minimal atypia, which are further divided into flat (PanIN-1A) and papillary types (PanIN-1B).[19] PanIN-2 lesions have moderate atypia with some nuclear abnormalities such as loss of polarity, nuclear crowding, enlarged nuclei, pseudostratification, and hyperchromasia. PanIN-3 lesions, also called as intraductal carcinoma or carcinoma in situ, are characterized by the presence of significant architectural and/or cytologic atypia. Strong evidence supports the facts that some of the invasive PCs that arise in patients with family history of PC evolve from PanIN lesions and sporadic PanIN3 lesions almost always progress to invasive cancer.[19]
ROLE OF ENDOSCOPIC ULTRASOUND IN DIAGNOSIS OF PANCREATIC CANCER
Endoscopic ultrasound (EUS)-fine-needle aspiration (FNA) has become an essential tool for the evaluation of pancreatic lesions. Since its first use in the early 1990s, it has evolved into an efficient technique with good safety profile and high diagnostic accuracy ranging 80%-90%.[20,21,22,23] A recent meta-analysis performed by Puli et al. to evaluate the accuracy of EUS-FNA in making diagnosis of solid pancreatic masses showed the sensitivity of EUS to be 86.8%, specificity of 95.8%, positive likelihood ratio of 15.2, and the negative likelihood ratio of 0.17.[24] EUS has been shown to have better accuracy in diagnosing pancreatic tumors than conventional computed tomography (CT) with greater sensitivity and specificity, particularly for small tumors.[25,26] EUS has proven to be particularly useful in patients with clinical suspicion of PC (pancreatic duct dilation) with no definitive mass seen on CT scan, especially when tumors are <2 cm in size.[27] In that study the sensitivity, specificity, positive predictive value (PPV), and accuracy of EUS-FNA to identify such masses was reported to be 87%, 98%, 98%, and 92%, respectively. EUS has also been proven very useful in the diagnosis of pancreatic neuroendocrine tumors (PNETs), especially for lesions that are small in size (<2 cm).[28] In one study, EUS was able to detect 91% of the PNETs that were missed on CT scan.[28] EUS also helps in characterization and differentiation of the tumors with its ability to perform FNA.[29] A meta-analysis to assess the diagnostic accuracy of EUS-FNA to diagnose PC was performed by Chen et al., which showed EUS-FNA to be a test with high sensitivity and specificity. The pooled sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio in that study were 89%, 96%, 16.88, and 0.13, respectively.[30] EUS is not only the best tool for tissue confirmation of PC but also helpful for preoperative staging.[31] Overall, the accuracy of EUS for T and N staging was 85% and 72% and for CT was 30% and 55%, respectively.
RISK FACTORS FOR PANCREATIC CANCER
Multiple risk factors have been associated with PC such as male gender, obesity, black race, Ashkenazi Jewish descent, smoking, diabetes mellitus, and high calorie intake.[32] Cigarette smoking is the most important environmental factor associated with PC and smokers have twofold increased risk of developing PC than nonsmokers and accounts for 25% of all PCs.[33] Smoking also adds to the increased risk of PC.[34] Apart from these, there are hereditary factors, which constitute patients called high-risk individuals (HRIs). A role for hereditary factors is suggested by the fact that around 10% of the patients have a positive family history of PC.[35,36] HRIs are individuals with strong family history of PC with no known hereditary syndrome (familial PC), inherited PC syndromes, and those carrying known genetic mutation such as BRCA2.[14] Other hereditary conditions that have low to moderate risk of predisposition to PC development are familial adenomatous polyposis, HNPCC, BRCA1 carrier, hereditary pancreatitis, and cystic fibrosis.
Familial pancreatic cancer (FPC) kindreds are defined as families with two or more first-degree relatives (FDRs) affected with PC without accumulation of other cancers or familial diseases. FPC is inherited in an autosomal dominant pattern in 58%-80% of families. Studies have shown that a considerable number of patients (approximately 10%) have a positive family history of disease and the degree of risk increases with the number of affected FDRs.[10] In a study conducted by Klein et al., the risk of developing PC was 4.5-fold versus 32-fold depending on the number of affected FDRs (single affected FDR vs. three or more affected FDRs).[37] It is anticipated that the younger generation of patients in this group develop the disease earlier than their affected parents.[38] Risk is also particularly high for individuals from families with a case of young-onset PC (age <50 years) in the kindred.[36] Some genetic mutations such as BRCA2 have been associated with some cases of FPC but most of these have no known genetic etiology.[39] Wang et al. proposed a Mendelian prediction model called PancPRO, which statistically assesses the probability of an individual to possess the genetic mutation responsible for PC development and the risk of developing PC in a future based on the individual's family history.[40]
Patients with inherited cancer syndromes such as familial atypical multiple mole melanoma (FAMMM) syndrome, Peutz-Jeghers syndrome (PJS), and hereditary pancreatitis constitute another important entity of HRIs. FAMMM is an autosomal dominant syndrome with multiple nevi, atypical nevi, and multiple melanomas. A subset of patients with this syndrome harbors mutations in CDKN2A gene (p16), which is found to be associated with PC.[41] The estimated cumulative risk of developing PC in CDKN2A carriers was reported to be 17%.[42]
PJS is an autosomal dominant disease characterized by hamartomatous gastrointestinal polyps and mucocutaneous pigmentation. These patients have mutation of STK11/LKB1 gene, which predisposes them to these neoplasms including PC. They have a markedly increased risk for PC with a relative risk of 132 and cumulative risk of 36% in the age of 15-64 years.[39,43]
Hereditary pancreatitis is also an autosomal dominant disorder associated with cationic trypsinogen gene (PRSS1) mutation. The cumulative risk of PC in various studies was reported to be 60-100-fold with a lifetime risk of 40%.[44,45] Smokers had nearly twofold increased risk for PC and they developed it 20 years earlier than nonsmokers in this cohort as well, which is in line with the findings from the general population.[46]
ROLE OF ENDOSCOPIC ULTRASOUND AND OTHER IMAGING STUDIES IN SCREENING HIGH-RISK INDIVIDUALS
The most commonly studied imaging modalities for PC screening in HRIs are EUS [Table 1], MRI/magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), and CT. Brentnall et al. reported the data on using imaging studies, along with clinical data, to identify dysplasia in patients with family history of PC.[35] Fourteen patients with >2 members in >2 generations with history of PC were included in this study. EUS, ERCP, CT, and CA 19-9 and carcinoembryonic antigen (CEA) assessments were performed in all patients. Seven out of 14 patients had abnormal finding on EUS and ERCP although the changes were nonspecific. Those patients were referred for pancreatectomy and all of them had histological evidence of dysplasia in the surgical specimen. CT and tumor markers were not helpful to identify the dysplastic lesions. The problem with identifying dysplasia on EUS imaging is that the changes are nonspecific and may be seen in other benign conditions such as chronic pancreatitis, increasing age,[47] asymptomatic alcohol abusers, and/or heavy alcohol consumption with concomitant smoking.[48,49]
Table 1.
The variable yield of EUS abnormalities during pancreatic cancer screening in high-risk individuals in 10 studies
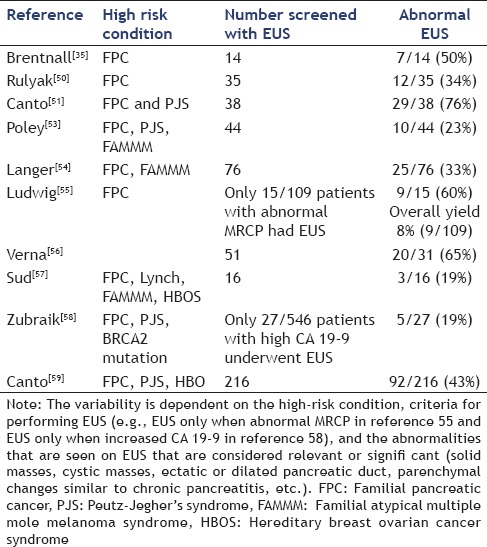
Rulyak et al. described their experience with screening HRIs.[50] They recruited 35 patients from 13 FPC kindreds and performed EUS in all of them. An abnormal EUS was followed by ERCP for further evaluation. A total of 12/35 patients (34.3%) were noted to have abnormal findings on EUS and ERCP and all of them underwent pancreatectomy. All 12 surgical specimens were identified as having pancreatic dysplasia (precursor lesion of PC) on histopathologic examination. None of the resected patients had PC at the time of resection or during the 48-month follow-up. They concluded that using EUS and ERCP for screening of HRI helps to identify precursor lesions prior to the onset of full blown cancer.
Canto et al. performed a pilot study in 38 asymptomatic HRIs (FPC:37 and PJS:1) to evaluate the feasibility of screening.[51] All patients underwent initial evaluation with EUS and abnormal EUS examination was followed by CT (in all patients) and ERCP (offered to all patients). EUS showed abnormal findings in 29 patients (76%) with six of them visualized as pancreatic masses. The diagnostic yield of EUS was 5.3% for detecting two clinically significant pancreatic neoplasms [one invasive pancreatic ductal adenocarcinoma (PDAC) and one IPMN]. Of note, the patient with adenocarcinoma remained disease-free >5 years after surgery.
Another larger study was performed by Canto et al. involving 78 HRIs (FPC:72 and PJS:6) using EUS and CT for screening.[52] Both examinations were performed at the baseline and 12-month interval and all abnormal EUS examinations were followed by EUS-FNA and ERCP. Surgery was offered to patients with potential neoplastic lesions. Eight patients with pancreatic neoplasms (six benign IPMN, one IPMN progressed to cancer, and one panIN) were identified by screening (diagnostic yield: 10%). EUS correctly diagnosed 7/8 pathologically confirmed malignancy and CT missed two of those lesions, which were small in size (<2 cm). As shown in their earlier study, there was a high prevalence of abnormalities suggestive of chronic pancreatitis in HRIs. They recommended screening HRI with EUS and CT to identify asymptomatic neoplasms and IPMN to be considered a phenotype of FPC.
Poley et al. investigated the role of EUS for first-time screening of HRIs (FPC, PJS, and FAMMM).[53] EUS imaging was abnormal in 10 patients (23%) and all abnormal EUS examinations were followed with MRI and/or CT scan. Three patients had mass lesions and all three of them were resected. Pathology from all these three lesions showed adenocarcinoma and one of the mass lesions was not detected on CT or MRI. EUS detected branch duct IPMN in seven patients. These IPMNs presenting as cystic lesions are identified at a higher frequency in HRI but the biological behavior of these lesions in HRI is still not clear. Overall, the yield of first-time EUS screening for identifying asymptomatic cancer was 7% and precursor lesions such as IPMN was 16%.
Langer et al. performed a prospective study to evaluate the yield of prospective screening in high-risk patients.[54] They enrolled 76 patients from families with FPC and FAMMM who underwent a total of 182 examinations during the 5-year time period. All patients underwent EUS, MRI, and MRCP. The screening tests detected abnormalities in 28 patients [abnormal EUS (N = 25) and/or abnormal MRI/MRCP (n = 12)]. Pancreatic resections were performed in seven patients and pathology revealed serous adenoma (n = 3), panIN 1 (n = 1), panIN 2 (n = 1), and IPMN (n = 1). They concluded that screening can detect precursor lesions but the yield was low as they detected low-risk precursor lesions for which carcinogenic progression potential is still unknown.
Ludwig et al. reported on the yield of screening at-risk relatives of familial PC.[55] All patients first underwent MRCP followed by EUS for abnormal examinations. The initial screening with MRI was abnormal in 18/109 patients (5%) and follow-up EUS confirmed the abnormality in nine patients with an overall diagnostic yield of 8.3%. The yield was significantly greater in individuals >65 years. Six patients underwent resection after EUS-FNA and pathology was the main duct IPMN (n = 2), PanIN 2 (N = 1), panIN 3 (N = 1), and adenocarcinoma (N = 1).
Verna et al. performed a study to evaluate the efficacy of screening programs in HRI.[56] A total of 51 patients were enrolled and screening with EUS; MRI and genetic testing were offered based on patients’ risk (high-risk patients screened with all three modalities). EUS imaging was abnormal in 20/31 patients with identification of two adenocarcinoms (resectable-1 and metastatic-1). Overall, six (12%) of the 51 patients had pancreatic neoplasia detected on screening. They concluded that comprehensive screening can identify curable neoplasms, which could be potentially resected.
Sud et al. in their study showed the significance of screening in patients with hereditary pancreatic syndromes with EUS.[57] A total of 30 patients were identified after genetic counseling to be at high risk of developing PDAC (lifetime risk of 5% or more) and 16 of them underwent EUS. Three patients had abnormalities detected on EUS (diagnostic yield: 19%), which led to further evaluation with EUS-FNA. The pathology results from those patients yielded adenocarcinoma. They suggested using genetic counseling to identify the most appropriate patients who would need screening and using EUS and/EUS-FNA for screening.
Zubarik et al. evaluated the role of EUS, along with CA 19-9 to identify early pancreatic neoplasia.[58] All patients included in that study were tested for CA 19-9 and those with elevated levels were evaluated with EUS-FNA. A total of 546 patients were enrolled and 27 (4.9%) of them had elevated CA 19-9. EUS was able to identify premalignant/malignant lesions in five patients (0.9%) and one of them had PDAC (0.2%). They concluded that using this protocol of CA 19-9 followed by EUS can diagnose early PDAC, which can potentially be cured by resection.
A multicenter prospective cohort study (CAPS 3) was performed by Canto et al. where they included three groups of HRIs (PJS patients n = 2, familial breast -ovarian cancer patients with at least one affected first- or second-degree relative with PC n = 19 and relatives of patients with FPC with at least two FDRs n = 195).[59] All patients underwent CT, MRI, and EUS evaluation and 42% (92/216) were found to have at least one pancreatic mass (84 cystic and 3 solid) or dilated pancreatic duct (n = 5) by one of the imaging modalities. Prevalence of these lesions increased with age. CT, MRI, and EUS detected pancreatic abnormality in 11%, 33.3%, and 42.6% of the patients, respectively. Out of all pancreatic lesions, 82 were IPMNs and three neuroendocrine tumors. Five patients underwent surgery and three of them had high grade dysplasia in <3 cm IPMNs and multiple intraepithelial neoplasms. They concluded that screening of asymptomatic HRI could detect curable noninvasive high-grade lesions alongside the detection of multiple cystic lesions. EUS and MRI were better diagnostic tests for screening HRI than CT.
There is variation in the prevalence and behavior of precursor lesions in high-risk groups [Table 1]. The prevalence of pancreatic cysts detected on MRI among the general population is reported to be 2.4%.[60] Potjer et al. compared the incidence of cystic lesions among two different groups of HRIs who were patients from FPC families (N = 125) and individuals with P16 Leiden germline mutation (N = 116).[61] Surveillance was performed annually with MRI and MRCP with/without EUS for a median period of 36 months. The prevalence of cystic lesions and specifically IPMN was high in the FPC cohort in comparison to the P16 Leiden cohort (42% vs. 16% and 42% vs. 28.5%, respectively). However, the prevalence of PDAC was greater in the P16 Leiden cohort (7% vs. 0.8%). This shows that the progression of cystic lesions to PDAC is variable among different groups of HRIs. They recommended using more strict surveillance for patients who are among those high-risk groups (e.g., P16 Leiden carriers).
The concept of a field effect is applicable to the risk of PC as individuals with IPMN are not only at risk of developing adenocarcinoma within the IPMN but also at risk of developing PDAC in another part of the pancreas away from the IPMN. In a study performed by Uehara et al. PDAC distinct from IPMN developed in five of 60 (8%) branch duct IPMNs during follow-up. The standardized incidence ratio of development of PDAC was 26 [95% confidence interval (CI) 3-48].[62]
SCREENING GUIDELINES
Screening for PC in the general population is not cost-effective as it accounts for an overall low incidence with 3% new cases each year and a lifetime risk of 1.3% in the United States.[63,64] It is estimated that 5%-10% of PCs arise as a result of genetic susceptibility and/or familial aggregation.[51,65] A 2007 consensus conference on inherited diseases of the pancreas proposed that screening for PC be restricted to individuals with a >10-fold increased risk of the disease.[66] An International Cancer of the Pancreas Screening (CAPS) Consortium was formed in 2010 with an objective to develop statements on screening, surveillance, and management of HRIs with an inherited predisposition to PC.[64] The group has recommended screening for the following individuals who are at high risk for disease: FDRs of patients with PC from a familial PC kindred with at least two affected FDRs; patients with PJS; p16, BRCA2, and hereditary nonpolyposis colorectal cancer (HNPCC) mutation carriers with >1 affected FDR. No consensus was reached on the age to initiate screening or stop surveillance. They suggested using EUS and/or MRI as screening tools and also to preclude using CT or ERCP for screening. They recommended using the same imaging studies for surveillance also. Although a consensus was not reached, most of the participants agreed on a screening interval of 6-12 months for nonsuspicious cysts and follow-up in 3 months for newly detected indeterminate solid lesion and indeterminate main pancreatic duct stricture. All participants agreed on resections to be performed only at high volume specialty centers but disagreed on which screening abnormalities were of sufficient concern for surgery to be recommended. The consortium concluded that the evidence supporting the screening and surveillance in HRI was limited and management of these patients should be done using an individualized approach using multidisciplinary programs. It is probably better to refer these HRIs to a center that is conducting the screening studies regularly or a center, which has ongoing trials addressing this issue [Figure 1].
Figure 1.
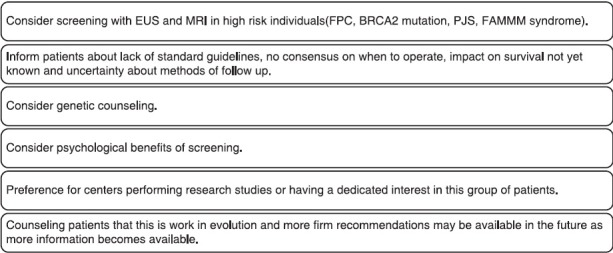
Considerations and key points regarding pancreatic cancer screening
PSYCHOLOGICAL ASPECTS
Individuals who belong to high-risk groups experience a lot of cancer-related anxiety and emotional distress from the loss of their close family members. The success of the screening programs not only depends on the technical feasibility of the procedures but also on the willingness of those individuals to participate in surveillance programs. In one study, genetic counseling for FPC was found to be helpful to more than 90% of the individuals at high risk despite the inability to identify a causative gene for PC.[67] Another study showed that patients who participated in a screening program did not experience significant increase in risk perception or cancer worry and in fact benefited from comprehensive risk assessment.[68] Harinck et al. studied the psychological impact of PC surveillance programs.[69] A significant proportion of patients perceive their risk of developing cancer to be much higher than the general population (58%) and most of them participate in surveillance hoping that cancer might be detected at an early stage. More than 80% of the participants thought that the advantages of surveillance outweighed the risks. EUS and MRI were the screening modalities used and EUS was not perceived as burdensome due to the invasiveness of the procedure. Overall surveillance programs for PC in HRIs seem to be feasible from psychological standpoint by decreasing cancer-related intrusive thoughts and cancer worry.
EMERGING AND FUTURE CONCEPTS
Molecular markers such as cathepsin E are overexpressed in PDAC and PanINs and the expression increases with progression of the disease. Identification of these markers may have a promising role in the monitoring of PDAC in a high-risk population.[70] Studies have shown that mutations in genes such as TP 53 are identified in increasing frequency in high-grade dysplasia and PC.[71] This could have a potential role in the identification of PC in high-risk groups. Other molecular markers such as DNA, mRNA, and microRNA can be assessed from EUS-FNA samples of pancreatic lesions, which might improve the diagnosis of PC.[72,73]
Japanese studies on EUS imaging follow-up of IPMNs have shown value in detecting early adenocarcinomas derived from IPMNs and concomitant with IPMNs.[74,75] Novel biomarkers in pancreatic cyst fluid collected by EUS have the potential to help predict which cystic lesions in the pancreas will likely progress to cancer. Mutations like K-ras can predict the aggressiveness of pancreatic cysts as patients with mutated K-ras were found to have cellular atypia on histopathology (mutated k-ras vs. wild type: 39% vs. 14%) and also elevated CEA levels.[76] A study by Kung et al. had shown that genetic analysis when used along with EUS- FNA and fluid CEA could be helpful to predict the biological behavior of pancreatic cysts.[77] Another potential marker is SPINK1, which can be extracted from the cyst fluid and can be used to differentiate benign (serous cystadenomas) from potentially malignant lesions (IPMN and mucinous cysts).[78] EUS-guided needle-based confocal laser endomicroscopy (nCLE) is another novel technique that has come into existence. The nCLE device has a miniprobe, which can be passed through an EUS-FNA needle that allows real time visualization of tissues at a microscopic level. This device has been used to differentiate pancreatic cystic lesions and the results thus far have been promising. The presence of villous structures on nCLE imaging is pathognomic for IPMN despite nondiagnostic histology.[79,80] Imaging of solid pancreatic lesions with EUS-guided nCLE is promising but needs more work. Combining nCLE with molecular markers such as cathepsin E may help predict which cysts or lesions in HRIs are likely to behave aggressively to need interventions such as surgery.
CONCLUSION
Screening strategies should include identification of the population at risk of developing PC, and an intense application of screening tools with adequate sensitivity to detect PC at an early, curable stage. EUS and MRI seem to be the most promising modalities for PC screening based on the data so far. EUS had an additional advantage over MRI by being able to obtain tissue sample during the same examination. Several questions remain unanswered at this time regarding the age to commence screening, frequency of screening, management of asymptomatic pancreatic lesions detected on screening, timing of resection, and extent of surgery, and impact of screening on survival. Novel techniques such as nCLE, along with biomarkers, may be helpful to identify pancreatic lesions with more aggressive malignant potential. Further studies will hopefully lead to the development of strategies combining EUS with other technological and biological advancements that will be cost-effective and have an impact on survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. 2015. [Last accessed on 2015 Mar 21]. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf .
- 3.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancuso A, Calabro F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58:231–41. doi: 10.1016/j.critrevonc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–9. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maire F, Sauvanet A, Trivin F, et al. Staging of pancreatic head adenocarcinoma with spiral CT and endoscopic ultrasonography: An indirect evaluation of the usefulness of laparoscopy. Pancreatology. 2004;4:436–40. doi: 10.1159/000079617. [DOI] [PubMed] [Google Scholar]
- 9.Karmazanovsky G, Fedorov V, Kubyshkin V, et al. Pancreatic head cancer: Accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488–500. doi: 10.1007/s00261-004-0279-z. [DOI] [PubMed] [Google Scholar]
- 10.Chari ST. Detecting early pancreatic cancer: Problems and prospects. Semin Oncol. 2007;34:284–94. doi: 10.1053/j.seminoncol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa H, Okada S, Saisho H, et al. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer. 1996;78:986–90. doi: 10.1002/(SICI)1097-0142(19960901)78:5<986::AID-CNCR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y, Yasui K, Matsueda K, et al. Small carcinoma of the pancreas is curable: New computed tomography finding, pathological study and postoperative results from a single institute. J Gastroenterol Hepatol. 2005;20:1591–4. doi: 10.1111/j.1440-1746.2005.03895.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhutani MS, Verma D, Guha S, et al. Is endoscopic ultrasound “sound” for pancreatic cancer screening? J Clin Gastroenterol. 2009;43:797–802. doi: 10.1097/MCG.0b013e3181b3ab58. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya R, Noda T, Harada N, et al. Collective review of small carcinomas of the pancreas. Ann Surg. 1986;203:77–81. doi: 10.1097/00000658-198601000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa O, Ohigashi H, Imaoka S, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter - collective review of Japanese case reports. Hepatogastroenterology. 1999;46:8–15. [PubMed] [Google Scholar]
- 17.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72. [PubMed] [Google Scholar]
- 18.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 19.Shi C, Klein AP, Goggins M, et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin Cancer Res. 2009;15:7737–43. doi: 10.1158/1078-0432.CCR-09-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 21.Bhutani MS, Hawes RH, Baron PL, et al. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854–8. doi: 10.1055/s-2007-1004321. [DOI] [PubMed] [Google Scholar]
- 22.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 23.Faigel DO, Ginsberg GG, Bentz JS, et al. Endoscopic ultrasound-guided real-time fine-needle aspiration biopsy of the pancreas in cancer patients with pancreatic lesions. J Clin Oncol. 1997;15:1439–43. doi: 10.1200/JCO.1997.15.4.1439. [DOI] [PubMed] [Google Scholar]
- 24.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?. A meta-analysis and systematic review. Pancreas. 2013;42:20–6. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 25.Muller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: Evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–51. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 26.Dewitt J, Devereaux BM, Lehman GA, et al. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: A systematic review. Clin Gastroenterol Hepatol. 2006;4:717–25. doi: 10.1016/j.cgh.2006.02.020. quiz 664. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Shpaner A, Krishna SG, et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc. 2013;78:73–80. doi: 10.1016/j.gie.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Khashab MA, Yong E, Lennon AM, et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest Endosc. 2011;73:691–6. doi: 10.1016/j.gie.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 29.Atiq M, Bhutani MS, Ross WA, et al. Role of endoscopic ultrasonography in evaluation of metastatic lesions to the pancreas: A tertiary cancer center experience. Pancreas. 2013;42:516–23. doi: 10.1097/MPA.0b013e31826c276d. [DOI] [PubMed] [Google Scholar]
- 30.Chen G, Liu S, Zhao Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: A meta-analysis. Pancreatology. 2013;13:298–304. doi: 10.1016/j.pan.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: A large single-center experience. Gastrointest Endosc. 1999;50:786–91. doi: 10.1016/s0016-5107(99)70159-8. [DOI] [PubMed] [Google Scholar]
- 32.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Silverman DT, Dunn JA, Hoover RN, et al. Cigarette smoking and pancreas cancer: A case-control study based on direct interviews. J Natl Cancer Inst. 1994;86:1510–6. doi: 10.1093/jnci/86.20.1510. [DOI] [PubMed] [Google Scholar]
- 34.Rulyak SJ, Lowenfels AB, Maisonneuve P, et al. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292–9. doi: 10.1016/s0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- 35.Brentnall TA, Bronner MP, Byrd DR, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 36.Brune KA, Lau B, Palmisano E, et al. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 2010;102:119–26. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 38.McFaul CD, Greenhalf W, Earl J, et al. European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC); German National Case Collection for Familial Pancreatic Cancer (FaPaCa). Anticipation in familial pancreatic cancer. Gut. 2006;55:252–8. doi: 10.1136/gut.2005.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–21. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Chen S, Brune KA, et al. PancPRO: Risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol. 2007;25:1417–22. doi: 10.1200/JCO.2006.09.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch HT, Fusaro RM, Lynch JF, et al. Pancreatic cancer and the FAMMM syndrome. Fam Cancer. 2008;7:103–12. doi: 10.1007/s10689-007-9166-4. [DOI] [PubMed] [Google Scholar]
- 42.Vasen HF, Gruis NA, Frants RR, et al. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden) Int J Cancer. 2000;87:809–11. [PubMed] [Google Scholar]
- 43.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–53. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 44.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–6. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 45.Lowenfels AB, Maisonneuve P, Whitcomb DC. Risk factors for cancer in hereditary pancreatitis. International Hereditary Pancreatitis Study Group. Med Clin North Am. 2000;84:565–73. doi: 10.1016/s0025-7125(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 46.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–70. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 47.Rajan E, Clain JE, Levy MJ, et al. Age-related changes in the pancreas identified by EUS: A prospective evaluation. Gastrointest Endosc. 2005;61:401–6. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 48.Bhutani MS. Endoscopic ultrasonography: Changes of chronic pancreatitis in asymptomatic and symptomatic alcoholic patients. J Ultrasound Med. 1999;18:455–62. doi: 10.7863/jum.1999.18.7.455. [DOI] [PubMed] [Google Scholar]
- 49.Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol. 2004;2:405–9. doi: 10.1016/s1542-3565(04)00126-0. [DOI] [PubMed] [Google Scholar]
- 50.Rulyak SJ, Brentnall TA. Inherited pancreatic cancer: Surveillance and treatment strategies for affected families. Pancreatology. 2001;1:477–85. doi: 10.1159/000055851. [DOI] [PubMed] [Google Scholar]
- 51.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: An EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 52.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 53.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–81. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 54.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410–8. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 55.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946–54. doi: 10.1038/ajg.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: A comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010;16:5028–37. doi: 10.1158/1078-0432.CCR-09-3209. [DOI] [PubMed] [Google Scholar]
- 57.Sud A, Wham D, Catalano M, et al. Promising outcomes of screening for pancreatic cancer by genetic testing and endoscopic ultrasound. Pancreas. 2014;43:458–61. doi: 10.1097/MPA.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 58.Zubarik R, Gordon SR, Lidofsky SD, et al. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: A feasibility study. Gastrointest Endosc. 2011;74:87–95. doi: 10.1016/j.gie.2011.03.1235. [DOI] [PubMed] [Google Scholar]
- 59.Canto MI, Hruban RH, Fishman EK, et al. American Cancer of the Pancreas. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–11. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 61.Potjer TP, Schot I, Langer P, et al. Leiden Familial Pancreatic Cancer Group; FaPaCa registry. Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin Cancer Res. 2013;19:442–9. doi: 10.1158/1078-0432.CCR-12-2730. [DOI] [PubMed] [Google Scholar]
- 62.Uehara H, Nakaizumi A, Ishikawa O, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561–5. doi: 10.1136/gut.2007.145631. [DOI] [PubMed] [Google Scholar]
- 63.Lami G, Biagini MR, Galli A. Endoscopic ultrasonography for surveillance of individuals at high risk for pancreatic cancer. World J Gastrointest Endosc. 2014;6:272–85. doi: 10.4253/wjge.v6.i7.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canto MI, Harinck F, Hruban RH, et al. International Cancer of Pancreas Screening (CAPS) Consortium. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–47. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brand RE, Lynch HT. Hereditary pancreatic adenocarcinoma. A clinical perspective. Med Clin North Am. 2000;84:665–75. doi: 10.1016/s0025-7125(05)70249-2. [DOI] [PubMed] [Google Scholar]
- 66.Brand RE, Lerch MM, Rubinstein WS, et al. Participants of the Fourth International Symposium of Inherited Diseases of the Pancreas. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460–9. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Axilbund JE, Brune KA, Canto MI, et al. Patient perspective on the value of genetic counselling for familial pancreas cancer. Hered Cancer Clin Pract. 2005;3:115–22. doi: 10.1186/1897-4287-3-3-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maheu C, Vodermaier A, Rothenmund H, et al. Pancreatic cancer risk counselling and screening: Impact on perceived risk and psychological functioning. Fam Cancer. 2010;9:617–24. doi: 10.1007/s10689-010-9354-5. [DOI] [PubMed] [Google Scholar]
- 69.Harinck F, Nagtegaal T, Kluijt I, et al. Feasibility of a pancreatic cancer surveillance program from a psychological point of view. Genet Med. 2011;13:1015–24. doi: 10.1097/GIM.0b013e31822934f5. [DOI] [PubMed] [Google Scholar]
- 70.Li H, Li Y, Cui L, et al. Monitoring pancreatic carcinogenesis by the molecular imaging of cathepsin E in vivo using confocal laser endomicroscopy. PLoS One. 2014;9:e106566. doi: 10.1371/journal.pone.0106566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol. 2013;11:719–30.e5. doi: 10.1016/j.cgh.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bournet B, Gayral M, Torrisani J, et al. Role of endoscopic ultrasound in the molecular diagnosis of pancreatic cancer. World J Gastroenterol. 2014;20:10758–68. doi: 10.3748/wjg.v20.i31.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhutani MS, Gupta V, Guha S, et al. Pancreatic cyst fluid analysis – A review. J Gastrointestin Liver Dis. 2011;20:175–80. [PubMed] [Google Scholar]
- 74.Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy. 2010;42:1077–84. doi: 10.1055/s-0030-1255971. [DOI] [PubMed] [Google Scholar]
- 75.Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2010;46:1–8. doi: 10.1055/s-0033-1353603. [DOI] [PubMed] [Google Scholar]
- 76.Mertz H. K-ras mutations correlate with atypical cytology and elevated CEA levels in pancreatic cystic neoplasms. Dig Dis Sci. 2011;56:2197–201. doi: 10.1007/s10620-010-1556-z. [DOI] [PubMed] [Google Scholar]
- 77.Kung JS, Lopez OA, McCoy EE, et al. Fluid genetic analyses predict the biological behavior of pancreatic cysts: Three-year experience. JOP. 2014;15:427–32. doi: 10.6092/1590-8577/2426. [DOI] [PubMed] [Google Scholar]
- 78.Raty S, Sand J, Laukkarinen J, et al. Cyst fluid SPINK1 may help to differentiate benign and potentially malignant cystic pancreatic lesions. Pancreatology. 2013;13:530–3. doi: 10.1016/j.pan.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 79.Konda VJ, Meining A, Jamil LH, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–13. doi: 10.1055/s-0033-1344714. [DOI] [PubMed] [Google Scholar]
- 80.Joshi V. nCLE (Needle Based Confocal Endomicroscopy) in the evaluation of indeterminate pancreatic cystic lesions: A single center experience. Am J Gastroenterol. 2014;109(Suppl 2):S101–23. [Google Scholar]


