Abstract
Introduction
Many of the symptoms and signs of Parkinson's disease (PD) arise from the death of midbrain dopamine neurons that utilize tyrosine hydroxylase (TH) as the rate-limiting enzyme in catecholamine biosynthesis.
Methods
We investigated whether the presence of a common TH polymorphism affects the clinical outcomes in 101 PD subjects. We further examined the effect of this polymorphism on the purified recombinant enzyme.
Results
PD subjects homozygous for the common V81M polymorphism, have higher overall freezing of gait scores after controlling for disease duration, although this polymorphism does not associate with the occurrence of PD or FOG. In vitro functional assays on pure recombinant wild type TH and V81M TH revealed that the Km of the mutant enzyme for tyrosine was twice that of the wild-type. This polymorphism, however, did not change the stability of the enzyme, nor did it affect the Vmax or Km for the co-substrate BH4.
Conclusion
The data suggest that presence of a homozygous V81M polymorphism is associated with more severe FOG, possibly due to lower catecholamine synthetic capacity. Further studies are warranted to investigate the role of subtle changes in catecholamine availability in the development of FOG.
Keywords: Polymorphism, SNP, Dopamine, Norepinephrine, Movement Disorders
Introduction
Parkinson's disease (PD), the second most common neurodegenerative disorder, presents initially with motor disabilities of resting tremor, bradykinesia, and rigidity[1]. As the disease progresses, gait and balance difficulties also occur. Freezing-of-gait (FOG) is one of common gait disorders in PD, and contributes to significant clinical disability such as falls. The pathological hallmark of PD is the loss of dopamine neurons in the substantia nigra pars compacta, including a reduction in tyrosine hydroxylase (TH), the rate limiting enzyme in catecholamine synthesis[2,3]. This protein loss is most significant at the nerve terminals.
TH is a homotetramer in which each of the subunits contains regulatory, catalytic, and tetramerization domains[2]. The enzyme utilizes tyrosine, BH4 and O2 as co-substrates, and Fe2+ as a cofactor. TH protein levels and activity can be regulated at several points, including mRNA expression, regulation of RNA stability, and translation including post-translational phosphorylation and binding of effector proteins. TH knockout mice are embryonically lethal, but one intact copy is sufficient to maintain dopamine synthesis, as indicated by the fact that heterozygote animals are viable and express normal levels of catecholamines[4,5]. There are genetic polymorphisms in several regions of the TH gene, and some of these have been postulated to be involved in the pathology of PD and other forms of parkinsonism[6,7].
A non-synonymous coding region polymorphism in the regulatory domain of TH, V81M (rs6356), has been described by independent studies with an average reported allelic frequency of 0.33[6,7], but neither the specific effect of this polymorphism on the enzyme, nor the potential functional consequences for PD patients, has been reported. This report is the first such comprehensive analysis on the effect of V81M on clinical presentations of PD and TH function.
Methods
Subjects
Blood samples were obtained from 101 PD patients and 68 controls for DNA analysis. Patients were selected from a large cohort currently followed at the Hershey Medical Center Neurology Outpatient Clinic for an ongoing longitudinal study. PD diagnosis[8] was performed by a movement disorder specialist. All PD subjects involved in this study are managed by a movement disorder specialist and on optimized medication regimens. Disease duration was obtained from subject history. Unified Parkinson's Disease Rating Scale part-3 (UPDRS-III) scores were used as a measure of general motor capability[9] and assessed twice: once 12 h after the last dose of PD medication (UPDRS-OFF) and then shortly after administration of the subject's regular PD medication (UPDRS-ON). The levodopa-equivalent daily dose (LEDD) was calculated to estimate the effect of combined dopaminergic medications on each subject[10]. FOG was assessed using the Freezing of Gait Questionnaire (FOG-Q)[11.12]. Written informed consent was obtained from all subjects and the study was approved by the Penn State Hershey Institutional Review Board (IRB# 40726) and conducted in accordance with the principles of the Declaration of Helsinki.
Genetic Analyses
Venous blood was collected from all participants and DNA extraction performed using the DNeasy Blood & Tissue Kit (QIAGEN; Valencia, CA). We utilized a TaqMan SNP Genotyping assay designed against the rs6356 SNP (Life Technologies, Thermo Fisher Scientific, Grand Island, NY). Follow-up and confirmation sequencing were performed with previously reported primers for exon 3 of the human tyrosine hydroxylase gene using Polymerase Chain Reaction (Hot Start Taq DNA Polymerase, QIAGEN) and Sanger amplicon sequencing to determine the presence of the V81M polymorphism[13]. Agarose gel electrophoresis (1.5% Agarose gel, Sigma-Aldrich, St. Louis, MO, in Tris/Acetic Acid/EDTA Buffer, Bio-Rad, Hercules, CA) of the PCR products (performed at 150 V for 1 h) was followed by gel extraction (NucleoSpin Gel and PCR Clean-up Kit, Macherey-Nagel, Bethlehem, PA). Sanger sequencing was performed to determine the DNA sequence of exon 3 (Eurofins MWG Operon, Huntsville, AL). The results were compared against the genomic sequence of native human TH using ClustalW2 by EBI (http://www.ebi.ac.uk/Tools/msa/clustalw2/), and the complementary strand sequence was obtained using Reverse Complement (http://www.bioinformatics.org/sms/rev_comp.html).
Site-Directed Mutagenesis and Expression of Mutant Recombinant TH Construct
The full length cDNA for hTH1 was purchased from OriGene in a custom vector (Rockville, MD), and the hTH1 coding sequence was cloned (as an N-terminal hexahistidine fusion protein) into the pET28-tev expression vector as described previously for the human tryptophan hydroxylase 2 gene[14]. Site-directed mutagenesis was performed using the QuikChange Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) with the following primers: 5’-GGGAAGGCCATGCTAAACCTG-3’ (forward) and 5’-GAAGAGCAGGTTTAGCATGGC-3’ (reverse) purchased from Integrated DNA Technologies (Coralville, IA). The following parameters were used in the thermal cycler: 30 seconds denaturation at 95°C, 1 minute annealing at 55°C, 8 minutes extension at 68°C, performed for 18 cycles. Following DpnI digestion, the plasmids were transformed into XL-1 Blue supercompetent cells, and positive colonies were selected using kanamycin (25 mg/mL) LB Agar plates (Sigma, St. Louis, MO). Selected colonies were grown overnight in liquid LB cultures at 37°C, and the plasmids isolated using the NucleoSpin Plasmid Isolation Kit (Macherey-Nagel, Bethlehem, PA). The sequencing of the plasmids was performed by Eurofins Operon (Huntsville, AL) with the following primers: 5’-ATGCCCACCCCCGACGCCACC-3’ (forward),5’-CTAGCCAATGGCACTCAGCGCATGG-3’ (reverse), as well as T7 promoter (forward) and T7 terminator (reverse) primers. Positive colonies then were transformed into the BL21-CodonPlus(DE3)-RIL E.coli strain for protein expression.
Bacterial Expression of Wild-Type Human TH
The expression of the recombinant protein in E. coli was established using ZYP-5052 medium as previously described[14,15]. The expression was performed by shaking the cultures at 215 rpm and 15°C until the saturation density (measured at 600 nm) reached 9, after which the cultures were harvested and stored at −80°C.
Purification of Recombinant hTH1
Harvested bacterial cultures were lysed with BugBuster, Amine Free (EMD Chemicals Inc., San Diego, CA) containing benzonase and r-lysozyme with EDTA-free protease inhibitor cocktail. The recombinant hexa-histidine fusion TH was purified on a 5 mL HT Nickel column (GE Healthcare, Pittsburgh, PA, USA), as described previously[14]. 50%-saturated ammonium sulfate was used to precipitate the Ni-column-purified proteins. The pellet then was dissolved in size exclusion chromatography buffer (25 mM HEPES, 100 mM NaCl, 1 mM DTT, 0.1 mM EDTA), and size exclusion chromatography was performed using a 25 mL Superdex 200 10/300 GL column (GE Healthcare). Protein standards were purchased from Sigma (MW-GF-1000 Protein Standard Kit).
SDS-PAGE Analysis
SDS-PAGE analysis was utilized to document the expression and purity of the recombinant proteins. Pre-cast 4-12% NuPAGE BisTris gels (Invitrogen) were used to resolve denatured proteins, as described previously[14]. Coomassie Blue dye was used to visualize the resolved proteins (Pierce).
Enzyme Activity Assay
Tyrosine hydroxylase activities of the pure recombinant proteins were assessed using a radioenzymatic assay that monitors the release of 3H2O, as described previously by Reinhard et al.[16]. Bradford protein assay (Bio-Rad) was used to determine protein concentration. Chemicals used for the activity assay were obtained from Sigma, except for the activated charcoal (Darco G-60, Fisher Scientific). In order to assay for the kinetic parameters, varying concentrations for either one of the substrates were employed in the presence of a fixed concentration of the counterpart (100 μM tyrosine or 100 μM BH4) with ambient oxygen. Resulting activity values were normalized to the amount of protein present to obtain specific activities. Prism Software V.5 (GraphPad, San Diego, CA) was used to calculate kinetic constants (Km, Vmax). The stability assay was performed in a similar manner, with the incubation of the protein at 37°C for varying periods of time up to 90 min. The activity values at different time points were plotted against time on a semi-log plot, and the enzyme activity half-life was calculated.
Statistical Analysis
The differences in Vmax, Km, and t1/2 values were analyzed using Student's t-tests with α = 0.05. These analyses were performed using GraphPad Prism Version 5 (GraphPad Inc, San Diego CA). Analysis of covariance (ANCOVA) was used to compare the FOG-Q scores of the 2 genotype groups (V81M homozygotes vs heterozygotes and homozygous wild type) while controlling for duration of illness. A square root transformation was applied to the FOG-Q score to meet normality assumptions. The statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). Wild type and heterozygous subjects were treated in a consolidated fashion, given that they are statistically indistinguishable, and a well-documented lack of gene-dosage effect, as reported previously[4,5].
Results
The Effect of the V81M Polymorphism on Disease Progression
The main purpose of this study was to investigate the effect of the hTH V81M polymorphism on the progression of PD. Thus, our analyses focused exclusively on PD subjects that were selected to cover a range of disease severity. The controls were only utilized for determination of allelic frequencies within populations with and without PD (Table 1). The minor allele (V81M) frequencies were similar for the PD (0.37) and control (0.38) populations, and consistent with the literature (Table 1) [6; http://www.ncbi.nlm.nih.gov/snp/?term=rs6356]. The V81M polymorphism resides in the regulatory domain of the TH protein (Suppl. Figure 1A), and is the result of a single G to A nucleotide change at position 241 of the coding region. Classic Sanger sequencing (Suppl. Figure 1B) of the samples was performed on exon 3 of the gene (containing the V81M SNP).
Table 1.
General characteristics of human subjects
| PD | Control | |
|---|---|---|
| Number | 101 | 68 |
| Age (year; mean ± SD) | 68.78 ± 9.36 | 65.92 ± 9.07 |
| Gender (M/F) | 55/46 | 32/36 |
| Minor Allele Frequency | 0.371 | 0.375 |
| Genotype (WT/HET/HOM) | 41/44/16 | 25/35/8 |
| FOG-Q (mean ± SD) | 4.86 ± 5.43 | |
| Disease Duration (years) | 6.20 ± 5.88 | |
| UPDRS-III-ON (mean ± SD) | 27.33 ± 19.52 | |
| UPDRS-III-OFF* (mean ± SD) | 34.76 ± 21.07 | |
| LEDD** (mean ± SD) | 659.06 ± 461.54 | |
| Minor Allele Frequency Subjects with Freezing of Gait | 0.381 | |
| Genotype (WT/HET/HOM) Subjects with Freezing of Gait | 32/35/13 | |
| FOG-Q (mean ± SD) Subjects with Freezing of Gait | 6.14 ± 5.42 | |
| Disease Duration (years) Subjects with Freezing of Gait | 7.23 ± 6.14 |
Only 71 subjects were able to complete the UPDRS-III while unmedicated.
The data were unavailable for 2 subjects.
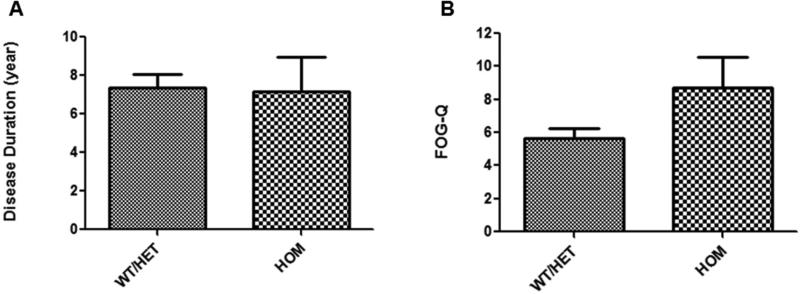
We found a significant association between the presence of the V81M genetic variant and the severity of FOG in PD subjects. As our hypothesis is that the polymorphism will make FOG-Q worse, and not necessarily cause FOG, we excluded patients that had no FOG. The results indicated that homozygous subjects (n=13) had significantly higher FOG-Q scores than individuals with 1 or 2 wild-type alleles (n=67), after controlling for disease duration (Figure 1, p=0.05). When the same analyses were performed with all of the PD subjects that were consented (even including those with no FOG), there was a similar trend, but the differences were not statistical significance (p=0.11, data not shown). This polymorphism did not affect other disease measures including UPDRS-ON, UPDRS-OFF, LEDD, daily levodopa intake or dyskinesia (data not shown). The possible molecular mechanisms underlying the observed association were explored using purified wild type and mutated TH enzymes. It has been previously established that heterozygous TH knock out animals behave similarly to wild type animals with regards to their catecholamine synthesis[4,5]. Hence, the WT and HET subjects were grouped together for analysis. In addition, when observed separately, there was no difference in mean FOG-Q scores for these two groups (p=0.88).
Figure 1.
Disease duration (A) and overall FOG-Q scores (B) of PD subjects that presented with freezing of gait at the time of analysis (mean ± SEM, p=0.05). These data represent subjects with FOG (80 total, 32 wild type [WT], 35 heterozygous [HET], 13 homozygous [HOM]).
Stability of the Mutant hTH Enzyme
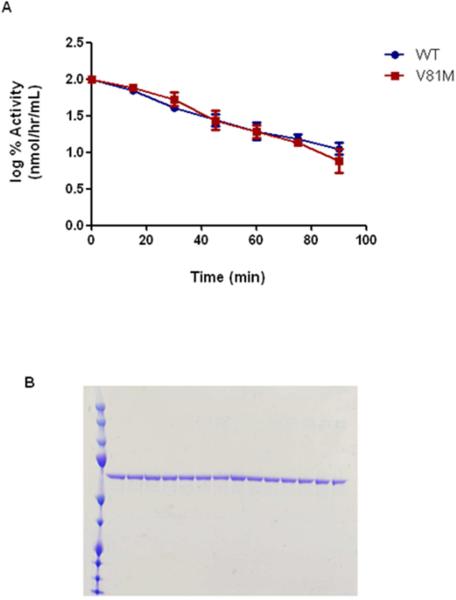
There was no difference between the activity half-lives of the recombinant enzymes (Supplementary Table 1 and Figure 2A). Using gel electrophoresis, the results demonstrated that differences in activity were not due to loss of protein quantity or a result of protein degradation (Figure 2B).
Figure 2.
A) Semi-log graph of the wild-type (WT) and polymorphic (V81M) hTH residual activity plotted against time of incubation at 37°C. B) SDS-PAGE gel of hTH samples assessed following increasing incubation times.
Change in the Enzyme Kinetic Parameters of the Mutant hTH
Table 2 describes the steady state kinetic parameters measured for the pure recombinant wild type and mutant TH. There was no change in the maximal velocity of these constructs or in the Km for BH4. Conversely, the V81M polymorphism resulted in a two-fold increase in the tyrosine Km compared to that of the wild type V81 protein (16 μM vs 33 μM; Table 2).
Table 2.
Steady-state kinetic parameters for purified wild type and mutant hTH enzymes
| Enzyme form | Km, L-Tyr (μM) | Vmax, L-Tyr (nmol/min/mg) | Km, BH4 (μM) | Vmax, BH4 (nmol/min/mg) |
|---|---|---|---|---|
| hTH WT | 16.0 ± 1.8 | 42.3 ± 12.2 | 82.2 ± 15.7 | 43.6 ± 8.9 |
| hTH V81M | 32.8 ± 3.5 | 48.3 ± 15.9 | 83.0 ± 28.3 | 47.7 ± 9.9 |
| p-value | 0.0049 | 0.78 | 0.98 | 0.77 |
Discussion
Although there is a small percentage of familial PD that results from specific polymorphisms in a dozen or so genes, the large majority of PD is sporadic and thought to be the result of a combination of environmental factors interacting with groups of susceptibility genes[1]. In addition, there are polymorphisms in genes that may affect specific clinical presentation of the disease (e.g., psychosis, dyskinesias, or personality disorders) in only small subsets of patients. As a result, it has been very difficult to associate polymorphisms in single genes with aspects of the clinical phenotype for a majority of patients.
FOG has emerged as a clinical measure of PD progression as it progresses proportionately with disease duration and its presence is independent of the type of medical management[17]. FOG arises in most PD patients in the later stages of the disease[18], and both norepinephrine (NE) and dopamine signaling dysfunctions are proposed to contribute to FOG. For example, NE levels are decreased significantly in patients with severe FOG (e.g., reviewed in[19,20]), and symptoms can be attenuated by administration of the norepinephrine precursor 3,4-dihydroxyphenylserine[21-24]. Although it is beyond the scope of this study, it should be noted that the cholinergic system also has been implicated in the pathology of FOG in PD[25]. It is also noteworthy that the TH V81M is associated with the clinical presentation of FOG, and not with the other clinical outcome measures such as levodopa induced dyskinesia and UPDRS-III scores, which are known to be modulated by dopaminergic therapies. This finding is consistent with the hypothesis that FOG may arise from extra-nigral dysfunctions (such as NE) during PD procession. As noted earlier, both dopaminergic and noradrenergic signaling may affect FOG and the latter has been linked to several other PD symptoms including dyskinesia and behavioral issues[26]. Thus, the effects of V81M may be due to changes in both dopamine and noradrenergic transmission. It should be noted that, in addition to the catecholaminergic systems, the cholinergic system and its dysfunction is an important target that has been proposed to underlie FOG [27]. For example, the peduncolopontine nucleus (PPN) has been implicated as a regulator of gait in humans, and PD patients display strong cholinergic neuron loss within this structure [27]. Artificial stimulation of the PPN has also been shown to improve gait difficulties and postural stability in PD subjects [27]. The work presented in this manuscript does not address non-catecholaminergic systems in FOG. It is possible that the association between TH V81M and more severe FOG-Q scores presented here is due to a change in interplay between different neurotransmitter systems, rather than an isolated catecholaminergic event. The current study does not fully elucidate the mechanism by which the TH V81M polymorphism is associated with more severe FOG. In fact, if the effects were solely due to changes in TH activity, L-DOPA would be expected to by-pass this step and result in a global increase in catecholamines in the brain. Therefore, it is likely that the current observation occurs due to the time-dependent impact of decreased noradrenergic function (e.g., neuroprotection or neuronal connectivity).
In the current study, we have also shown that the relatively common V81M polymorphism doubles the Km of TH for its tyrosine substrate, but does not affect enzyme stability or the enzyme's utilization of the pterin co-substrate (BH4). It is known that the plasma and tissue concentrations of tyrosine are in the range of the measured Km values[28,29]. Thus, although the doubling of Km in V81M is a relatively small change, this could potentially decrease the production of l-DOPA. It should be noted, however, that this particular study does not measure catecholamine levels in polymorphic tissues. When there is already limited dopamine and norepinephrine, this kinetic effect might play a role in FOG. Accordingly, the subject group that was homozygous for the TH V81M polymorphism had higher FOG-Q scores; hence, their FOG symptoms were worse compared to their counterparts. It is not surprising that the change in FOG-Q scores was observed significantly only in the homozygous PD subjects, because it is known that one intact copy of the TH allele is sufficient to compensate and achieve optimal catecholamine synthesis in heterozygous TH knock out mice[4,5].
Polymorphisms in TH have not been associated with the occurrence of PD nor FOG[30,31], and prior work suggests that V81M does not play an etiological role in PD[32]. Recently, however, Greenbaum and co-workers reported an association of V81M carriers and lower UPDRS scores in subjects that were in early disease stages as assessed by dopamine transporter SPECT imaging[33].
In conclusion, this report describes a significant association between a common TH polymorphism and a more severe presentation of FOG in PD. Our functional characterization of this polymorphism on the native enzyme provides a potential kinetic explanation (reduced tyrosine substrate utilization) for the clinical observation (increased severity of freezing of gait). The work presented in this manuscript does not establish a causative effect of the TH V81M polymorphism with PD FOG. Future studies, designed and executed longitudinally, will better address the direct relation between this TH polymorphism and the severity of FOG in PD. Nevertheless, this is the first study showing a relation between an SNP in the rate-limiting enzyme for catecholamine synthesis and the severity of a PD symptom.
Supplementary Material
Highlights.
We have shown a significant association between a common TH polymorphism and FOG in PD.
The V81M mutation causes the doubling of the Michaelis-Menten constant for the enzyme's substrate; tyrosine.
This supports a role for NE in the generation of FOG and supports a role of this system as a target for PD therapies.
Acknowledgements
The authors thank Dr. Guangwei Du, Ms. Eleanore Hernandez, Ms. Brittany Jones, Ms. Melissa Santos, Ms. Grace Shyu and Ms. Raghda Clayiff for assistance with aspects of subject selection and Mr. Eugene Gonzales-Lopez for technical assistance. We also thank Ms. Christy Stetter for her assistance with statistical analyses. This work was supported by grants from the National Institutes of Health (GM38931, NIH NS060722, NS082151, Penn State Hershey Medical Center CTSI [NIH UL1 TR000127], GCRC Construction [C06 RR016499], and CTSI [TL1 TR000125] research grants) and the Penn State Institute for Personalized Medicine (04-017-52 HY 8A1HO; under a grant from the Pennsylvania Department of Health using Tobacco CURE Funds). The PA Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 2.Tekin I, Roskoski R, Jr., Carkaci-Salli N, et al. Complex molecular regulation of tyrosine hydroxylase. J Neural Transm. 2014;121:1451–1481. doi: 10.1007/s00702-014-1238-7. [DOI] [PubMed] [Google Scholar]
- 3.Levitt M, Spector S, Sjoerdsma A, et al. Elucidation of the Rate-Limiting Step in Norepinephrine Biosynthesis in the Perfused Guinea-Pig Heart. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- 4.Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K, Morita S, Sawada H, et al. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem. 1995;270:27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- 6.Haavik J, Blau N, Thony B. Mutations in human monoamine-related neurotransmitter pathway genes. Hum Mutat. 2008;29:891–902. doi: 10.1002/humu.20700. [DOI] [PubMed] [Google Scholar]
- 7.Bademci G, Vance JM, Wang L. Tyrosine hydroxylase gene: another piece of the genetic puzzle of Parkinson's disease. CNS Neurol Disord Drug Targets. 2012;11:469–481. doi: 10.2174/187152712800792866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. 1992;32(Suppl):S125–127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 9.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 11.Giladi N, Shabtai H, Simon ES, et al. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000;6:165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 12.Giladi N, Tal J, Azulay T, et al. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord. 2009;24:655–661. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- 13.Janssen RJ, Wevers RA, Haussler M, et al. A branch site mutation leading to aberrant splicing of the human tyrosine hydroxylase gene in a child with a severe extrapyramidal movement disorder. Ann Hum Genet. 2000;64:375–382. doi: 10.1046/j.1469-1809.2000.6450375.x. [DOI] [PubMed] [Google Scholar]
- 14.Carkaci-Salli N, Flanagan JM, Martz MK, et al. Functional domains of human tryptophan hydroxylase 2 (hTPH2). J Biol Chem. 2006;281:28105–28112. doi: 10.1074/jbc.M602817200. [DOI] [PubMed] [Google Scholar]
- 15.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Reinhard JF, Jr., Smith GK, Nichol CA. A rapid and sensitive assay for tyrosine-3-monooxygenase based upon the release of 3H2O and adsorption of [3H]-tyrosine by charcoal. Life Sci. 1986;39:2185–2189. doi: 10.1016/0024-3205(86)90395-4. [DOI] [PubMed] [Google Scholar]
- 17.Giladi N, McDermott MP, Fahn S, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;556:1712–1721. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- 18.Achiron A, Ziv I, Goren M, et al. Primary progressive freezing gait. Mov Disord. 1993;8:293–297. doi: 10.1002/mds.870080307. [DOI] [PubMed] [Google Scholar]
- 19.Panisset M. Freezing of gait in Parkinson's disease. Neurol Clin. 2004;22:S53–62. doi: 10.1016/j.ncl.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Lewitt PA. Norepinephrine: the next therapeutics frontier for Parkinson's disease. Transl Neurodegener. 2012;1:4. doi: 10.1186/2047-9158-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tohgi H, Abe T, Takahashi S. The effects of L-threo-3,4-dihydroxyphenylserine on the total norepinephrine and dopamine concentrations in the cerebrospinal fluid and freezing gait in parkinsonian patients. J Neural Transm Park Dis Dement Sect. 1993;5:27–34. doi: 10.1007/BF02260912. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa N, Kuroda H, Yamamoto M, Nukina I, Ota Z. Improvement in freezing phenomenon of Parkinson's disease after DL-threo-3, 4-dihydroxyphenylserine. Acta Med Okayama. 1984;38:301–304. doi: 10.18926/AMO/30351. [DOI] [PubMed] [Google Scholar]
- 23.Narabayashi H, Kondo T, Yokochi F, Nagatsu T. Clinical effects of L-threo-3,4-dihydroxyphenylserine in cases of parkinsonism and pure akinesia. Adv Neurol. 1987;45:593–602. [PubMed] [Google Scholar]
- 24.Fukada K, Endo T, Yokoe M, Hamasaki T, Hazama T, Sakoda S. L-threo-3,4-dihydroxyphenylserine (L-DOPS) co-administered with entacapone improves freezing of gait in Parkinson's disease. Med Hypotheses. 2013;80:209–212. doi: 10.1016/j.mehy.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Karachi C, Grabli D, Bernard FA, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 27.Devos D, Defebvre L, Bordet R. Dopaminergic and non-dopaminergic pharmacological hypotheses for gait disorders in Parkinson's disease. Fund Clin Pharmacol. 2010;24:407–421. doi: 10.1111/j.1472-8206.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 28.Wurtman RJ, Larin F, Mostafapour S, Fernstrom JD. Brain catechol synthesis: control by train tyrosine concentration. Science. 1974;185:183–184. doi: 10.1126/science.185.4146.183. [DOI] [PubMed] [Google Scholar]
- 29.Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137:1539S–1547. doi: 10.1093/jn/137.6.1539S. [DOI] [PubMed] [Google Scholar]
- 30.Hertz JM, Ostergaard K, Juncker I, et al. Low frequency of Parkin, Tyrosine Hydroxylase, and GTP Cyclohydrolase I gene mutations in a Danish population of early-onset Parkinson's Disease. Eur J Neurol. 2006;13:385–390. doi: 10.1111/j.1468-1331.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 31.Plante-Bordeneuve V, Davis MB, Maraganore DM, Marsden CD, Harding AE. Tyrosine hydroxylase polymorphism in familial and sporadic Parkinson's disease. Mov Disord. 1994;9:337–339. doi: 10.1002/mds.870090312. [DOI] [PubMed] [Google Scholar]
- 32.Kunugi H, Kawada Y, Hattori M, Ueki A, Otsuka M, Nanko S. Association study of structural mutations of the tyrosine hydroxylase gene with schizophrenia and Parkinson's disease. Am J Med Genet. 1998;81:131–133. [PubMed] [Google Scholar]
- 33.Greenbaum L, Lorberboym M, Melamed E, et al. Perspective: identification of genetic variants associated with dopaminergic compensatory mechanisms in early Parkinson's disease. Front Neurosci. 2013;7:52. doi: 10.3389/fnins.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.