Abstract
OBJECTIVES
To examine the prospective relationship between self-reported physical activity and aerobic fitness in the Health, Aging and Body Composition (Health ABC) study using the Long Distance Corridor Walk (LDCW).
DESIGN
Cohort study with 7 years follow-up.
SETTING
Two U.S. clinical sites.
PARTICIPANTS
Community dwelling older adults enrolled in Health ABC (n=3075, age 70–79, 52% women, 42% black) with no self-reported difficulty walking one-quarter mile or climbing 10 steps.
MEASURES
Participants were classified based on a physical activity questionnaire as being inactive (≤1,000 kcal/week exercise activity and ≤2,719 kcal/week total physical activity), lifestyle active (≤1,000 kcal/week exercise activity and >2,719 kcal/week total physical activity), or exercisers (reporting ≥ 1,000 kcal/week exercise activity). The Long Distance Corridor Walk,an endurance walking test (400m), was administered at Year 1 (baseline), 2, 4, 6, and 8 to assess aerobic fitness.
RESULTS
At baseline, LDCW completion times (adjusted for age and sex) were 351.8 (95% Confidence Interval= 346.9–356.8), 335.9 (95% CI= 332.7–339.1), and 307.7 (95% CI= 303.2–312.3) seconds for the inactive, lifestyle active, and exerciser groups, respectively (P<0.001). Slowing from baseline to Year 8 was 36.1 (95% CI= 28.4–43.8), 38.1 (95% CI= 33.6–42.4), and 40.8 (95% CI= 35.2–46.5) seconds for the inactive, lifestyle active, and exerciser groups, respectively and did not differ significantly between groups. In linear mixed-effects models, the rate of change in LDCW time did not differ across groups, although exercisers consistently had the fastest completion times (P<0.001 for all pair wise comparisons).
CONCLUSIONS
Decline in the LDCW time occurred regardless of baseline activity. However, exercisers maintained higher aerobic fitness, which may delay reaching critically low threshold of aerobic fitness where independence is impaired.
Keywords: aerobic fitness, physical activity, 400m walk
INTRODUCTION
Declines in aerobic fitness and associated cardiorespiratory changes are hallmarks of the aging process.1–7 Previous studies have suggested that aerobic fitness—a measure of maximal aerobic capacity—peaks in the early to mid-20s and decreases thereafter, with the steepest decline observed after the age of 45.5,7–11 Age-related declines in maximal heart rate, forced expiratory volume, and lean body tissue seem to explain much of the observed aerobic fitness deterioration.12–14 Low aerobic fitness in older adults is independently associated with functional limitations and disability.15 For those with very low fitness levels, basic household activities may require a considerable percentage of an individual’s maximal aerobic capacity,16 making basic tasks difficult and fatiguing—potentially threatening independence. Additionally, poor fitness is an important predictor for all-cause mortality.17
Early exercise physiology studies suggested that those who participate in high levels of physical activity have slower relative rates of decline in aerobic capacity compared to sedentary participants.9–11,18 However, these studies were often focused on highly specialized groups, limiting their generalizability to the general public. Although physical activity and exercise increase aerobic fitness,18–20 epidemiologic evidence suggests that the rate of decline in aerobic fitness (maximal aerobic capacity from a treadmill based test) does not vary by physical activity level.8 However, there are many limitations to using maximal aerobic capacity treadmill-based tests to measure aerobic fitness in older adults.21 These vigorous tests have a high subject burden, require expensive equipment and specialized staff training, and it is difficult for older adults to reach a true maximal effort.22,23 Further, maximal exercise tests have stringent eligibility criteria22,24—particularly related to cardiovascular risk factors—which exclude a large portion of older adults.
Due to these limitations, other performance-based tests have been developed to measure aerobic fitness that may be more appropriate for older adults. One such measure, the Long-Distance Corridor Walk (LDCW), provides a valid estimate of peak aerobic capacity for older adults25 and has been shown to be associated with the development of cardiovascular disease, mobility limitations, mobility disability, and total mortality.26,27
This study aims to examine longitudinal changes in LDCW performance with respect to baseline physical activity status, defined using both type and intensity of activities and established cut-points.28 We hypothesized that the observed longitudinal decline in LDCW performance will vary by physical activity group, with the most active participants having a slower decline in LDCW performance compared to the least physically active participants.
METHODS
Participants
The study population was participants in the Health, Aging and Body Composition (Health ABC) study. Briefly, Health ABC is a longitudinal cohort study of 3075 community-dwelling older adults (age 70–79 at baseline; 52% women; 42% black.) from Pittsburgh, PA and Memphis, TN aimed at investigating factors related to the development of functional limitation and disability. White participants were recruited by mailing to a random sample of Medicare beneficiaries in selected zip codes, while black participants were recruited from all age-eligible residents in these areas. To be eligible for the study, participants had to report no difficulty in walking ¼ mile, climbing 10 steps, or performing any basic activity of daily living; be free of any life-threatening cancers; and plan to remain in the study area for at least three years.29 Participants were recruited between April 1997 and June 1998 and provided written informed consent. All protocols associated with the Health ABC study were approved by institutional review boards at the respective sites.
Physical Activity Assessment
A modified version of the Minnesota Leisure Time Physical Activity Questionnaire (MLTPAQ)30 was administered at baseline. Physical activity measured using this modified questionnaire developed for Health ABC has been shown to be associated with physical function,28 incident mobility limitation31 and brain structure.32 Participants were first asked if they performed specific activities at least 10 times in the past year. Follow-up questions for affirmative responses included if they performed the activity in the past 7 days and the number of hours spent in the activity. Activities from the questionnaire were then divided to create two components: physical activity, which included household chores, paid and volunteer work, care giving, stair climbing, routine walking, and other lifestyle activities; and intentional exercise, which consisted of walking for exercise, aerobic dance, weight lifting, eight specific moderate-intensity activities and 10 specific high-intensity exercise activities (e.g. exercise classes and weight lifting). Energy costs were calculated in kcal/week for both physical activity and intentional exercise using the metabolic equivalent for each task 33 and multiplying by the number of hours spent in the activity and by participant body weight in kilograms. Participants were grouped based on calculated energy expenditure for physical activity and exercise.28 Groups included inactive participants (those reporting <1,000 kcal/week of exercise activity and ≤2,719 kcal/week of total physical activity), lifestyle active (reporting <1,000 kcal/week of exercise activity, but >2,719 kcal/week of total physical activity) and exercisers (≥1,000 kcal/week of exercise activity, regardless of physical activity energy expenditure).
Walking Endurance Assessment for Aerobic Fitness
The LDCW, an endurance walking test, was administered at baseline (Year 1) and follow-up Years 2, 4, 6, and 8 to assess aerobic fitness, 25 an indicator of aerobic capacity. The test was administered in a dedicated corridor with two traffic cones spaced 20 meters apart. Participants walked 10 laps around the cones for a total of 400 meters and were given standard encouragement at each lap. Heart rate was recorded for each lap and blood pressure was measured at the end of the test. This test included a two minute warm-up walk where the participant was instructed to “cover as much ground as possible” followed immediately by the LDCW performed “as quickly as possible at a pace that can be maintained for 400 meters.”34 Distance walked in the two-minute warm-up was measured and completion time for the 400m walk was recorded in seconds. Exclusion criteria included: systolic blood pressure >200 mmHg, resting pulse of ≥120 beats per minute, electrocardiogram abnormality, or cardiac surgery, worsening of chest pain or shortness of breath in the prior three months. The test was stopped if heart rate surpassed 135 beats per minute, or for lightheadedness, dizziness, chest pain, shortness of breath or leg pain.
Physical Function
Lower extremity function was assessed using self-reported ease of walking ¼ mile and the Short Physical Performance Battery (SPPB) score. Briefly, the SPPB consists of three components: standing balance (standing with feet together, semi-tandem stand, and tandem stand, each for 10 seconds), usual paced walking time over 6 meters, and 5 chair stands performed as quickly as possible without using hands or arms to push off. 35 Each component carries a score of 0–4, with total scores ranging from 0–1235 Higher scores indicate better physical functioning.
Health History
History of specific diseases and conditions were included as potential confounders based on their potential influence on physical activity participation or aerobic fitness. These included baseline self-report history of: heart attack, stroke, congestive heart failure and lung disease (chronic obstructive pulmonary disease, asthma, or emphysema), depression, osteoarthritis at the hip or knee, peripheral artery disease, diabetes and osteoporosis. Self-reported symptoms of pain in the lower extremities while walking, back pain, or shortness of breath while walking were also included.
Covariates
Clinical site, baseline age, sex, and race were included as demographic characteristics. Smoking history (never, former, current) and health status (excellent, very good, good, fair, or poor) were reported by questionnaire at baseline. Body mass index (BMI) was calculated in weight in kilograms per squared height in meters using a standard physician’s balance scale and stadiometer, respectively. Season of baseline visit was included as a covariate due to the seasonal variability in physical activity patterns.36
Statistical Analysis
Descriptive statistics for each group were calculated using chi-square tests for categorical variables and analysis of variance for continuous variables. Tests were conducted to detect a linear trend across the three ordered groups. Participants (n=3,075) were classified as not attempting the test due to meeting exclusion criteria, being eligible for the LDCW but being unable to complete 10 laps, or as completing the LDCW. The odds of baseline LDCW completion status were calculated using logistic regression models. First an unadjusted model was run, and then a second model adjusting for age, sex, race and other factors which varied across the ordered physical activity groups or were related to completion status was built.
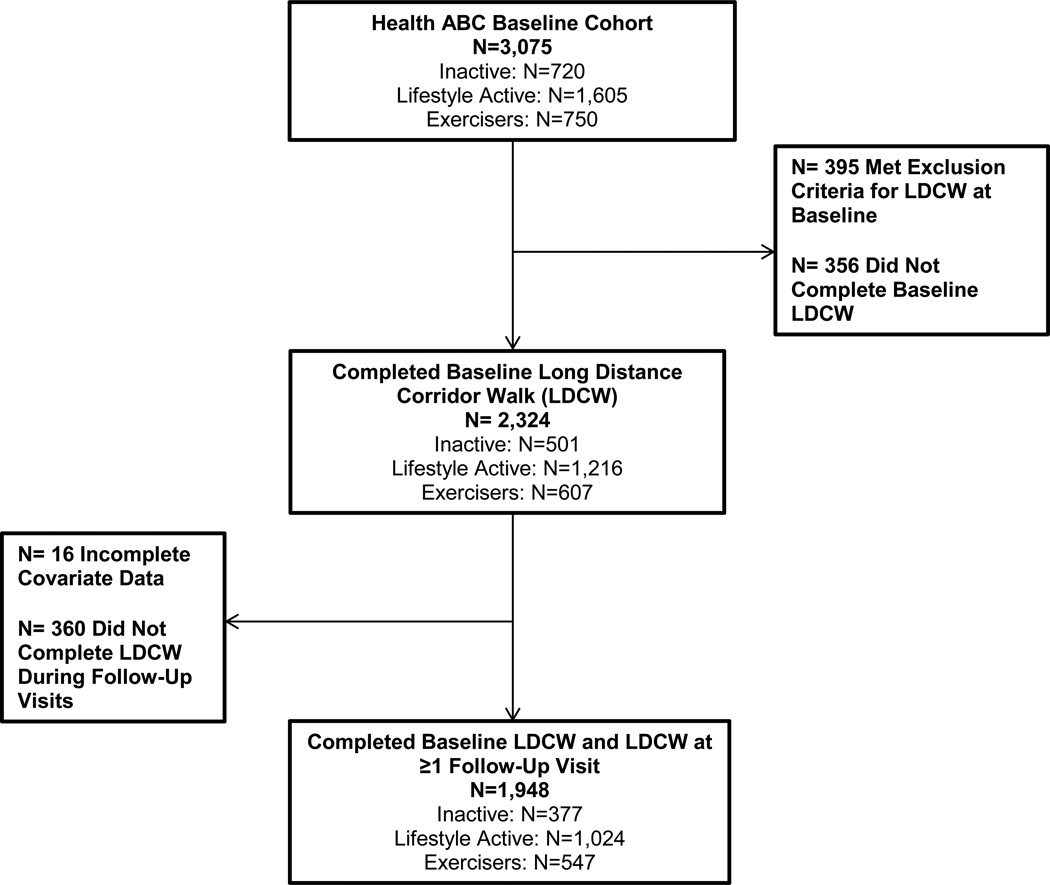
Linear mixed-effects models were used to assess the association of baseline physical activity category with rate of continuous LDCW time change. Because only a subset of participants had LDCW completion times at all follow-up visits (n=592), we chose mixed models to maximize all available data. Only participants with a baseline LDCW completion time and at least one follow-up completion time were included in the models (n=1,948). Figure 1 describes the number of participants who completed the LDCW at baseline and during at least one follow-up visit. We used t-tests or Wilcoxon signed rank tests for non-normally distributed continuous variables, two-sample t-tests for normally distributed continuous variables and chi-square tests for categorical variables to compare those who were included in the model or not.
Figure 1.
Participant Flow Diagram from Health ABC Cohort
Models were built progressively, starting with an unadjusted model for LDCW completion time predicted by physical activity group and an interaction term for activity group and visit. Health ABC visit year (1, 2, 4, 6, and 8) was used in the model as the time parameter. Interaction terms of the variable and time indicate the contribution of that variable with the rate of LDCW time over the course of the study. We then built a second model adding common covariates (age, sex, race, site, body mass index, baseline health status, and season of baseline visit) and interaction terms for each of the covariates with visit. The third model included all factors from the second model reaching a significance of P<0.10 as well as health history, self-reported symptoms, and physical functioning variables as well as the interaction terms of visit with these covariates. We then used backwards selection to build a final parsimonious model with only factors reaching a significance of P<0.05. All models included a random intercept for each participant and a random slope for the visit parameter. We did not impose any structure on the covariance matrix of the random effects. Finally, we compared the slopes and LDCW completion times for each group at all visit points. All data analyses were performed using STATA version 12.1 (StataCorp, College Station, TX).
RESULTS
When grouping participants into physical activity categories, 23% were classified as inactive, 52% as lifestyle active and 25% as exercisers (Table 1). Inactive participants were slightly older, more likely to be women, black, and self-report fair or poor baseline health status (P<0.05 for all trends). Inactive participants also had lower SPPB scores, were more likely to report back pain at least fairly often, shortness of breath or pain in the lower extremities while walking and a history of osteoporosis, stroke, lung disease, or diabetes (P<0.01). The exerciser group, however, was more likely to have a history of heart attack compared to the other lifestyle active and inactive participants (P=0.01).
Table 1.
Baseline Characteristics of Health ABC Participants by Baseline Physical Activity Category
| Characteristic | Inactive (N= 720) |
Lifestyle Active (N= 1605) |
Exerciser (N=750) |
P-value for trend |
|---|---|---|---|---|
| Age (years), Mean ± SD | 74.0 ± 2.92 | 73.5 ± 2.86 | 73.6 ± 2.85 | 0.03 |
| Pittsburgh Site | 41.6 (300) | 49.8 (800 ) | 56.9 (427) | <0.001 |
| Female Sex, % (N) | 56.3 (405) | 57. (922) | 34.3 (257) | <0.001 |
| Black Race, % (N) | 50.3 (362) | 44.6(715) | 27.2 (204) | <0.001 |
| BMIa (kg/m2), Mean ± SD | 26.6 ± 5.47 | 27.5 ± 4.96 | 26.85 ± 4.37 | 0.63 |
| Ever Smoker, % (N) | 58.6 (422) | 53.2 (853) | 59.6 (447) | 0.67 |
| Baseline Health Fair or Poor, % (N) | 23.2 (167) | 16.9 (271) | 7.5 (56) | <0.001 |
| Season of Baseline Visit, % (N) | ||||
| Spring | 31.5 (227) | 33.5 (538) | 38.5 (289) | 0.02 |
| Summer | 18.8 (136) | 23.0 (369) | 20.1 (151) | 0.77 |
| Autumn | 25.6 (184) | 21.2 (341) | 21.3 (160) | 0.02 |
| Winter | 22.4 (161) | 22.7 (365) | 20.5 (154) | 0.55 |
| Health History, % (N) | ||||
| Heart Attack | 10.4 (75) | 10.7 (172) | 14.7 (110) | 0.01 |
| Stroke | 3.5 (25) | 2.3 (37) | 1.3 (10) | 0.01 |
| Congestive Heart Failure | 8.2 (59) | 192 (12.0) | 73 (9.7) | 0.55 |
| Lung Diseaseb | 21.5 (155) | 19.2(308) | 14.7(110) | 0.001 |
| Osteoporosis | 9.0 (65) | 9.5(152) | 5.1 (38) | 0.005 |
| Depression | 10.4 (75) | 9.3 (149) | 9.1 (68) | 0.38 |
| Osteoarthritis in knee or hip | 8.2 (59) | 12. (192) | 9.7 (73) | 0.36 |
| Peripheral Artery Disease | 4.2 (30) | 3.9 (62) | 4.8 (36) | 0.54 |
| Diabetes | 17.9 (129) | 15.2 (244) | 11.6 (87) | 0.001 |
| Self-Reported Symptoms, % (N) | ||||
| Lower Extremity Pain While Walking | 25.8 (186) | 22.0 (353) | 15.7 (118) | <0.001 |
| Back Pain Fairly Often or More | 24.2 (174) | 22.5(361) | 16.0 (120) | <0.001 |
| Shortness of Breath While Walking | 36.9 (266) | 34.0 (546) | 21.6 (162) | <0.001 |
| Physical Functioning | ||||
| Short Physical Performance Battery | 7.5 ± 2.98 | 8.1 ± 2.85 | 9.0 ± 2.39 | <0.001 |
| Report Walking ¼ Mile is “Very Easy”, % (N) |
63.5 (457) | 65.4(1050) | 73.7 (553) | <0.001 |
BodyMass Index
Lung disease includes a history of asthma, chronic obstructive pulmonary disease, or emphysema.
At baseline, 395 participants of the original 3,075 met exclusion criteria for the LDCW, 356 were eligible but unable to complete the walk, and 2,324 participants completed the full LDCW. Of these, a total of 1,948 participants were included in the longitudinal analyses, after excluding those with missing data (n=16) or those who did not have at least one other LDCW completion time after baseline (n=360). Those in the exercise group were 1.9 times (P<0.001) more likely to complete the walk at baseline compared to inactive participants in the unadjusted model. Differences in completion status at baseline between groups were attenuated by poorer health and functional characteristics of the inactive group.
Baseline LDCW completion times adjusted for age and sex were 351.8 (95% Confidence Interval= 346.9–356.8), 335.9 (95% CI= 332.7–339.1), and 307.7 (95% CI= 303.2–312.3) seconds for the inactive, lifestyle active, and exerciser groups, respectively (P<0.001). At baseline, 13%, 8%, and 3% of the inactive, lifestyle active, and exerciser participants required greater than 7 minutes to complete the LDCW, respectively (P<0.001 for trend). Slowing from baseline to Year 8 was 36.1 (95% CI= 28.4–43.8), 38.1 (95% CI= 33.6–42.4), and 40.8 (95% CI= 35.2–46.5) seconds for the inactive, lifestyle active, and exerciser groups, respectively and did not differ significantly between groups (P=0.76).
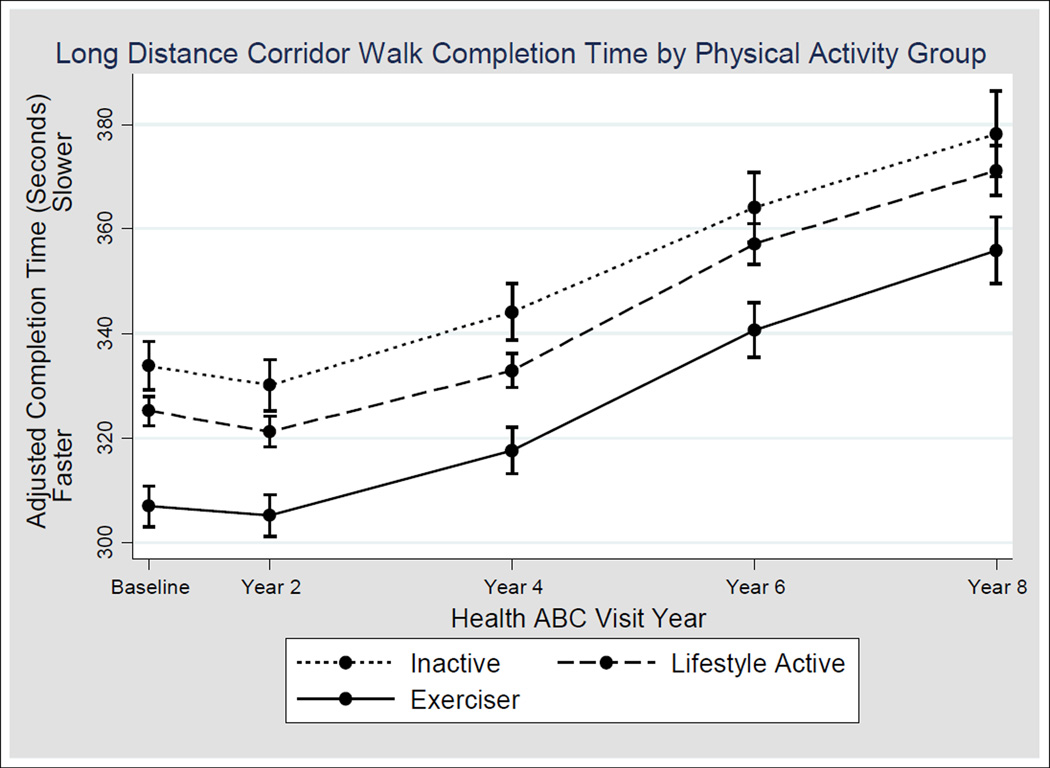
Although all groups slowed during the study follow up (Figure 2), interaction terms in the mixed models indicating varying rates of slowing by physical activity group were not significant in any of the models at any visit. Overall, in final adjusted model, the effect of being in the exerciser and lifestyle active group was a 26.9 (95% CI= 20.7–32.9) seconds and 8.6 (95% CI= 3.2–14.0) seconds faster completion time, respectively, compared to the inactive group (P<0.001 for both). As is evident in Figure 4, completion times for the inactive group at baseline are slower than completion for the exerciser group at Year 4, and this trend remains throughout the study period. No statistically significant difference in completion existed time at Year 2 compared to baseline, despite a slight decrease in time. The mean number of observations in the model for each participant was 3.7. However, the number of observations per participant varied by group, with a mean of 3.5, 3.6, and 3.8 completion times in the inactive, lifestyle active, and exerciser groups, respectively (P<0.01 for all pair wise comparisons.) A significant trend was found at each follow-up visit that exercisers were most likely to complete the LDCW while the inactive participants were the least likely (P-value for trend was 0.05 for all follow-up visits). Those who could not be included in the longitudinal analysis (n=1,127) were older (baseline age 74.0 vs. 73.4 years), more likely to be women (41.8% vs. 31.2%), black (48.0% vs. 28.5%), had a higher baseline BMI (28.3 vs. 26.8 kg/m2) and were less likely to be in the exerciser group compared to those who were included in the model (P<0.001 for all comparisons).
Figure 2.
Change in Long Distance Corridor Walk (LDCW) Completion Time by Physical Activity Group. The figure represents the longitudinal completion times (mean and 95% confidence interval) based upon final model (adjusted for age, sex, body mass index, Short Physical Performance Battery (SPPB) score, poor health at baseline, reporting pain while walking, reporting that walking ¼ mile is easy, SPPB score and associated interactions with time as well as main effects for race and indicating that walking ¼ mile is easy) for the 1948 participants who had a baseline LDCW completion time and at least one other completion time observation. The number of participants with a completion time at each visit is as follows: Baseline visit: n=1948 (n=377 inactive, n=1024 lifestyle active, n=547 exercisers), Year 2: n=1635 (n=304 inactive, n=806 lifestyle active, n=471 exercisers), Year 4: n=1419 (n=267 inactive, n=732 lifestyle active, n=420 exercisers), Year 6: n=1201 (n=197 inactive, n=636 lifestyle active, n=368 exercisers), Year 8: n=924 (n=157 inactive, n=477 lifestyle active, n=290 exercisers).
DISCUSSION
This study found that the rate of decline in LDCW performance for older adults over 7 years follow-up was similar for participants regardless of physical activity status, though exercisers had consistently better performance throughout the study period. In particular, more than a four-year difference in mean times existed between the exerciser and inactive groups. Loss of endurance and aerobic fitness over time may be a fundamental aspect of aging, and clinicians should be aware that even the most active older adults may be experiencing these declines. Never the less, older adults who reach higher peak aerobic fitness levels in life may delay crossing thresholds of very low fitness where activities of daily living are impaired. Future studies should examine an ideal time and methodology for intervening before impairment.
Results from the initial validation study of the LDCW indicated that requiring >7 minutes (420 seconds) to complete the LDCW is approximately equivalent to an aerobic capacity level of <12 mL O2 per kilogram of body weight per minute.25 This is a critical threshold of aerobic capacity where community living may be seriously compromised.37 At baseline, a larger proportion of participants in the inactive group (13%) had completion times greater than >7 minutes compared to the lifestyle active (8%) and exerciser groups (3%). Given the adverse outcomes related to very low fitness levels—including death, cardiovascular disease, and mobility limitations and disability—this trend is likely at least a partial explanation as to why fewer inactive participants had follow-up data. Furthermore, other previous work with the LDCW in this cohort revealed that each additional 30 seconds needed to complete the LDCW was associated with a higher likelihood of incident mobility disability in two years by 65% in women and 37% in men27. At baseline, on average the exerciser group completed the LDCW slightly above five minutes (mean 307.7 seconds), while the lifestyle active and inactive groups needed an additional 35.9 and 51.8 seconds, respectively, above five minutes (adjusted for age and sex). Even in the final adjusted mixed model, being in the inactive group was associated with completing the LDCW nearly 30 seconds slower than the exerciser group, indicating the importance of participating in physical activities of higher duration and intensity compared to inactivity.
The lifestyle active group did not have significantly different completion times compared to the inactive group; however, evidence of a dose-response relationship existed between higher physical activity and LDCW faster completion times. Lifestyle activity was beneficial over inactivity, indicated by the faster completion times the lifestyle active group had compared to the inactive group. Given that the population as a whole is becoming less active over time,38 morbidity and mortality related to low aerobic fitness may increase as the highly inactive population reaches older ages. Extremely low aerobic fitness levels in old age lead to a diminished energy reserve for performing daily activities and sustaining homeostasis, potentially making even basic tasks difficult and fatiguing.39
The findings in this study are consistent with previous work by Fleg and colleagues examining longitudinal changes in aerobic fitness based on aerobic capacity measured using maximal treadmill testing in the BLSA,8 though fundamental differences exist between the methods used in defining physical activity groups and assessing aerobic fitness. In this study, physical activity categories were classified using the same methods that have been associated with physical functioning and incident mobility limitation in previous studies and take into account type and intensity of activities,40,28 rather than population based cutoffs. Population based-cutoffs can be problematic since participants in the highest percentile may still be considered inactive by traditional definitions. Additionally, the use of a performance-based test with broad inclusion criteria adds to the clinical relevance of this work. Over-ground walking tests are much more feasible to incorporate into clinical, rehabilitation, and research settings compared to maximal treadmill testing. Additionally, over-ground walking tests can be used for assessing fitness of many older adults who may be excluded from maximal exercise testing, allowing for participants with a wider range of functional and health status to be examined‥ The large sample size, well characterized study population, and several years of follow-up time also add to the strengths of this study.
Although the use of mixed models helped us maximize the available data, the proportion of participants who were not included in the models differed between physical activity group, with inactive participants being the most likely to be excluded. Additionally, those in the exerciser group had more observations over the time period compared to the lifestyle active and inactive groups. At baseline, exercisers were nearly twice as likely to complete the LDCW compared to inactive participants. This relationship was attenuated by the poor health factors of the inactive group, though the exerciser group may have been in better health because they exercise. Likely, participants excluded from the analysis had lower aerobic fitness levels than those who were included. Thus, the observed differences between the physical activity groups may in fact be an underestimate of the true relationship. The most inactive participants may have truly had a faster decline in aerobic fitness compared to the most active participants, but if those very inactive participants could not complete the LDCW, we could not detect the trend.
A limitation to this study is that physical activity was not measured in a consistent manner throughout the study follow-up period and we were unable to use the physical activity category as a time-varying predictor. Although possibly participants may not have remained in the same physical activity category over time, the trend is that older adults become less active over time, rather than more active.41 Limitations exist in assessing physical activity via self-report in older adults, particularly in regards to recall issues. Advances in activity monitoring have greatly changed the field of physical activity epidemiology, though these monitors are likely not feasible for use in many clinical settings—especially given their cost and the time and analytic skills needed to process activity monitor data. Well designed and validated physical activity questionnaires can still be important to use in clinical settings in order to screen participants for physical activity counseling or interventions.
These results indicate that the level of self-reported physical activity is related to LDCW completion and walking speed, but is not predictive of the rate of change in LDCW performance. This suggests that the rate of decline in aerobic fitness associated with aging may not be avoidable. However, older adults who reach higher peak aerobic fitness levels earlier in life could potentially experience a delay in crossing thresholds where activities of daily living are impaired. This is an area worthy of future investigation. Future studies should determine whether interventions increasing physical activity can slow the reduction in fitness over time, and whether a delay in crossing below thresholds of low aerobic fitness also prevent impairments of activities of daily living.
Acknowledgments
ABN and ESS declare grant support from the National Institute on Aging (NIA) grants N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, R01-AG028050, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. BLM declares funding by T32AG000181. JSB declares grant support from PCORI (#6301).
Sponsor’s Role: The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest:
| Elements of Financial/Personal Conflicts |
Lange-Maia, Brittney | Strotmeyer, Elsa S. | Harris, Tamara B. | Simonsick, Eleanor M. | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts |
Glynn, Nancy W. | Brach, Jennifer S. | Cauley, Jane A. | Richey, Phyllis A. | Schwartz, Ann V. | Newman, Anne B. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
|
Employment or Affiliation |
x | x | x | x | x | x | ||||||
| Grants/Funds | x | x | x | x | x | x | ||||||
| Honoraria | x | x | x | x | x | x | ||||||
| Speaker Forum | x | x | x | x | x | x | ||||||
| Consultant | x | x | x | x | x | x | ||||||
| Stocks | x | x | x | x | x | x | ||||||
| Royalties | x | x | x | x | x | x | ||||||
| Expert Testimony | x | x | x | x | x | x | ||||||
| Board Member | x | x | x | x | x | x | ||||||
| Patents | x | x | x | x | x | x | ||||||
|
Personal Relationship |
x | x | x | x | x | x | ||||||
Author Contributions: BLM, JAC, and ABN were involved in the study concept and design, analysis, interpretation of data, and preparation of the manuscript. ABN was involved in acquisition of the data. All other coauthors were involved in the interpretation of data and preparation of the manuscript.
REFERENCES
- 1.Buskirk ER, Hodgson JL. Age and aerobic power: the rate of change in men and women. Fed Proc. 1987;46:1824–1829. [PubMed] [Google Scholar]
- 2.Gerstenblith G, Lakatta EG, Weisfeldt ML. Age changes in myocardial function and exercise response. Prog Cardiovasc Dis. 1976;19:1–21. doi: 10.1016/0033-0620(76)90005-0. [DOI] [PubMed] [Google Scholar]
- 3.Astrand I, Astrand PO, Hallback I, et al. Reduction in maximal oxygen uptake with age. J Appl Physiol. 1973;35:649–654. doi: 10.1152/jappl.1973.35.5.649. [DOI] [PubMed] [Google Scholar]
- 4.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand. 1960;49(Suppl):1–92. [PubMed] [Google Scholar]
- 5.Ogawa T, Spina RJ, Martin WH, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 6.Fleg J, Strait J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Fail Rev. 2012;17:545–554. doi: 10.1007/s10741-011-9270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inbar O, Oren A, Scheinowitz M, et al. Normal cardiopulmonary responses during incremental exercise in 20- to 70-yr-old men. Med Sci Sports Exerc. 1994;26:538–546. [PubMed] [Google Scholar]
- 8.Fleg J, Morrell C, Bos A, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 9.Astrand PO, Bergh U, Kilbom A. A 33-yr follow-up of peak oxygen uptake and related variables of former physical education students. J Appl Physiol. 1997;82:1844–1852. doi: 10.1152/jappl.1997.82.6.1844. [DOI] [PubMed] [Google Scholar]
- 10.Rogers MA, Hagberg JM, Martin WH, et al. Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol. 1990;68:2195–2199. doi: 10.1152/jappl.1990.68.5.2195. [DOI] [PubMed] [Google Scholar]
- 11.Eskurza I, Donato AJ, Moreau KL, et al. Changes in maximal aerobic capacity with age in endurance-trained women: 7-yr follow-up. J Appl Physiol. 2002;92:2303–2308. doi: 10.1152/japplphysiol.01124.2001. [DOI] [PubMed] [Google Scholar]
- 12.Hollenberg M, Yang J, Haight T, et al. Longitudinal changes in aerobic capacity: Implications for concepts of aging. J Gerontol A Biol Sci Med Sci. 2006;61A:851–858. doi: 10.1093/gerona/61.8.851. [DOI] [PubMed] [Google Scholar]
- 13.Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1988;65(3):1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 14.Booth FW, Weeden SH, Tseng BS. Effect of aging on human skeletal muscle and motor function. Med Sci Sports Exerc. 1994;26:556–560. [PubMed] [Google Scholar]
- 15.Morey M, Pieper C, Cornoni-Huntley J. Physical fitness and functional limitations in community-dwelling older adults. Med Sci Sports and Exerc. 1998;30:715–723. doi: 10.1097/00005768-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Arnett SW, Laity JH, Agrawal SK, et al. Aerobic reserve and physical functional performance in older adults. Age and ageing. 2008;37:384–389. doi: 10.1093/ageing/afn022. [DOI] [PubMed] [Google Scholar]
- 17.Blair S, Kampert J, Kohl H, et al. Influences of Cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 18.Dehn MM, Bruce RA. Longitudinal variations in maximal oxygen intake with age and activity. J Appl Physiol. 1972;33:805–807. doi: 10.1152/jappl.1972.33.6.805. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher G, Balady G, Blair S, et al. Statement on exercise: Benefits and recommendations for physical activity programs for all Americans. Circulation. 1996;96:857–862. doi: 10.1161/01.cir.94.4.857. [DOI] [PubMed] [Google Scholar]
- 20.Astrand PO. Physical activity and fitness. Am J Clin Nutr. 1992;55:1231S–1236S. doi: 10.1093/ajcn/55.6.1231S. [DOI] [PubMed] [Google Scholar]
- 21.Huggett DL, Connelly DM, Overend TJ. Maximal aerobic capacity testing of older adults: A critical review. J Gerontol A Biol Sci Med Sci. 2005;60:57–66. doi: 10.1093/gerona/60.1.57. [DOI] [PubMed] [Google Scholar]
- 22.Church T, Gill T, Newman A, et al. Maximal fitness testing in sedentary elderly at substantial risk of disability: LIFE-P Study experience. J Aging Phys Act. 2008;16:408–415. doi: 10.1123/japa.16.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollenberg M, Ngo LH, Turner D, et al. Treadmill exercise testing in an epidemiologic study of elderly subjects. J Gerontol A Biol Sci Med Sci. 1998;53A:B259–B267. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- 24.ACSM. ACSM’s Guidelines for Exercise Testing and Prescription. Ninth. American College of Sports Medicine; 2013. [DOI] [PubMed] [Google Scholar]
- 25.Simonsick E, Fan E, Fleg J. Estimating Cardiorespiratory ritness in well-functioning older adults: Treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006 Jan;54:127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 26.Newman A, Simonsick E, Naydeck B, et al. Association of Long-Distance Corridor Walk Performance with Mortality, Cardiovascular Disease, Mobility Limitation, and Disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 27.Simonsick EM, Newman AB, Visser M, et al. Mobility limitation in self-described well-functioning older adults: Importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63:841–847. doi: 10.1093/gerona/63.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brach J, Simonsick E, Kritchevsky S, et al. The association between physical function and lifestyle activity and exercise in the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 29.Simonsick E, Newman A, Nevitt M, et al. Measuring higher level physical function in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2001:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 30.Taylor H, Jacobs D, Shucker B. A questionnaire for the assessment of leisure-time physical activities. J Chronic Dis. 1978:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 31.Koster A, Patel KV, Visser M, et al. Joint effects of adiposity and physical activity on incident mobility limitation in older adults. J Am Geriatr Soc. 2008;56:636–643. doi: 10.1111/j.1532-5415.2007.01632.x. [DOI] [PubMed] [Google Scholar]
- 32.Tian Q, Erickson KI, Simonsick EM, et al. Physical activity predicts microstructural integrity in memory-related networks in very old adults. J Gerontol A Biol Sci Med Sci Oct. 2014;69:1284–1290. doi: 10.1093/gerona/glt287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ainsworth B, Haskell W, Leon A. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993:71–83. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Simonsick E, Montgomery P, Newman A, et al. Measuring fitness in healthy older adults: The Health ABC long distance corridor walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 35.Guralnik J, Simonsick E, Ferrucci L, et al. Short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 36.Tucker P, Gilland J. The effect of season and weather on physical activity: A systematic review. Public Health. 2007;121:909–922. doi: 10.1016/j.puhe.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Binder EF, Birge SJ, Spina R, et al. Peak aerobic power is an important component of physical performance in older women. J Gerontol A Biol Sci Med Sci. 1999;54:M353–M356. doi: 10.1093/gerona/54.7.m353. [DOI] [PubMed] [Google Scholar]
- 38.Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: What are the contributors? Annu Rev Publ Health. 2005;26:421–443. doi: 10.1146/annurev.publhealth.26.021304.144437. [DOI] [PubMed] [Google Scholar]
- 39.Schrack J, Simonsick E, Ferrucci L. The energetic pathway to mobility loss: An emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58:S329–S336. doi: 10.1111/j.1532-5415.2010.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser M, Simonsick E, Colbert L, et al. Type and intensity of activity and risk of mobility limitation: The mediating role of muscle parameters. J Am Geriatr Soc. 2005;53:762–770. doi: 10.1111/j.1532-5415.2005.53257.x. [DOI] [PubMed] [Google Scholar]
- 41.Xue Q-L, Bandeen-Roche K, Mielenz TJ, et al. Patterns of 12-year change in physical activity levels in community-dwelling older women: Can modest levels of physical activity help older women live longer? Am J Epidemiol. 2012;176:534–543. doi: 10.1093/aje/kws125. [DOI] [PMC free article] [PubMed] [Google Scholar]