Abstract
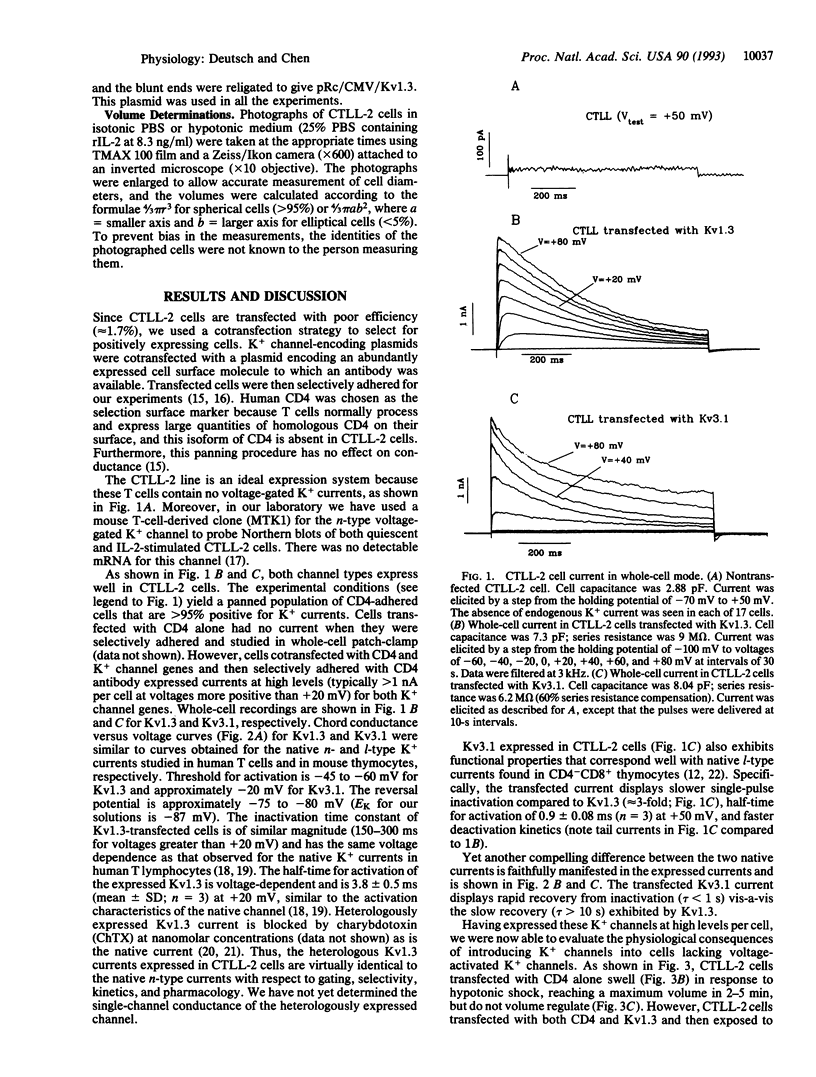
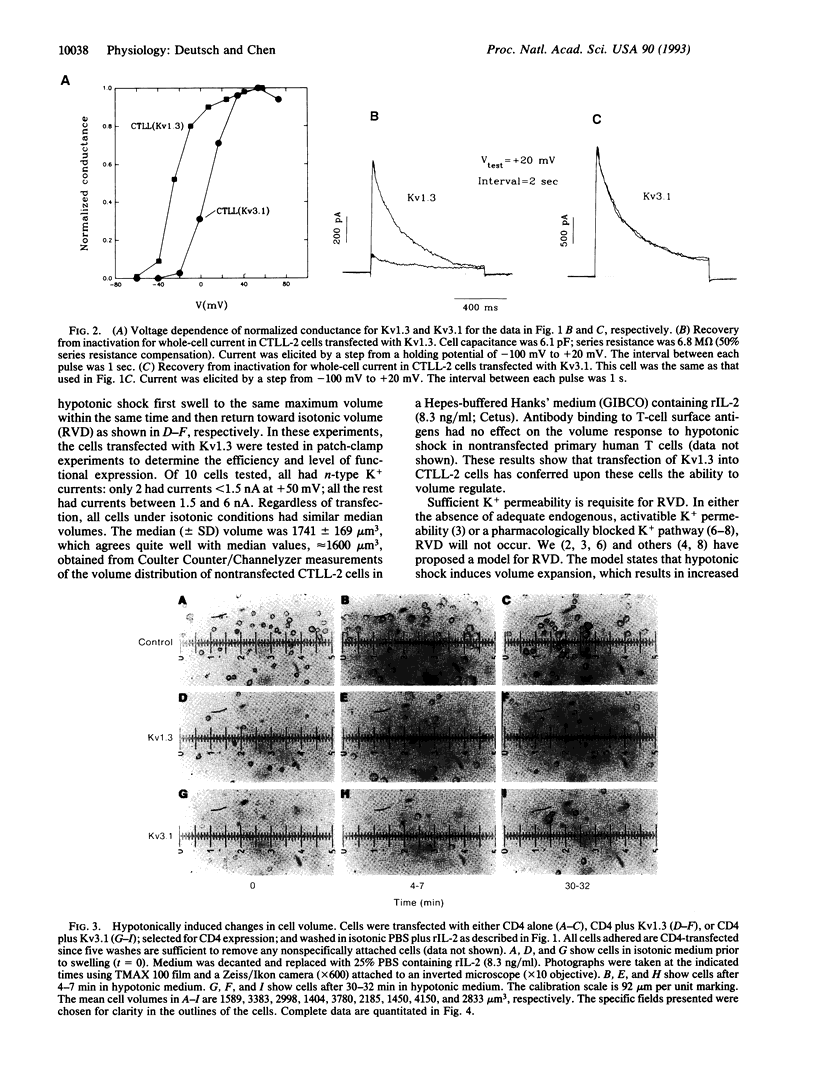
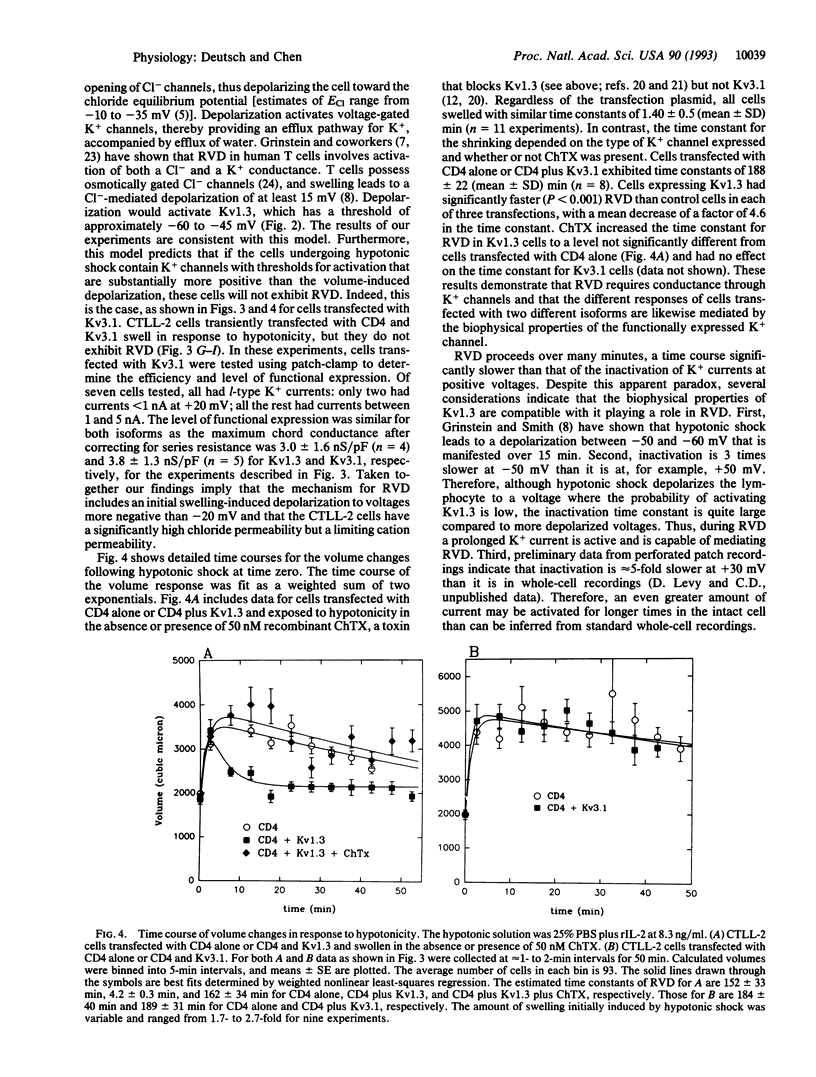
It has been postulated that the K+ channel isoform Kv1.3 plays a role in regulatory volume decrease (RVD) in response to hypotonic shock. We show that a mouse cytotoxic T-lymphocyte line, CTLL-2, is devoid of voltage-dependent K+ channels and is unable to volume regulate. Transient transfection of these cells with Kv1.3 reconstitutes their ability to volume regulate. As predicted by our model, this ability depends critically on volume-induced changes in membrane potential and the isoform of the K+ channel used. When the cells were transfected with Kv3.1, an isoform believed to be expressed in a specific subclass of mouse thymocytes, the CTLL-2 cells did not show RVD. The difference in the ability of the two isoforms to confer the capacity for RVD is expected from differences in the voltage dependence of activation of the channels, according to our proposed model for RVD. The experimental approach that we use, transient transfection and panning to select positive transfectants, is highly effective; it has a > 95% efficiency. This method, and this cell line, may be important tools in studying lymphocyte K+ channels and their function in situ.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attali B., Romey G., Honoré E., Schmid-Alliana A., Mattéi M. G., Lesage F., Ricard P., Barhanin J., Lazdunski M. Cloning, functional expression, and regulation of two K+ channels in human T lymphocytes. J Biol Chem. 1992 Apr 25;267(12):8650–8657. [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G., DeCoursey T. E., Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985 Jan;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. C., Osborne P. B., North R. A., Dooley D. C., Douglass J. Characterization and functional expression of genomic DNA encoding the human lymphocyte type n potassium channel. DNA Cell Biol. 1992 Mar;11(2):163–172. doi: 10.1089/dna.1992.11.163. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Krause D., Lee S. C. Voltage-gated potassium conductance in human T lymphocytes stimulated with phorbol ester. J Physiol. 1986 Mar;372:405–423. doi: 10.1113/jphysiol.1986.sp016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C., Lee S. C. Cell volume regulation in lymphocytes. Ren Physiol Biochem. 1988 May-Oct;11(3-5):260–276. doi: 10.1159/000173166. [DOI] [PubMed] [Google Scholar]
- Douglass J., Osborne P. B., Cai Y. C., Wilkinson M., Christie M. J., Adelman J. P. Characterization and functional expression of a rat genomic DNA clone encoding a lymphocyte potassium channel. J Immunol. 1990 Jun 15;144(12):4841–4850. [PubMed] [Google Scholar]
- Freedman B. D., Price M. A., Deutsch C. J. Evidence for voltage modulation of IL-2 production in mitogen-stimulated human peripheral blood lymphocytes. J Immunol. 1992 Dec 15;149(12):3784–3794. [PubMed] [Google Scholar]
- Grinstein S., Clarke C. A., Dupre A., Rothstein A. Volume-induced increase of anion permeability in human lymphocytes. J Gen Physiol. 1982 Dec;80(6):801–823. doi: 10.1085/jgp.80.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Dixon S. J. Ion transport, membrane potential, and cytoplasmic pH in lymphocytes: changes during activation. Physiol Rev. 1989 Apr;69(2):417–481. doi: 10.1152/physrev.1989.69.2.417. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Smith J. D. Calcium-independent cell volume regulation in human lymphocytes. Inhibition by charybdotoxin. J Gen Physiol. 1990 Jan;95(1):97–120. doi: 10.1085/jgp.95.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Dethlefs B., Wasmuth J. J., Goldin A. L., Gutman G. A., Cahalan M. D., Chandy K. G. Expression and chromosomal localization of a lymphocyte K+ channel gene. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9411–9415. doi: 10.1073/pnas.87.23.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Ghanshani S., Dethlefs B., McPherson J. D., Wasmuth J. J., Gutman G. A., Cahalan M. D., Chandy K. G. The Shaw-related potassium channel gene, Kv3.1, on human chromosome 11, encodes the type l K+ channel in T cells. J Biol Chem. 1992 Oct 15;267(29):20971–20979. [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Deutsch C. Temperature dependence of K(+)-channel properties in human T lymphocytes. Biophys J. 1990 Jan;57(1):49–62. doi: 10.1016/S0006-3495(90)82506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Levy D. I., Deutsch C. Diverse K+ channels in primary human T lymphocytes. J Gen Physiol. 1992 May;99(5):771–793. doi: 10.1085/jgp.99.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Price M., Prystowsky M. B., Deutsch C. Volume response of quiescent and interleukin 2-stimulated T-lymphocytes to hypotonicity. Am J Physiol. 1988 Feb;254(2 Pt 1):C286–C296. doi: 10.1152/ajpcell.1988.254.2.C286. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Garcia M. L., Slaughter R. S., Reuben J. P. Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10094–10098. doi: 10.1073/pnas.89.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. Subset-specific expression of potassium channels in developing murine T lymphocytes. Science. 1988 Feb 12;239(4841 Pt 1):771–775. doi: 10.1126/science.2448877. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Deutsch C. K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984 Feb 2;307(5950):468–471. doi: 10.1038/307468a0. [DOI] [PubMed] [Google Scholar]
- Price M., Lee S. C., Deutsch C. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10171–10175. doi: 10.1073/pnas.86.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands S. B., Lewis R. S., Cahalan M. D. Charybdotoxin blocks voltage-gated K+ channels in human and murine T lymphocytes. J Gen Physiol. 1989 Jun;93(6):1061–1074. doi: 10.1085/jgp.93.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]




