Abstract
Objective:
Surgical treatment of retrochiasmatic craniopharyngioma still remains a challenge. While complete removal of the tumor with preservation of the vital neurovascular structures is often the goal of the treatment, there is no optimal surgical approach available to achieve this goal. Transcranial and transsphenoidal microsurgical approaches, commonly used in the past, have considerable technical limitations. The extended endonasal endoscopic surgical route, obtained by removal of tuberculum sellae and planum sphenoidale, offers direct midline access to the retrochiasmatic space and provides excellent visualization of the undersurface of the optic chiasm. In this report, we describe the technical details of the extended endoscopic approach, and review our results using this approach in the surgical management of retrochiasmatic craniopharyngiomas.
Methods:
Fifteen children, including 9 girls and 6 boys, aged 8 to 15 years underwent surgery using extended endoscopic transsphenoidal approach between 2008 and 2014. Nine patients had a surgical procedure done previously and presented with recurrence of symptoms and regrowth of their residual tumors.
Results:
A gross total or near total excision was achieved in 10 (66.7%) patients, subtotal resection in 4 (26.7%), and partial removal in 1 (6.7%) patient. Postoperatively, headache improved in 93.3%, vision recovered in 77.3%, and the hormonal levels stabilised in 66.6%. Three patients (20%) developed postoperative CSF leaks which were managed conservatively. Three (20%) patients with diabetes insipidus and 2 (13.3%) with panhypopituitarism required long-term hormonal replacement therapy.
Conclusions:
Our early experience suggests that the extended endonasal endoscopic approach is a reasonable option for removal of the retrochiasmal craniopharyngiomas. Compared to other surgical approaches, it provides better opportunities for greater tumor removal and visual improvement without any increase in risks.
Keywords: Endoscopic endonasal approach, extended transsphenoidal approach, retrochiasmatic craniopharyngioma, transplanum transtuberculum
Introduction
Craniopharyngiomas are complex benign tumors that arise from the rests of the metaplastic adenohypophyseal cells of the pituitary stalk and have highly variable growth patterns. Retrochiasmatic craniopharyngiomas represent approximately 11–46% of all craniopharyngiomas[1,2,3,4] and are believed to have a supradiaphragmatic origin with a tendency to grow under the optic nerves and chiasm into the third ventricle, interpeduncular cistern, and retrosellar region.[4,5,6,7,8] Radical tumor resection with preservation of neurological and endocrinological functions is the ultimate goal in the treatment of craniopharyngioma.[1,9,10,11,12] However, surgical management of retrochiasmatic craniopharyngioma presents a formidable challenge to neurosurgeons due to its difficult location. While radical tumor removal is frequently associated with high surgical mortality and morbidity due to injury to the adjacent critical neurovascular structures, an incomplete tumor removal often results in high recurrence rate.[3,4,5,6]
Traditionally, various transcranial[9,11,12,13,14,15,16,17,18] and transsphenoidal microsurgical[19,20,21,22] approaches have been used to remove these tumors with variable surgical outcome and results. Recently, the endoscopic techniques have been introduced in the surgical removal of craniopharyngioma.[6,23] The extended endoscopic endonasal transsphenoidal approach, the most recent advent in the endoscopic surgery, provides a direct midline exposure to retrochiasmatic craniopharyngiomas and allows safe bimanual microsurgical dissection of the tumor from the undersurface of the optic chiasm (OC) and hypothalamus.[24,25,26,27,28,29,30,31,32,33]
In this report, we describe our surgical technique for removal of retrochiasmatic craniopharyngiomas in children using the endoscopic endonasal transplanum transtuberculum approach, and review early results of surgery.
Methods
Fifteen children, 6 males and 9 females, aged between 8 and 15 years, who underwent surgery for retrochiasmatic craniopharyngiomas using extended endonasal endoscopic approach at our institute between January 2008 and March 2014, were identified from the database and were included in this study [Table 1]. Nine patients (60%) were symptomatic because of the recurrence of their tumors after previous surgery elsewhere. Headache and diminished vision were the most common presenting features, followed by isolated cranial nerve deficits, seizures, and altered sensorium. Mild to moderate hydrocephalus was observed on the magnetic resonance (MR) imaging in 4 (26.7%) patients. The follow-up period of this study ranged from 20 months to 6 years (mean: 32 months).
Table 1.
Clinical features
Surgical technique
Patient positioning
After induction of general anesthesia, the patient is positioned supine on the operating table with his head flexed toward the left shoulder by 15°, and rotated by 15° to the right side to achieve an appropriate trajectory and a comfortable access to the nasal cavity. The head is also extended by 5–10° to enhance accessibility to the anterior skull base. A lumbar drain is placed for postoperative controlled cerebrospinal fluid (CSF) drainage in patients who do not have a preoperative ventriculoperitoneal shunt placement. Intraoperative image guidance provides anatomical orientation and is extremely helpful during bone removal, dural opening, and tumor removal. The nostrils are prepared externally with betadine, and the nasal cavity is packed with adrenaline-soaked pledgets for 7–10 min. The thigh is also prepared to harvest autologous fascia lata and fat for closure and reconstruction of the sellar floor. Intravenous antibiotics and dexamethasone are administered.
Endoscopic endonasal transsphenoidal exposure
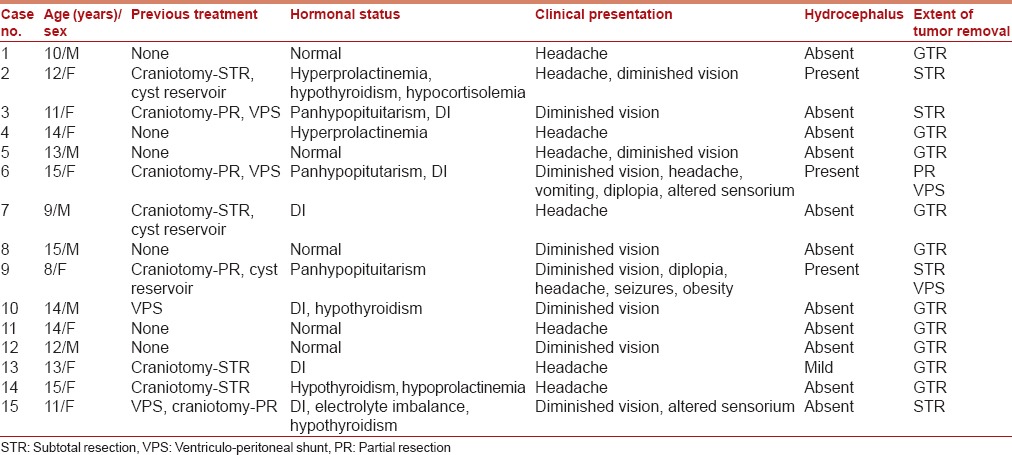
The surgery is performed by a team comprising a neurosurgeon and an otolaryngologist, both experienced and well-versed with the endoscopic skull base surgery. Both surgeons are positioned on the right side of the patient's head and use binostril access for the entire procedure. The endoscope is placed in the upper part of the right nostril (12 o' clock position) by one surgeon, and the other surgeon uses the lower half of the right nostril for suction (6 o' clock position) and the entire left nasal cavity for instruments required for tumor dissection. We use the bimanual technique that includes one surgeon holding the scope in his left hand and saline irrigation in the other, and the other surgeon performing dissection using both hands under the endoscopic guidance provided by the first surgeon [Figure 1]. The dynamic visualization provided by manual maneuvering of the scope facilitates three-dimensional perception of the surgical target.
Figure 1.
Intraoperative photograph showing binostril bimanual (4-hand) technique for endonasal endoscopic surgery. One surgeon holds the scope in his left hand and saline irrigation in the other hand, and the other surgeon performs dissection using both hands under the endoscopic guidance provided by the first surgeon
Using a 0° endoscope with 4-mm diameter and 18-cm length, the otolaryngologist performs the initial part of the approach which consists of right middle turbinectomy, posterior ethmoidectomy on right side, creation of nasoseptal flap, posterior septectomy, and a wide sphenoidotomy, followed by removal of bony septations and mucosa within the sphenoid sinus [Figure 2]. The nasoseptal flap should always be created before widening the sphenoid ostia to avoid injury to the vascular pedicle of the flap which is located in the sphenoethmoid recess.[34,35] The flap is then mobilized into nasopharynx until the time of closure.
Figure 2.
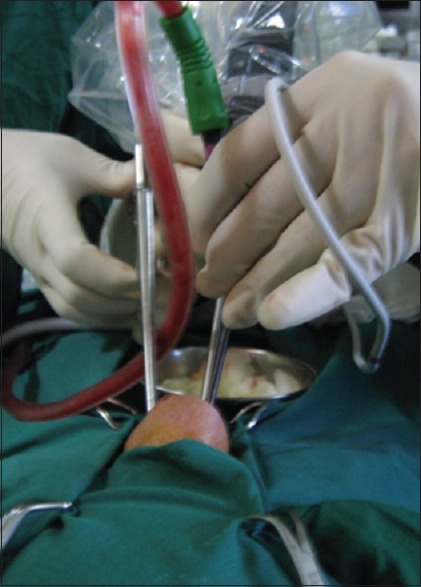
Endoscopic view of the skull base showing important bony landmarks in the roof of the sphenoid sinus including the planum sphenoidale (PS), tuberculum sellae (TS), floor of the sella turcica (SF), optic protuberance (OP), carotid protuberance (CP), medial opticocarotid recess (M), lateral opticocarotid recess (L), and the clivus (C)
The medial opticocarotid recess is a very important landmark [Figure 2]. It represents the ventral aspect of a pneumatized middle clinoid process and marks the medial aspect of the parasellar carotid canal and the cavernous sinus, the lateral edge of the sella, and the inferomedial aspect of the optic nerve. It also forms the most lateral extent of the tuberculum sellae (TS). Removal of bone over the medial opticocarotid recess widens intradural exposure and provides better anatomical orientation by early identification of the optic nerves and paraclinoid internal carotid artery (ICA), and thereby easy identification and protection of the superior hypophyseal artery and perforating arteries to OC. The sella, which is often normal in configuration, is identified between the carotid prominences on both sides, and the clival recess is visible inferior to the sella. In the event of a nonpneumatized sphenoid sinus, as in young children, identification of these landmarks is extremely difficult. Careful drilling of the bone under image guidance is essential until the landmarks become distinguishable.
Transplanum transtuberculum exposure
The primary steps of the extended endoscopic endonasal technique that we used in these cases have been described in detail in other publications.[28,30,33,36,37] The bone of the sellar floor, TS, and planum sphenoidale (PS) is first thinned down with a high-speed diamond drill and is then removed carefully using Kerrison rongeur [Figure 2]. The optic canals mark the lateral limits, whereas the posterior ethmoidal arteries mark the anterior limit of the bony resection. Resection of the skull base anterior to these arteries risks injury to the olfactory fibers and epithelium. Copious irrigation is used during drilling over the medial opticocarotid recess, which is the most critical anatomical landmark, to prevent thermal injury to the optic nerve. Venous bleeding, occasionally vigorous, is usually controlled with Gelfoam or Surgicel packing, or other local hemostatic agents.
The optimal exposure usually includes bone removal from the carotid artery on one side to the carotid artery on the other side in horizontal plane. The extent of bony exposure in the sagittal plane is determined by the size and location of the tumor and can be further evaluated during surgery using navigation. Craniopharyngiomas confined to the sella require removal of the anterior sellar wall only. In cases of preinfundibular tumors, more bone removal over TS and PS, rather than the anterior sellar wall, is necessary. It is important to expose the areas above, below, and over the superior intercavernous sinus to achieve vascular control and to open the diaphragm sella. Transinfundibular craniopharyngiomas require a steeper working angle to reach the superior ventricular extent of the tumors and, therefore, additional bone removal from the anterior sella is often helpful. Extensive bone removal from the sellar floor along the inferior intercavernous sinus, and sometimes the posterior clinoid processes and dorsum sella, may be required to expose the retroinfundibular craniopharyngiomas extending from the infundibulum into the prepontine and interpeduncular cisterns.
Dural opening
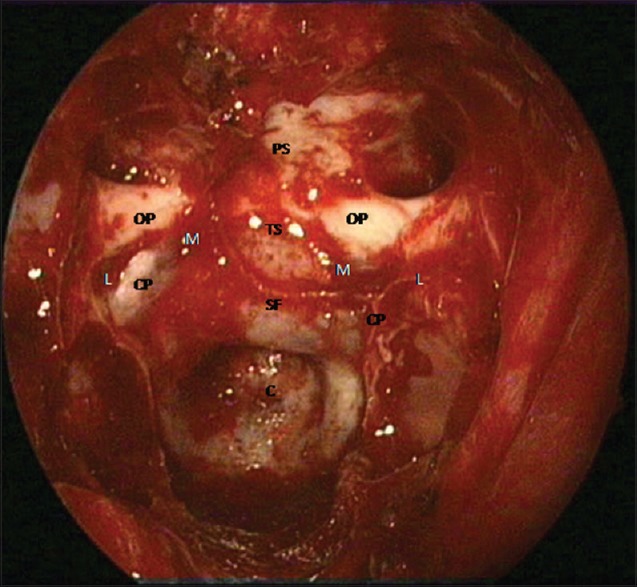
We open the dura in a transdiaphragmatic fashion with two incisions in the dura, parallel to each other, immediately superior and inferior to the superior intercavernous sinus. The superior intercavernous sinus is coagulated with bipolar forceps and is divided under direct vision to obtain access to the suprasellar cistern. The sellar dura is incised in the midline from the lower horizontal incision and can be extended down to the lower end of the sella, if necessary. The dural edges are coagulated and shrunk or excised to widen the dural opening [Figure 3]. Opening the dura in the planum and sellar regions in this manner provides an advantage of simultaneously exposing the suprasellar cistern containing optic chiasm and anterior cerebral arterial complex, the diaphragm sella, and the supradiaphragmatic or subchiasmatic space containing infundibulum, superior hypophyseal artery and perforating vessels, and the infradiaphragmatic space containing pituitary gland (PG). It also provides two distinct surgical corridors for tumor dissection, the superior or intra-arachnoidal or suprasellar route, and the inferior or extra-arachnoidal or endosellar route.
Figure 3.
Dural opening: The dura is with two incisions in the dura, parallel to each other, immediately superior and inferior to the superior intercavernous sinus. The superior intercavernous sinus is coagulated with bipolar forceps and is divided under direct vision to obtain access to the suprasellar cistern. The dural edges are coagulated and shrunk or excised to widen the dural opening. T = tumor, OC = optic chiasm
Tumor removal
Our technique of endoscopic craniopharyngioma removal follows the same steps as that of the standard transcranial microsurgery, which include identification of the tumor, internal tumor debulking, extracapsular dissection in the arachnoid-capsular plane, and protection of the neurovascular structures [Figure 4]. Selection of an appropriate intradural corridor for tumor dissection and intraoperative decision-making regarding the extent of tumor removal pertaining to neurological and endocrinological morbidity are some of the crucial issues that influence the overall outcome.
Figure 4.
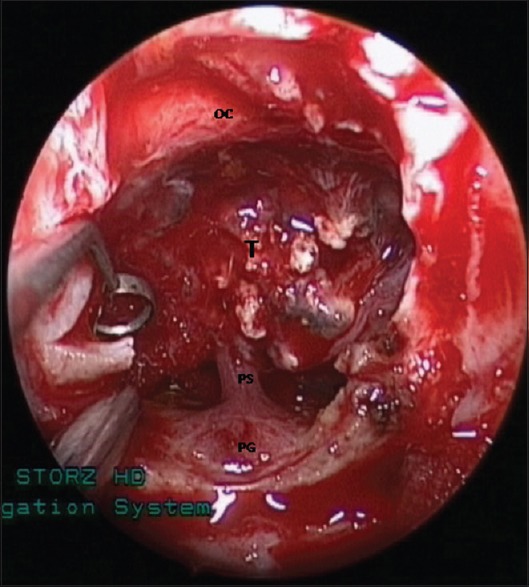
Internal decompression of a calcified tumor, which is adherent to the optic nerves and chiasm, pituitary stalk and gland, and hypothalamus
The classification of craniopharyngiomas according to their location in relation to the infundibulum provided by Kassam et al.[30] is simple and useful because it facilitates surgical planning and intraoperative decision-making. Type I or preinfundibular tumors are located anterior to the pituitary stalk, tend to displace the OC posteriorly and superiorly, and are visible as soon as the dura is opened. Tumor removal, thus, can be performed under direct vision through the superior part of the transtuberculum transplanum corridor. Type II or trans-infundibular craniopharyngiomas involve the pituitary stalk and usually grow along its axis. These tumors extend superiorly into the third ventricle, and therefore, need a steeper working angle through the lower part of the bony opening. A gross total resection of these tumors often requires sectioning of the pituitary stalk. Type III or retroinfundibular tumors are located posterior to the stalk and tend to grow either superiorly into the third ventricle (Subtype IIIa) or posteriorly and inferiorly into the interpeduncular or prepontine cisterns (Subtype IIIb). Multiple surgical corridors, including superior subchiasmatic, straight supradiaphragmatic, and inferior routes through dorsum sellae, either single or in combination, can be used for this group of tumors. Occasionally, a lateral transposition of the PG or an “above and below” approach is needed for a complete tumor exposure.[38] Type IV craniopharyngiomas are purely intraventricular tumors and should be treated through the transcranial microscopic/endoscopic approaches.
The thin arachnoid layer of the suprasellar cistern over the tumor should be opened sharply, and a plane of dissection between the overlying arachnoid and the tumor capsule must be identified. The arachnoid covering is usually in two layers and, it is thus, important to identify and distinguish the tumor arachnoid plane from the cisternal arachnoid plane. The optimal plane of safe dissection is between the tumor capsule and the tumor arachnoid. An attempt must be made to identify the pituitary stalk and the superior hypophyseal arteries as early as possible to ensure their protection from inadvertent injury. The capsule, after confirming its relationship with optic nerves and chiasm, is incised allowing drainage of the cyst or internal decompression of the solid mass. Occasionally, firm consistency of the tumor mass due to the presence of calcification makes tumor decompression slow and difficult.
Once the tumor cyst has been drained or the mass has been debulked adequately, mobilization of the redundant capsule is carried out using a gentle traction technique similar to one used in microscopic resection. Likewise, sharp dissection is used to divide arachnoid adhesions from the undersurface of the optic nerves and chiasm, while meticulously preserving the perforators supplying the chiasm. Injury to the perforators is the main cause of visual deterioration after craniopharyngioma surgery. The process of capsule mobilization followed by more tumor debulking is continued and repeated until complete relaxation of the tumor capsule is achieved. Care must be exercised to avoid amputation of the tumor capsule prematurely and to ensure enough tumor capsule is available to be grasped with tumor forceps for providing counter traction during extracapsular dissection.
The arachnoid dissection is continued on each side of the tumor laterally until the opticocarotid cistern is entered, following which the ICA is traced superiorly preserving the superior hypophyseal arteries and other small perforating vessels. Extra care should be taken in larger tumors which are frequently in contact with the A1-A2 junction, anterior communicating artery, the recurrent arteries of Heubner, and A2 segments of the anterior cerebral arteries. We recommend the use of a 30° endoscope for capsular dissection at this stage because it allows a direct “looking-up” view of the retrochiasmatic space. We also change the position of the scope from 12 o' clock to 6 o' clock in the right nostril to obtain a superior trajectory and an optimal upward visualization of the area.
As dissection continues posteriorly, great care is required to separate the tumor capsule from the floor of the third ventricle [Figure 5]. When the floor of the third ventricle is involved, the tumor dissection is continued from the lateral and superior walls until most of the tumor has been removed. One can inspect the third ventricular cavity with a 30° endoscope for the presence of any residual tumor, which, if present, can be removed carefully. When the tumor has significant third ventricular extension and a clear plane of dissection is difficult to find, it is safer to leave some tumor behind to prevent traction injury to the hypothalamic nuclei.
Figure 5.
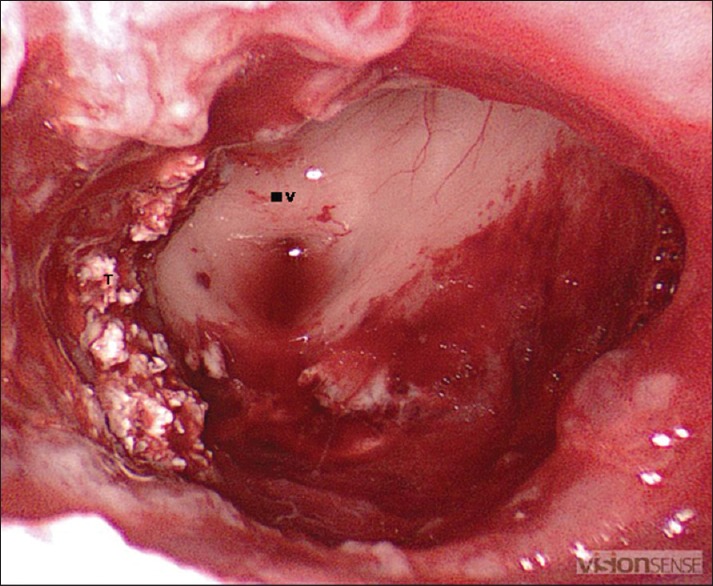
Intraoperative endoscopic views showing tumor cavity and the anterior third ventricle after tumor removal. III V = anterior third ventricle, T = smaller flakes of calcification
Next, the attention should be paid to the inferior pole of the tumor, which is separated from the diaphragm sellae and PG. The diaphragm is transacted if the tumor is extending into the sella. Management of the pituitary stalk is crucial and depends on several factors, including the patient's preoperative endocrinological status, and probabilities of safe gross total tumor resection based on the intraoperative findings, and surgeon's assessment. Ideally, every attempt should be made to preserve the pituitary stalk [Figure 4]. However, if the surgeon's impression during surgery is strongly in favor of the possibility of a gross total resection, the stalk should be transacted, and a complete tumor removal should be attempted. Poor preoperative hormonal status of diabetes insipidus (DI) may also justify the decision to section the pituitary stalk.[32,37,38] For the same reason, in cases of Type II or trans-infundibular craniopharyngiomas, it is preferable to perform a low stalk section to achieve a more aggressive removal of the tumor and plan a postoperative hormonal replacement therapy. In the event in which the preoperative endocrinological functions are normal and/or when complete resection appears unlikely on inspection during surgery, it is appropriate to leave behind small portion of the tumor in order to avoid any risk of stalk injury during an attempt of tumor removal.
In most cases, the Liliequist membrane is intact and serves as a protective barrier for the basilar artery (BA), posterior cerebral arteries, and P1 perforating vessels [Figure 6]. However, the residual tumor may be occasionally found tethered posteriorly to the mammillary bodies or BA and its branches in the interpeduncular fossa. Holding the tumor capsule by tumor forceps in one hand, a gentle sharp dissection is performed in the arachnoid covering the mammillary bodies, optic tracts, posterior cerebral arteries, posterior communicating arteries and thalamoperforators, and the pituitary stalk, to remove the tumor completely. The resection cavity is then inspected carefully with an angled endoscope for any residual tumor or active bleeding [Figure 6] and is irrigated thoroughly with copious saline as the tumor contents can cause local irritation and may incite chemical meningitis.
Figure 6.
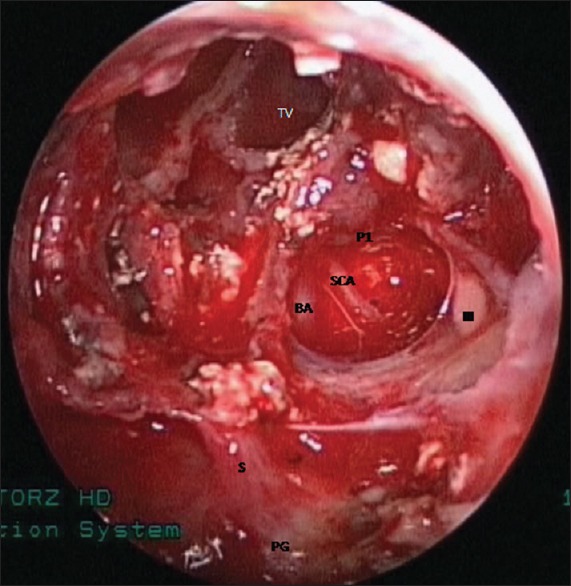
Endoscopic view showing tumor cavity after complete removal of the tumor. TV = third ventricle, BA = basilar artery, P1 = left posterior cerebral artery, SCA = left superior cerebellar artery, III = left oculomotor nerve, S = pituitary stalk, and PG = pituitary gland
Closure
A meticulous closure of the cranial base defect after tumor removal is of critical importance to prevent a postoperative CSF leak, pneumocephalus, and their potential complications. We perform multilayered reconstruction to achieve a watertight closure as described by most authors [Figure 7].[39,40] As the first step, we prefer to place autologous fat in the sellar cavity to cover the arachnoid defect. Care must be taken to avoid too tight packing or too deep placement of the graft. A fascia lata graft harvested from the thigh is then placed intradurally as an underlay graft, carefully tucked underneath the dural edges. Where possible, a small piece of thin bone or cartilage obtained from the nasal septum is placed snugly over the bony defect in the skull base. The final layer of a previously prepared vascularized pedicled nasoseptal flap is then rotated to cover the entire defect. We apply a thin layer of fibrin glue (TISSEEL, Baxter Healthcare Corp.) over the flap edges to prevent its displacement or migration. The flap is supported by the nasal Merocel (Medtronic Xomed) pack which is left in place for 3–4 days. A controlled drainage of CSF using a lumbar catheter is continued postoperatively for 5 days. We have found this method of closure very effective in preventing postoperative CSF leaks.
Figure 7.
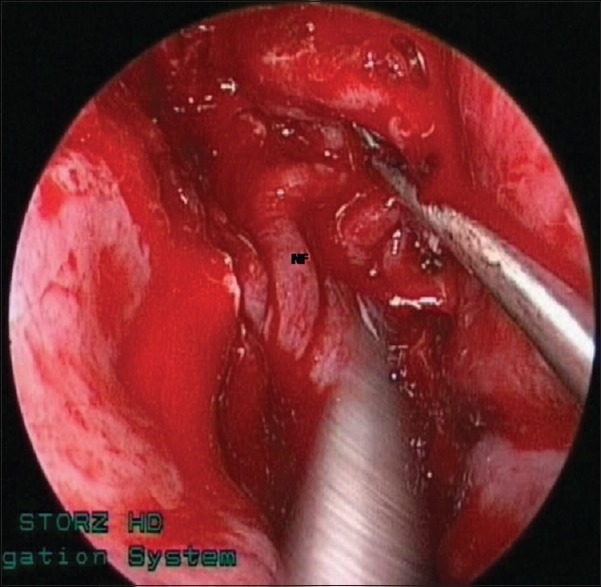
Intraoperative photographs showing multi-layered closure of the dural and skull base defects: NF = nasoseptal pedicled flap
Results
The extent of tumor resection was assessed by the surgeon's observation and the findings on the postoperative MR imaging. Gross total or near total tumor removal was achieved in 10 (66.7%) [Figure 8], subtotal in 4 (26.7%), and partial in 1 (6.7%) patient [Table 2]. Postoperatively, headache and vision improved in 93.3% and 77.3% of patients, respectively. Improvement or stabilization of hormonal levels was observed in 66.6% of the patients [Table 3]. The most common postoperative complications were CSF rhinorrhea (20%), DI (20%), and panhypopituitarism (13.3%) [Table 4].
Figure 8.
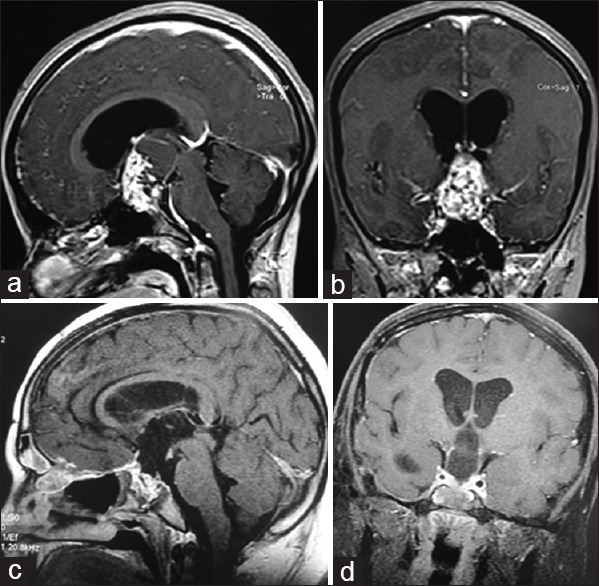
Contrast-enhancing magnetic resonance imaging of a 14-year-old girl, sagittal (a) and coronal (b) views, showing a large solid, cystic, and calcified retrochiasmatic craniopharyngioma. Postoperative magnetic resonance sagittal (c) and coronal (d) imaging showing gross-total resection of the tumor
Table 2.
Postoperative results: Extent of tumor removal

Table 3.
Postoperative results: Outcome

Table 4.
Postoperative complications

Discussion
Gross total resection of craniopharyngiomas offers highest rates of recurrence-free survival.[1,9,10,11,12] Therefore, radical tumor resection with preservation of neurological function is considered as the ultimate goal in the treatment of craniopharyngiomas. However, craniopharyngiomas in retrochiasmatic region are difficult to access surgically because of their critical location, i.e., ventral to the OC and hypothalamus, and intimate anatomical relationship with vital neurovascular structures, including optic nerves and chiasm, PG and stalk, arteries of the circle of Willis, brainstem, hypothalamus, third ventricle, and frontal/temporal lobes.
Conventionally, transcranial[9,11,12,13,14,15,16,17,18] and transsphenoidal microsurgical[19,20,21,22] approaches have been employed to remove these tumors, although with considerable limitations. Conventional anterior skull base transcranial approaches usually provide a restricted exposure of retrochiasmatic craniopharyngiomas, which are often hidden behind the anteriorly displaced or prefixed chiasm.[14] Intracranial corridors available through the interoptic, opticocarotid, or lateral carotid cisterns; however, do not allow adequate visualization of the tumors in the subchiasmatic and retrochiasmatic spaces.[4,9,10] Although partial access to the retrochiasmatic portion of the tumor can be made by opening the critical lamina terminalis,[2,5,9,10,14,15,16,41] a large portion of the tumor lying under the optic nerves and chiasm remains essentially inaccessible. The posterior petrosal approach described by Al-Mefty et al.,[42] and Hakuba et al.,[43] which has the advantage of providing better exposure of the upper pole of the tumor, hypothalamus, and pituitary stalk, has the disadvantage of carrying higher risks of complications due to prolonged temporal lobe retraction, injury to the vein of Labbe, and loss of midline orientation.
The transnasal transsphenoidal approach, which provides direct vision to the suprasellar region, has been used primarily for craniopharyngiomas located within the sella turcica.[19,20,21,22] For a long time, transsphenoidal microsurgery was considered unsuitable for lesions originating in or extending to the extrasellar space with a normal sized sella. Weiss[44] in 1987 described the technique of extended transsphenoidal microsurgical approach, which involves removal of the TS and PS, and has the advantage of providing direct visualization and access to the lesions located in the supra sellar and supradiaphragmatic regions. Although successful tumor removal has been reported using microsurgical extended transsphenoidal approach, the use of the speculum-based techniques is generally limited by the compromised field of vision and line of sight, deep and narrow working channel, and restricted instrument maneuverability caused by the nasal speculum.[25,45,46]
With recent advances in endoscopic technology, craniopharyngioma surgery has evolved considerably in the past few years. The endoscope offers the advantages of better illumination with a much wider field of view at the skull base, from the PS to the clival recess and from one medial opticocarotid recess to the other. Increased experience in transnasal surgery has led to the introduction of endoscopic approaches in the surgical management of tumors at locations other than the sellar cavity.[16,18,19,20,23,24,25,27,29,30,47] The pure extended (transtuberculum transplanum) endoscopic endonasal approach is a more recent modification in the endoscopic approaches and is believed to provide a direct midline exposure to the craniopharyngiomas located under the OC and retrochiasmatic region.[26,29,31,32,33,37] Extracapsular dissection of the tumor from the visual apparatus, hypothalamus, pituitary stalk, and perforating vessels can be performed using bimanual technique in the similar fashion as with the microsurgical tumor dissection. Recent literature suggests that for most craniopharyngiomas, the degree of resection via endonasal endoscopic approach, in experienced hands, is comparable or superior to those of transcranial routes.[23,28,29,48] It is also now evident that the endoscopic surgery is less invasive, has the potential to improve visual outcome, and requires shorter postoperative hospital stays.[6,18,29,47,48,49,50,51]
Our results further support the literature, which suggests that, radical resection of retrochiasmatic craniopharyngiomas can be performed using extended endonasal endoscopic approach with minimal surgical morbidity. The major criticism of the endoscopic approach is the high risk of postoperative CSF rhinorrhea. However, this complication has been reduced significantly with the use of the vascularized nasoseptal flap which provides excellent coverage of skull base defect and prevents CSF leaks.[34,35] With improved closure techniques, it has now been possible to reduce CSF leak rates to 0–15%.[51,52,53] DI is also a common postoperative complication found in 42–64% of the patients.[52,53] Postoperative DI is often temporary and recovers completely over time. We completely agree with the views of other authors that the transnasal extended endoscopic approach provides better opportunities to identify and preserve the pituitary stalk during surgery.[30,32,37] There were only 3 (20%) patients in our study who experienced permanent DI. Dysregulation of anterior pituitary hormone is reported in 28–46% patients.[52,53] The anterior pituitary deficiency was observed in 2 (13.3%) patients in our series. Hyperphagia due to hypothalamic injury, which may lead to morbid obesity, is a well-described complication following craniopharyngioma resection in children. However, we have not observed any complication of obesity, decreased vision, or vascular injury in our patients.
In young children, a conchal non-pneumatized sphenoid sinus can make the endonasal approach more difficult because of the lack of natural bony landmarks. Careful bone drilling under the guidance of intraoperative neuronavigation is extremely important to deal with this problem.
Conclusions
The endoscopic endonasal transplanum transtuberculum approach offers good visualization and direct midline access to the retrochiasmatic craniopharyngiomas, and facilitates binostril bimanual extracapsular tumor dissection from the vital neurovascular structures. Our early experience indicates that the results with respect to the extent of tumor removal and visual improvement are superior to the conventional transcranial and transsphenoidal microscopic techniques, with no increase in surgical morbidity. The steep learning curve, however, remains to be a major concern.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
- 1.Baskin DS, Wilson CB. Surgical management of craniopharyngiomas. A review of 74 cases. J Neurosurg. 1986;65:22–7. doi: 10.3171/jns.1986.65.1.0022. [DOI] [PubMed] [Google Scholar]
- 2.Ammirati M, Samii M, Sephernia A. Surgery of large retrochiasmatic craniopharyngiomas in children. Childs Nerv Syst. 1990;6:13–7. doi: 10.1007/BF00262259. [DOI] [PubMed] [Google Scholar]
- 3.Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas: Experience with 168 patients. J Neurosurg. 1999;90:237–50. doi: 10.3171/jns.1999.90.2.0237. [DOI] [PubMed] [Google Scholar]
- 4.Van Effenterre R, Boch AL. Craniopharyngioma in adults and children: A study of 122 surgical cases. J Neurosurg. 2002;97:3–11. doi: 10.3171/jns.2002.97.1.0003. [DOI] [PubMed] [Google Scholar]
- 5.Patterson RH, Jr, Danylevich A. Surgical removal of craniopharyngiomas by the transcranial approach through the lamina terminalis and sphenoid sinus. Neurosurgery. 1980;7:111–7. doi: 10.1227/00006123-198008000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Maira G, Anile C, Rossi GF, Colosimo C. Surgical treatment of craniopharyngiomas: An evaluation of the transsphenoidal and pterional approaches. Neurosurgery. 1995;36:715–24. doi: 10.1227/00006123-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Villani RM, Tomei G, Bello L, Sganzerla E, Ambrosi B, Re T, et al. Long-term results of treatment for craniopharyngioma in children. Childs Nerv Syst. 1997;13:397–405. doi: 10.1007/s003810050108. [DOI] [PubMed] [Google Scholar]
- 8.Wang KC, Kim SK, Choe G, Chi JG, Cho BK. Growth patterns of craniopharyngioma in children: Role of the diaphragm sellae and its surgical implication. Surg Neurol. 2002;57:25–33. doi: 10.1016/s0090-3019(01)00657-7. [DOI] [PubMed] [Google Scholar]
- 9.Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990;73:3–11. doi: 10.3171/jns.1990.73.1.0003. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI. Aggressive surgical management of craniopharyngiomas in children. J Neurosurg. 1992;76:47–52. doi: 10.3171/jns.1992.76.1.0047. [DOI] [PubMed] [Google Scholar]
- 11.Mortini P, Losa M, Pozzobon G, Barzaghi R, Riva M, Acerno S, et al. Neurosurgical treatment of craniopharyngioma in adults and children: Early and long-term results in a large case series. J Neurosurg. 2011;114:1350–9. doi: 10.3171/2010.11.JNS10670. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann BM, Höllig A, Strauss C, Buslei R, Buchfelder M, Fahlbusch R. Results after treatment of craniopharyngiomas: Further experiences with 73 patients since 1997. J Neurosurg. 2012;116:373–84. doi: 10.3171/2011.6.JNS081451. [DOI] [PubMed] [Google Scholar]
- 13.Golshani KJ, Lalwani K, Delashaw JB, Selden NR. Modified orbitozygomatic craniotomy for craniopharyngioma resection in children. J Neurosurg Pediatr. 2009;4:345–52. doi: 10.3171/2009.5.PEDS09106. [DOI] [PubMed] [Google Scholar]
- 14.Liu JK, Christiano LD, Gupta G, Carmel PW. Surgical nuances for removal of retrochiasmatic craniopharyngiomas via the transbasal subfrontal translamina terminalis approach. Neurosurg Focus. 2010;28:E6. doi: 10.3171/2010.1.FOCUS09309. [DOI] [PubMed] [Google Scholar]
- 15.Maira G, Anile C, Colosimo C, Cabezas D. Craniopharyngiomas of the third ventricle: Trans-lamina terminalis approach. Neurosurgery. 2000;47:857–63. doi: 10.1097/00006123-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Shibuya M, Takayasu M, Suzuki Y, Saito K, Sugita K. Bifrontal basal interhemispheric approach to craniopharyngioma resection with or without division of the anterior communicating artery. J Neurosurg. 1996;84:951–6. doi: 10.3171/jns.1996.84.6.0951. [DOI] [PubMed] [Google Scholar]
- 17.Shirane R, Ching-Chan S, Kusaka Y, Jokura H, Yoshimoto T. Surgical outcomes in 31 patients with craniopharyngiomas extending outside the suprasellar cistern: An evaluation of the frontobasal interhemispheric approach. J Neurosurg. 2002;96:704–12. doi: 10.3171/jns.2002.96.4.0704. [DOI] [PubMed] [Google Scholar]
- 18.Dusick JR, Fatemi N, Mattozo C, McArthur D, Cohan P, Wang C, et al. Pituitary function after endonasal surgery for nonadenomatous parasellar tumors: Rathke's cleft cysts, craniopharyngiomas, and meningiomas. Surg Neurol. 2008;70:482–90. doi: 10.1016/j.surneu.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Laws ER., Jr Transsphenoidal removal of craniopharyngioma. Pediatr Neurosurg. 1994;21(Suppl 1):57–63. doi: 10.1159/000120863. [DOI] [PubMed] [Google Scholar]
- 20.Kouri JG, Chen MY, Watson JC, Oldfield EH. Resection of suprasellar tumors by using a modified transsphenoidal approach. Report of four cases. J Neurosurg. 2000;92:1028–35. doi: 10.3171/jns.2000.92.6.1028. [DOI] [PubMed] [Google Scholar]
- 21.Maira G, Anile C, Albanese A, Cabezas D, Pardi F, Vignati A. The role of transsphenoidal surgery in the treatment of craniopharyngiomas. J Neurosurg. 2004;100:445–51. doi: 10.3171/jns.2004.100.3.0445. [DOI] [PubMed] [Google Scholar]
- 22.Kaptain GJ, Vincent DA, Sheehan JP, Laws ER., Jr Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2008;62(6 Suppl 3):1264–71. doi: 10.1227/01.neu.0000333791.29091.83. [DOI] [PubMed] [Google Scholar]
- 23.Jane JA, Jr, Kiehna E, Payne SC, Early SV, Laws ER., Jr Early outcomes of endoscopic transsphenoidal surgery for adult craniopharyngiomas. Neurosurg Focus. 2010;28:E9. doi: 10.3171/2010.1.FOCUS09319. [DOI] [PubMed] [Google Scholar]
- 24.Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: Surgical experience in 105 cases. Neurosurgery. 2004;55:539–47. doi: 10.1227/01.neu.0000134287.19377.a2. [DOI] [PubMed] [Google Scholar]
- 25.Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP, et al. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102:832–41. doi: 10.3171/jns.2005.102.5.0832. [DOI] [PubMed] [Google Scholar]
- 26.Frank G, Pasquini E, Doglietto F, Mazzatenta D, Sciarretta V, Farneti G, et al. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery. 2006;59(1 Suppl 1):ONS75–83. doi: 10.1227/01.NEU.0000219897.98238.A3. [DOI] [PubMed] [Google Scholar]
- 27.de Divitiis E, Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A. Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery. 2007;61(5 Suppl 2):219–27. doi: 10.1227/01.neu.0000303220.55393.73. [DOI] [PubMed] [Google Scholar]
- 28.Laufer I, Anand VK, Schwartz TH. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106:400–6. doi: 10.3171/jns.2007.106.3.400. [DOI] [PubMed] [Google Scholar]
- 29.Gardner PA, Kassam AB, Snyderman CH, Carrau RL, Mintz AH, Grahovac S, et al. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: A case series. J Neurosurg. 2008;109:6–16. doi: 10.3171/JNS/2008/109/7/0006. [DOI] [PubMed] [Google Scholar]
- 30.Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: A new classification based on the infundibulum. J Neurosurg. 2008;108:715–28. doi: 10.3171/JNS/2008/108/4/0715. [DOI] [PubMed] [Google Scholar]
- 31.Cappabianca P, Cavallo LM, Esposito F, De Divitiis O, Messina A, De Divitiis E. Extended endoscopic endonasal approach to the midline skull base: The evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008;33:151–99. doi: 10.1007/978-3-211-72283-1_4. [DOI] [PubMed] [Google Scholar]
- 32.Liu JK, Christiano LD, Patel SK, Eloy JA. Surgical nuances for removal of retrochiasmatic craniopharyngioma via the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurg Focus. 2011;30:E14. doi: 10.3171/2011.1.FOCUS10297. [DOI] [PubMed] [Google Scholar]
- 33.Cavallo LM, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, et al. The endoscopic endonasal approach for the management of craniopharyngiomas: A series of 103 patients. J Neurosurg. 2014;121:100–13. doi: 10.3171/2014.3.JNS131521. [DOI] [PubMed] [Google Scholar]
- 34.Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–6. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 35.Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63(1 Suppl 1):ONS44–52. doi: 10.1227/01.neu.0000297074.13423.f5. [DOI] [PubMed] [Google Scholar]
- 36.Sankhla SK, Jayashankar N, Khan GM. Surgical management of selected pituitary macroadenomas using extended endoscopic endonasal transsphenoidal approach: Early experience. Neurol India. 2013;61:122–30. doi: 10.4103/0028-3886.111114. [DOI] [PubMed] [Google Scholar]
- 37.Conger AR, Lucas J, Zada G, Schwartz TH, Cohen-Gadol AA. Endoscopic extended transsphenoidal resection of craniopharyngiomas: Nuances of neurosurgical technique. Neurosurg Focus. 2014;37:E10. doi: 10.3171/2014.7.FOCUS14364. [DOI] [PubMed] [Google Scholar]
- 38.Silva D, Attia M, Kandasamy J, Alimi M, Anand VK, Schwartz TH. Endoscopic endonasal transsphenoidal “above and below” approach to the retroinfundibular area and interpeduncular cistern – Cadaveric study and case illustrations. World Neurosurg. 2014;81:374–84. doi: 10.1016/j.wneu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 39.McCoul ED, Anand VK, Singh A, Nyquist GG, Schaberg MR, Schwartz TH. Long-term effectiveness of a reconstructive protocol using the nasoseptal flap after endoscopic skull base surgery. World Neurosurg. 2014;81:136–43. doi: 10.1016/j.wneu.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005;19:E8. [PubMed] [Google Scholar]
- 41.Dehdashti AR, de Tribolet N. Frontobasal interhemispheric trans-lamina terminalis approach for suprasellar lesions. Neurosurgery. 2005;56(2 Suppl):418–24. doi: 10.1227/01.neu.0000157027.80293.c7. [DOI] [PubMed] [Google Scholar]
- 42.Al-Mefty O, Ayoubi S, Kadri PA. The petrosal approach for the total removal of giant retrochiasmatic craniopharyngiomas in children. J Neurosurg. 2007;106(2 Suppl):87–92. doi: 10.3171/ped.2007.106.2.87. [DOI] [PubMed] [Google Scholar]
- 43.Hakuba A, Nishimura S, Inoue Y. Transpetrosal-transtentorial approach and its application in the therapy of retrochiasmatic craniopharyngiomas. Surg Neurol. 1985;24:405–15. doi: 10.1016/0090-3019(85)90300-3. [DOI] [PubMed] [Google Scholar]
- 44.Weiss MH. The transnasal transsphenoidal approach. In: Apuzzo ML, editor. Surgery of the Third Ventricle. Baltimore: Williams and Wilkins; 1987. pp. 476–94. [Google Scholar]
- 45.Dehdashti AR, Ganna A, Witterick I, Gentili F. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: Indications and limitations. Neurosurgery. 2009;64:677–87. doi: 10.1227/01.NEU.0000339121.20101.85. [DOI] [PubMed] [Google Scholar]
- 46.Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: Experience with 50 patients. J Neurosurg. 1997;87:44–51. doi: 10.3171/jns.1997.87.1.0044. [DOI] [PubMed] [Google Scholar]
- 47.Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery. 2009;64(5 Suppl 2):269–84. doi: 10.1227/01.NEU.0000327857.22221.53. [DOI] [PubMed] [Google Scholar]
- 48.Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Tyler-Kabara EC, Wang EW, Snyderman CH. Endoscopic endonasal surgery for craniopharyngiomas: Surgical outcome in 64 patients. J Neurosurg. 2013;119:1194–207. doi: 10.3171/2013.6.JNS122259. [DOI] [PubMed] [Google Scholar]
- 49.Campbell PG, McGettigan B, Luginbuhl A, Yadla S, Rosen M, Evans JJ. Endocrinological and ophthalmological consequences of an initial endonasal endoscopic approach for resection of craniopharyngiomas. Neurosurg Focus. 2010;28:E8. doi: 10.3171/2010.1.FOCUS09292. [DOI] [PubMed] [Google Scholar]
- 50.Zada G, Kelly DF, Cohan P, Wang C, Swerdloff R. Endonasal transsphenoidal approach for pituitary adenomas and other sellar lesions: An assessment of efficacy, safety, and patient impressions. J Neurosurg. 2003;98:350–8. doi: 10.3171/jns.2003.98.2.0350. [DOI] [PubMed] [Google Scholar]
- 51.Leng LZ, Greenfield JP, Souweidane MM, Anand VK, Schwartz TH. Endoscopic, endonasal resection of craniopharyngiomas: Analysis of outcome including extent of resection, cerebrospinal fluid leak, return to preoperative productivity, and body mass index. Neurosurgery. 2012;70:110–23. doi: 10.1227/NEU.0b013e31822e8ffc. [DOI] [PubMed] [Google Scholar]
- 52.Patel KS, Komotar RJ, Szentirmai O, Moussazadeh N, Raper DM, Starke RM, et al. Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J Neurosurg. 2013;119:661–8. doi: 10.3171/2013.4.JNS13124. [DOI] [PubMed] [Google Scholar]
- 53.Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, et al. Endoscopic endonasal skull base surgery: Analysis of complications in the authors' initial 800 patients. J Neurosurg. 2011;114:1544–68. doi: 10.3171/2010.10.JNS09406. [DOI] [PubMed] [Google Scholar]


