Abstract
Purpose
We compared culture independent assessment of microbiota of the lower urinary tract in standard culture negative female patients with urological chronic pelvic pain syndrome who reported symptom flare vs those who did not report a flare.
Materials and Methods
Initial stream (VB1) and midstream (VB2) urine specimens (233 patients with urological chronic pelvic pain syndrome) were analyzed with Ibis T-5000 Universal Biosensor system technology for comprehensive identification of microorganism species. Differences between flare and nonflare groups for presence or number of different species within a higher level group (richness) were examined by permutational multivariate analysis of variance and logistic regression.
Results
Overall 81 species (35 genera) were detected in VB1 and 73 (33) in VB2. Mean (SD) VB1 and VB2 species count per person was 2.6 (1.5) and 2.4 (1.5) for 86 flare cases and 2.8 (1.3) and 2.5 (1.5) for 127 nonflare cases, respectively. Overall the species composition did not significantly differ between flare and nonflare cases at any level (p=0.14 species, p=0.95 genus in VB1 and VB2, respectively) in multivariate analysis for richness. Univariate analysis, unadjusted as well as adjusted, confirmed a significantly greater prevalence of fungi (Candida and Saccharomyces) in the flare group (15.7%) compared to the nonflare group in VB2 (3.9%) (p=0.01). When adjusted for antibiotic use and menstrual phase, women who reported a flare remained more likely to have fungi present in VB2 specimens (OR 8.3, CI 1.7–39.4).
Conclusions
Among women with urological chronic pelvic pain syndrome the prevalence of fungi (Candida and Saccharomyces sp.) was significantly greater in those who reported a flare compared to those who did not.
Keywords: microbiota; infection; cystitis, interstitial; symptom assessment
Interstitial cystitis/bladder pain syndrome is characterized by waxing and waning symptoms of bladder pain and storage urinary symptoms.1 Symptom exacerbations among patients with IC/BPS, often called flares, well recognized by physicians and patients, have not been well characterized. Many patients and physicians attribute flares to an infection, and antibiotics are often prescribed.1,2 Following the culture status of women with IC/BPS for almost 2 years failed to provide evidence to support the bacterial etiology flare hypothesis.3 However, standard culture techniques have many limitations, including the fact that 99% of known bacteria cannot be cultivated using standard culture media techniques.4 Enhanced culture techniques are reportedly better (larger volumes, multiple media, multiple atmospheric conditions, longer incubation times) at capturing more bacterial and fungal species,5,6 while new culture independent methods (such as Ibis T-500 Universal Biosensor technology7) allow for the detection of up to 1% to 3% of the total microbiome without needing an a priori hypothesis of which species are present.
In this study we use a novel, state-of-the-art, culture independent method to compare the micro-biota of the lower urinary tract in standard culture negative (for bacteria) female patient with UCPPS (ie IC/BPS) enrolled in the MAPP-EP study8,9 who reported a current flare at study entry compared to those women who did not.
Methods
The Trans-MAPP EP Study
The MAPP-EP study recruited UCPPS participants for baseline phenotyping and 12-month longitudinal followup of the treated history of UCPPS symptoms, with standardized data acquisition and analysis and biological sample collection across network sites.8 Inclusion and exclusion criteria have been described.9 This study evaluated female participants with a diagnosis of IC/BPS, with urological symptoms present a majority of the time during any 3 of the last 6 months. Further details of the study design are available, including descriptions of the study population enrollment criteria, and disease specific questionnaires are available8–10 or can be accessed online at http://www.biomedcentral.com/content/pdf/1471-2490-14-58.pdf.
Participants and Specimens
At the time of enrollment (baseline) each participant was asked the question, “Are you currently experiencing a flare of your urologic or pelvic pain symptoms? By this we mean are you currently experiencing symptoms that are much worse than usual?” (possible responses yes or no). Participants who responded with yes were included in the flare group. Patients with a flare at baseline were compared to women who did not report a flare. Each participant was asked to provide an initial stream urine specimen (VB1, approximately 20 ml) and classic midstream clean catch specimen (VB2, approximately 20 ml) at the baseline clinic visit. Participants were asked to clean the genital area using saline wipes before urine collection. A second VB2 urine taken at the same in-clinic visit was cultured (standard culture technique) on-site to identify active infection. Participants with a positive urine culture (traditional uropathogen colony forming units 105/ml or greater) were excluded from the EP study.
Specimen Handling
Urine specimens were collected using standardized collection kits and frozen as soon as possible (85% were frozen within 15 minutes, more than 95% within 30 minutes). After collection at MAPP Network Discovery sites,8,9 specimens were transferred from urine cups to 50 ml conical tubes and frozen at −80C until shipping to the central MAPP Network TATC (Tissue Analysis and Technology Core). Upon receipt at the TATC the specimens were thawed, thoroughly mixed and aliquoted into 1 and 3 ml aliquots. Specimens were frozen at −80C until used.
DNA Extraction and Ibis Eubacterial and Fungal Domain Ibis T-5000 Assays
We subjected VB1 and VB2 specimens to next generation molecular diagnostic Ibis T-5000 Universal Biosensor technology.7 Total DNA was extracted using 3 ml from VB1 and VB2 urine samples, and the bacterial and fungal DNA was amplified by polymerase chain reaction using the 16 primer pair BAC (Bacterial:Anti-biotic resistance genes:Candida)/Fungal detection systems developed by Ibis.7 The individual amplicons were weighed using the Ibis instrumentation for electrospray ionization time-of-flight mass spectrometry, which reports molecular mass. The species identities of the amplicons were then revealed using a database containing base composition data on virtually all bacterial/fungal species sequenced to date. Details of the methodology, including limitations, are available (supplementary Appendix 2, http://jurology.com/).7
Statistical Analysis
Demographic characteristics and relevant clinical factors were compared between flare and nonflare cases by chi-square tests. Differences in the overall microbial composition for flare vs nonflare IC/BPS cases were assessed by permutational multivariate analysis (PERMANOVA).11 This procedure is a nonparametric analogue of multivariate ANOVA that uses resampling for inference. The presence or absence of particular taxa for each subject is converted into a numerical matrix from which distance matrices are calculated and compared between groups according to a selected distance measure. The Euclidean distance was chosen as the basis of this analysis. Differences in the representation of individual taxa were tested using logistic regression for the presence or absence and PERMANOVA for differences in species richness in a higher level classification such as genus. All testing was conducted on a specific species and genus if it was detected in at least 10 subjects (the number required to detect a difference in a specific species or genus between flare and nonflare cases). Tests for differences in overall microbial composition between participants who reported flares and those who did not at the species and genus level were adjusted for antibiotic use (previous 2 years) and menstrual cycle phase. Tests of individual taxa were adjusted for multiple comparisons by controlling the false discovery rate.12
Results
Baseline urine specimens were obtained from 233 UCPPS female participants. We studied 213 participants with reported flare status and full specimen collection. Baseline demographics and data are presented in table 1. Considering clinical factors, women in the flare group reported significantly more pain and urinary symptoms as measured by the SYMQ-1, ICSI and GUPI total scores. There was no significant difference with respect to menstrual cycle phase (p=0.201) and 2-year antibiotic use (p=0.523) between the 2 groups.
Table 1. Baseline demographics.
| No Flare | Flare | Overall | p Value | |
|---|---|---|---|---|
| No. participants | 127 | 86 | 213 | |
| Mean age (SD) | 41.7 (15.0) | 41.2 (14.0) | 0.190 | |
| No. Caucasian (%) | 107 (84.2) | 78 (90.6) | 0.022 | |
| No. employed (%) | 81 (63.8) | 46 (53.5) | 0.01 | |
| Mean (SD) SYMQ-1* | 4.6 (2.0) | 6.4 (1.8) | 5.3 (2.1) | <0.001 |
| Mean (SD) ICSI score (0-20) | 10.1 (4.1) | 12.2 (4.8) | 10.9 (4.5) | 0.001 |
| Mean (SD) GUPI score (0-45) | 24.7 (8.1) | 30.8 (8.4) | 27 (8.7) | <0.001 |
| No. menstrual cycle phase (%): | ||||
| No cycle | 46 (36.2) | 38 (44.2) | 84 (39.4) | 0.201 |
| Follicular (0-12) | 23 (18.1) | 13 (15.1) | 36 (16.9) | |
| Ovulation (13-14) | 3 (2.4) | 2 (2.3) | 5 (2.3) | |
| Luteal (15-40) | 40 (31.5) | 19 (22.1) | 59 (27.7) | |
| Greater than 40 days | 7 (5.5) | 11 (12.8) | 18 (8.5) | |
| Missing | 8 (6.3) | 3 (3.5) | 11 (5.2) | |
| No. antibiotic use in last 2 yrs (%): | ||||
| Yes | 71 (82.61) | 61 (85.92) | 132 | 0.523 |
| No | 16 (18.39) | 10 (14.08) | 26 | |
| Missing | 55 |
Pain, pressure and discomfort associated with the bladder/pelvis rated 0 to 10 (with 10 being the most severe discomfort imagined).
We detected at least 1 species in 97% of the VB1 specimens (flare 93.0% vs nonflare 98.4%) and 88.0% of VB2 specimens (flare 86.7% vs nonflare 90.0%). Overall 81 species (35 genera) were detected in VB1 and 73 in VB2 (33 genera) (tables 2 and 3). Mean (SD) VB1 and VB2 species count per person was 2.6 (1.5) and 2.4 (1.5) for flare cases and 2.8 (1.3) and 2.5 (1.5) for nonflare cases, respectively.
Table 2. Prevalence of species in women with vs without flare in VB1.
| VB1 | No. No Flare (%) | No. Flare (%) | p Unadjusted (richness) | p Unadjusted (presence/absence) | p Adjusted (richness)* | p Adjusted (presence/absence)* |
|---|---|---|---|---|---|---|
| Overall | 127 | 86 | ||||
| Species: | 0.128 | 0.142 | ||||
| Bifidobacterium subtile | 17 (13.4) | 8 (9.3) | 0.366 | 0.6091 | ||
| Candida albicans | 3 (2.4) | 7 (8.1) | 0.0655 | 0.0664 | ||
| Escherichia coli | 6 (4.7) | 4 (4.7) | 0.9802 | 0.8632 | ||
| Finegoldia magna | 10 (7.9) | 7 (8.1) | 0.9441 | 0.9263 | ||
| Lactobacillus acidophilus | 18 (14.2) | 7 (8.1) | 0.1847 | 0.2996 | ||
| Lactobacillus crispatus | 44 (34.6) | 32 (37.2) | 0.7016 | 0.6713 | ||
| Lactobacillus gasseri | 12 (9.4) | 5 (5.8) | 0.3413 | 0.5272 | ||
| Lactobacillus johnsonii | 37 (29.1) | 24 (27.9) | 0.8459 | 0.9183 | ||
| Lactobacillus sp. | 5 (3.9) | 6 (7) | 0.3316 | 0.227 | ||
| Staphylococcus epidermidis/haemolyticus | 45 (35.4) | 42 (48.8) | 0.0517 | 0.0265 | ||
| Staphylococcus haemolyticus | 16 (12.6) | 2 (2.3) | 0.0184 | 0.0212 | ||
| Staphylococcus hominis | 29 (22.8) | 16 (18.6) | 0.4587 | 0.4719 | ||
| Streptococcus agalactiae | 7 (5.5) | 5 (5.8) | 0.9252 | 0.9817 | ||
| Genus: | 0.923 | 0.938 | 0.954 | 0.931 | ||
| Bifidobacterium | 23 (18.1) | 11 (12.8) | 0.277 | 0.3005 | 0.262 | 0.3868 |
| Candida | 9 (7.1) | 11 (12.8) | 0.291 | 0.1669 | 0.324 | 0.1917 |
| Corynebacterium | 8 (6.3) | 3 (3.5) | 0.550 | 0.3698 | 0.662 | 0.7284 |
| Escherichia | 6 (4.7) | 4 (4.7) | 1.000 | 0.9802 | 0.87 | 0.8632 |
| Finegoldia | 10 (7.9) | 7 (8.1) | 1.000 | 0.9441 | 0.926 | 0.9263 |
| Lactobacillus | 83 (65.4) | 56 (65.1) | 0.788 | 0.9714 | 0.882 | 0.7414 |
| Staphylococcus | 83 (65.4) | 56 (65.1) | 0.929 | 0.9714 | 0.924 | 0.7465 |
| Streptococcus | 19 (15) | 13 (15.1) | 0.854 | 0.9751 | 0.866 | 0.8971 |
| Fungi† | 14 (11) | 14 (16.3) | 0.334 | 0.268 | 0.38 | 0.3646 |
Only taxa present in at least 10 subjects were tested individually.
Adjusted for employment (employed, disabled or other) and race.
Ibis BAC screen only detects Candida and Saccharomyces sp.
Table 3. Prevalence of species in women with and without flares in VB2.
| VB2 | No. No Flare (%) | No. Flare (%) | p Unadjusted (richness) | p Unadjusted (presence/absence) | p Adjusted (richness)* | p Adjusted (presence/absence)* |
|---|---|---|---|---|---|---|
| Overall | 127 | 83 | ||||
| Species: | 0.697 | 0.752 | ||||
| Bifidobacterium subtile | 15 (11.8) | 10 (12) | 0.9586 | 0.8616 | ||
| Finegoldia magna | 8 (6.3) | 5 (6) | 0.9355 | 0.7899 | ||
| Lactobacillus acidophilus | 13 (10.2) | 8 (9.6) | 0.8878 | 0.6615 | ||
| Lactobacillus crispatus | 34 (26.8) | 28 (33.7) | 0.2803 | 0.3235 | ||
| Lactobacillus gasseri | 7 (5.5) | 7 (8.4) | 0.4099 | 0.477 | ||
| Lactobacillus johnsonii | 36 (28.3) | 22 (26.5) | 0.7706 | 0.784 | ||
| Lactobacillus sp. | 10 (7.9) | 5 (6) | 0.6118 | 0.7961 | ||
| Propionibacterium acnes | 8 (6.3) | 4 (4.8) | 0.6524 | 0.9557 | ||
| Staphylococcus epidermidis/haemolyticus | 41 (32.3) | 32 (38.6) | 0.3514 | 0.2587 | ||
| Staphylococcus haemolyticus | 9 (7.1) | 4 (4.8) | 0.5075 | 0.8359 | ||
| Staphylococcus hominis | 20 (15.7) | 17 (20.5) | 0.3798 | 0.4221 | ||
| Streptococcus agalactiae | 8 (6.3) | 4 (4.8) | 0.6524 | 0.5768 | ||
| Genus: | 0.846 | 0.513 | 0.871 | 0.407 | ||
| Bifidobacterium | 22 (17.3) | 12 (14.5) | 0.757 | 0.5821 | 0.73 | 0.5122 |
| Candida | 5 (3.9) | 8 (9.6) | 0.130 | 0.1042 | 0.121 | 0.1071 |
| Finegoldia | 8 (6.3) | 5 (6) | 1.000 | 0.9355 | 0.803 | 0.7899 |
| Lactobacillus | 74 (58.3) | 55 (66.3) | 0.601 | 0.2452 | 0.533 | 0.1251 |
| Propionibacterium | 8 (6.3) | 4 (4.8) | 0.759 | 0.6524 | 0.976 | 0.9557 |
| Staphylococcus | 70 (55.1) | 48 (57.8) | 0.927 | 0.6985 | 0.998 | 0.5355 |
| Streptococcus | 18 (14.2) | 11 (13.3) | 0.716 | 0.8501 | 0.567 | 0.7927 |
| Fungi† | 5 (3.9) | 13 (15.7) | 0.003 | 0.0058 | 0.007 | 0.0103 |
Only taxa present in at least 10 subjects were tested individually.
Adjusted for employment (employed, disabled or other) and race.
Ibis BAC screen only detects Candida and Saccharomyces sp.
Overall species composition did not significantly differ between flare and nonflare cases at any level (p=0.14, 0.75 species level, p=0.95, 0.87 genus level, p=0.42 in VB1 and VB2, respectively, in multivariate analysis for richness). Tables 2 and 3 describe differences in the occurrence of species and genus, respectively, that were represented in at least 10 subjects (tests between flare and nonflare cases using logistic regression).
There was also no significant difference in the number (percent) or specific genus composition of traditional uropathogens detected between groups (table 4). In patients with IC/BPS with negative standard plate cultures uropathogens were detected in 8.1% vs 9.4% of VB1 in flare vs nonflare cases, respectively. The corresponding values for VB2 specimens were 1.2% vs 3.9%. Univariate analysis revealed a significantly greater prevalence of fungi (Candida and Saccharomyces sp.) in the flare group (15.7%) compared to the nonflare group in VB2 (3.9%) (table 3, p=0.01). When adjusted for antibiotic use and menstrual phase, women who reported a flare remained more likely to have fungi present in VB2 specimens than women who did not report a flare (OR 8.3, CI 1.7–39.4). Representative VB2 heat maps by flare status were developed to illustrate the differences in VB2 between groups (see figure).
Table 4. Prevalence of uropathogens in women with UCPPS with and without flares in VB1 and VB2.
| No. (%) | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Enterobacter | Enterococcus | Escherichia | Klebsiella | Proteus | Pseudomonas | Any Uropathogen | |
| VB1: | |||||||
| No flare (127) | 0 (0) | 4 (3.1) | 6 (4.7) | 2 (1.6) | 0 (0) | 1 (0.8) | 12 (9.4) |
| Flare (86) | 0 (0) | 4 (4.7) | 4 (4.7) | 1 (1.2) | 0 (0) | 1 (1.2) | 7 (8.1) |
| p Unadjusted | - | 0.574 | 0.9802 | 0.803 | - | 0.7818 | 0.7424 |
| p Adjusted* | - | 0.4996 | 0.8632 | 0.6449 | - | 0.7253 | 0.5677 |
| VB2: | |||||||
| No flare (127) | 0 (0) | 1 (0.8) | 2 (1.6) | 0 (0) | 2 (1.6) | 0 (0) | 5 (3.9) |
| Flare (83) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 1 (1.2) | 2 (2.4) | 1 (1.2) |
| p Unadjusted | 1.000 | 0.7625 | 0.9957 | - | 0.9957 | 0.9966 | 0.5505 |
| p Adjusted* | 1.000 | 0.6369 | 0.9971 | - | 0.9971 | 0.9978 | 0.9059 |
These data refer to nonculture identification technique since all subjects had standard culture sterile VB2 urine sample.
Adjusted for employment (employed, disabled or other) and race.
Fig 1.
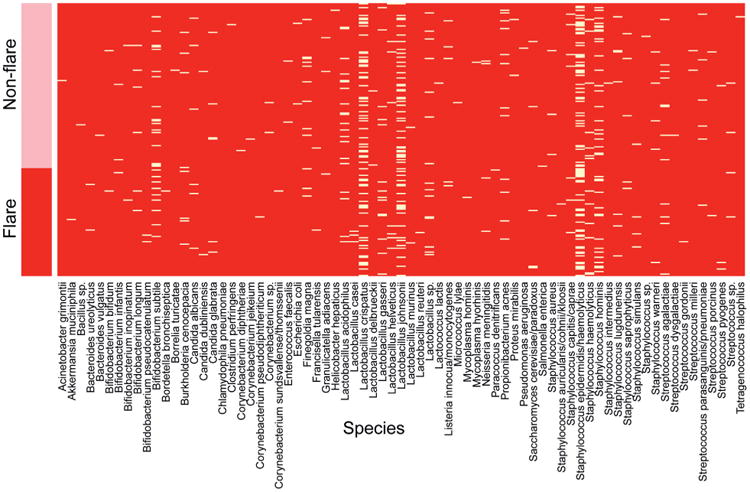
Presence of species by flare status, VB2. Pink bar denotes nonflare group participants and red bar denotes flare group participants. White lines in heat map indicate presence of species listed alphabetically along bottom of heat map.
Discussion
In our study symptom exacerbations were referred to as flares and were defined as “symptoms that are much worse than usual.” This definition was based on expert clinical opinion as, to our knowledge, no published data are available to objectively define flares. Almost 40% of female patients with IC/BPS evaluated in this Trans-MAPP EP study reported such a symptom exacerbation at baseline evaluation. In a condition in which patient reported pain and voiding symptoms wax and wane over time, it is difficult to develop a generalizable definition for flare or understand the relevance of flares to the course of disease. However, it has been documented that most patients with IC/BPS do report flares,13,14 and there is little doubt that flares have a negative impact on several aspects of patients' lives, including their work lives and their relationships.14 During a 6-month period in a longitudinal study of 400 patients with IC, symptom severity improved in 15% to 20% and deteriorated in another 15% to 20%, often attributed to changes in stress, diet, sexual intercourse and premenstrual flares.15,16 The proportion of patients with flares is consistent with the 32% reported in a cohort of more than 100 newly diagnosed female patients with IC/BPS followed for 2 years.17 However, other cohort data suggest that flares may be more frequent in the IC/BPS population, ranging in duration from 1 day to more than 2 weeks.13,14
We failed to identify a difference in the bacterial microbiota of women with IC/BPS who reported flares at study entry compared to those who did not report a flare. Approximately 2.5 species per patient on average were detected in both groups. On multivariate analysis there was no difference in bacterial species or genus composition between flare and nonflare cases. However, in VB2, univariate analysis did show a significant difference in the prevalence of fungi between flare and nonflare groups. A significantly greater prevalence of fungi (Candida and Saccharomyces sp.) was detected in the flare group (15.7%) compared to the nonflare group in VB2 (3.9%) (p=0.006). It could be argued that this finding, which was not observed in the VB1 analyses, suggests bladder involvement rather than just a urethral/introital difference. However, this conclusion would require analysis of specific bladder (catheterized) urine specimens and vaginal/introital swab specimens before such a conclusion could be drawn. It is possible that the presence of fungi might be influenced by previous antibiotic use or menstrual cycle status. However, controlling for these 2 variables, women who reported a flare remained 8 times more likely to have fungi present in VB2 specimens than those who did not report a flare.
If this discovery is replicated in future studies, the ramifications for patient care are obvious, even if we have not yet proven causality. Our standard cultures did not detect fungi (in this case Candida and Saccharomyces sp.) in patients enrolled in this study implying that nonculture techniques are more sensitive and may be required to detect a clinically significant fungal presence in patients with IC/BPS. If detected or suspected, antifungal therapy may be appropriate. This suggestion has not been proven in this study.
In a questionnaire study evaluating flare triggers, patients reported that a UTI episode was associated with a flare in 54% of patients with UCPPS (included male and female patients).12 However, there has been a paucity of attempts to use standard culture techniques to document this association with flares. In one of the few longitudinal studies reported evaluating bacteriuria and UTI in patients with IC/BPS, 106 consecutive women with newly diagnosed IC/BPS were followed for 24 months.17 Overall 34 women (32%) presented with 54 flares, of which 44 were standard culture negative and 10 were culture positive with gram-negative uropathogens. The investigators suggested that a small proportion of the symptom flares of IC/BPS might be associated with recurrent UTI, although data were not provided regarding the status after bacterial eradication. Nickel et al were not able to show an association between significant bacteriuria with uropathogenic bacteria and flares.3 That study also suggested a minimal response with antibiotic treatment in terms of ameliorating flare symptoms in patients with positive bacterial cultures.
We hypothesized that flares might be due to unculturable microorganisms, microorganisms that were overlooked or not identified as a uropathogen or a change (or imbalance) in the microbial ecology of an individual's lower urinary tract microbiota (dysbiosis). We proposed that the application of next generation molecular diagnostics (in this case, Ibis T-5000 Universal Biosensor technology) to analyze MAPP Network specimens of patients with flare9 and no flare status would more definitively address questions regarding the presence of unique microorganisms or ecological communities of microorganisms than previous approaches and, thus, potentially identify 1 possible etiology for flares in IC/BPS. (Supplementary Appendix 2, http://jurology.com/, has an explanation and description of this technique, its comparison to other nonculture microbial identification techniques and limitations of the technique.)
Our study has some important weaknesses and limitations. We excluded patients with positive standard cultures at baseline and, therefore, cannot speculate that significant bacteriuria at baseline might have been associated with flares in some excluded study candidates. The specimens were voided urine specimens and, therefore, do not necessarily represent the microbiome of the bladder, but rather the lower urinary tract and introitus/vagina. We reported only on the detection of microorganisms and, therefore, could only assess the presence rather than the dominance of any species. The available antibiotic use data were not comprehensive (yes or no only) and we could not account for the scope of antibiotic use during the previous 2 years. We did not comprehensively identify all fungal species (a full Ibis fungal panel would be required to identify all fungal organisms). There is a risk of type II error in analyzing our results, with the possibility that we did not have enough subjects to detect minor differences for some bacterial species.
The strengths of our analyses lie in the comprehensive clinical assessment of patients with IC/BPS in the Trans-MAPP EP study and the fact that, a priori, we collected reported flare status at initial evaluation. The analysis also adjusted and/or corrected for demographic differences, including menstrual status and antibiotic use, although antibiotic use was measured fairly coarsely as any use in the last 2 years. The next generation technology used to analyze IC/BPS specimens in this Trans-MAPP EP flare study was the Ibis T-5000 Universal Biosensor technology, which has demonstrated the ability to provide extremely accurate and comprehensive data in clinical studies involving complex infectious processes.18,19
Our finding that the prevalence of fungi was greater in participants who reported flares needs to be replicated. We are planning hypotheses driven studies to examine flares in this same cohort (MAPP 1 EP study participants) at 6 and 12 months as well as in the upcoming MAPP-2 Microbiome Project using similar methodology with the addition of a full fungal screen.
Conclusions
Assessment of baseline culture independent microbiological data from female subjects enrolled in the MAPP Research Network has identified a higher prevalence of certain fungi (Candida and Saccharomyces sp.) in the urine microbiota that may be involved in the etiology or pathogenesis of a subset of patients with IC/BPS related flares. This preliminary discovery is currently being tested further to validate the findings.
Supplementary Material
Acknowledgments
Supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health MAPP Network Awards U01DK82370, U01DK82342, U01DK82315, U01DK82344, U01DK82325, U01DK82345, U01DK82333 and U01DK82316.
Abbreviations and Acronyms
- EP
Epidemiology and Phenotyping
- GUPI
Genitourinary Pain Index
- IC/BPS
interstitial cystitis/bladder pain syndrome
- ICSI
Interstitial Cystitis Symptom Index
- MAPP
Multidisciplinary Approach to the study of Pelvic Pain
- SYMQ-1
pain, pressure, discomfort scale
- UCPPS
urological chronic pelvic pain syndrome
- UTI
urinary tract infection
- VB1
voided bladder 1 or initial stream urine (urethral) specimen
- VB2
voided bladder 2 or midstream urine (bladder) specimen
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
References
- 1.Nickel JC. Interstitial cystitis: a chronic pelvic pain syndrome. Med Clin North Am. 2004;88:467. doi: 10.1016/S0025-7125(03)00151-2. [DOI] [PubMed] [Google Scholar]
- 2.Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61:29. doi: 10.1016/j.eururo.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 3.Nickel JC, Shoskes D, Irvine-Bird K. Prevalence and impact of bacteriuria and/or urinary tract infection in interstitial cystitis/painful bladder syndrome. Urology. 2010;76:799. doi: 10.1016/j.urology.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich GD, Greenberg SJ. PCR-Based Diagnostics in Infectious Disease. Boston: Blackwell Scientific Publications; 1994. [Google Scholar]
- 5.Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52:871. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014;5:e01283. doi: 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ecker DJ, Sampath R, Massire C, et al. Ibis T5000: a universal biosensor approach for microbiology. Nat Rev Microbiol. 2008;6:553. doi: 10.1038/nrmicro1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens JQ, Mullins C, Kusek JW, et al. The MAPP Research Network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landis JR, Williams DA, Lucia MS, et al. The MAPP Research Network: design, patient characterization and operations. BMC Urol. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickel JC, Stephens A, Landis JR, et al. Search for microorganisms in men with urologic chronic pelvic pain syndrome: a culture-independent analysis in the MAPP Research Network. J Urol. 2015;194:127. doi: 10.1016/j.juro.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32. [Google Scholar]
- 12.Yoav B, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B Methodol. 1995;57:289. [Google Scholar]
- 13.Sutcliffe S, Colditz GA, Pakpahan R, et al. Changes in symptoms during urologic chronic pelvic pain syndrome symptom flares: findings from one site of the MAPP Research Network. Neurourol Urodyn. 2015;34:188. doi: 10.1002/nau.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutcliffe S, Colditz GA, Goodman MS, et al. Urological chronic pelvic pain syndrome symptom flares: characterization of the full range of flares at two sites in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. BJU Int. 2014;114:916. doi: 10.1111/bju.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Propert KJ, Schaeffer AJ, Brensinger CM, et al. A prospective study of interstitial cystitis: results of longitudinal followup of the Interstitial Cystitis Data Base cohort. The Interstitial Cystitis Data Base Study Group. J Urol. 2000;163:1434. doi: 10.1016/s0022-5347(05)67637-9. [DOI] [PubMed] [Google Scholar]
- 16.Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832. doi: 10.1097/01.ju.0000176747.40242.3d. [DOI] [PubMed] [Google Scholar]
- 17.Stanford E, McMurphy C. There is a low incidence of recurrent bacteriuria in painful bladder syndrome/interstitial cystitis patients followed longitudinally. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:551. doi: 10.1007/s00192-006-0184-9. [DOI] [PubMed] [Google Scholar]
- 18.Jacovides C, Kreft R, Adeli B, et al. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94:2247. doi: 10.2106/JBJS.L.00210. [DOI] [PubMed] [Google Scholar]
- 19.Palmer MP, Altman DT, Altman GT, et al. Can we trust intraoperative culture results in nonunions? J Orthop Trauma. 2014;28:384. doi: 10.1097/BOT.0000000000000043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


