Abstract
Background
cardiac dysfunction is frequently observed after severe traumatic brain injury (sTBI), however its significance is poorly understood. Our study sought to elucidate the association of cardiac troponin I (cTnI) elevation with all cause in-hospital mortality following isolated sTBI (brain AIS ≥ 3 and admission GCS ≤ 8, no AIS ≥3 to any other bodily regions).
Methods
we retrospectively reviewed all adult patients (≥ 18 years) with isolated sTBI admitted to a level one trauma center between June 2007 and January 2014. Patients must have cTnI values within 24 hours of admission. Mortality risks were examined by Cox proportional hazard model.
Results
of the 580 patients identified, 30.9% had detectable cTnI in 24 hours of admission. The median survival time was 4.19 days (IQR: 1.27 – 11.69). When adjusted for potential confounders, patients in the highest cTnI category (≥ 0.21 ng/mL) had significantly higher risk of in-hospital mortality (HR: 1.39; 95% CI: 1.04 – 1.88) compared to patients with undetectable cTnI. Mortality risk increased with higher troponin levels (p-trend < 0.0001). This association was more pronounced in patients ≤65 years (HR: 2.28; 95% CI: 1.53 – 3.40; p-trend < 0.0001), while interestingly, insignificant in those > 65 years (p-trend = 0.0826).
Conclusions
among patients with sTBI, cTnI elevation is associated with all cause in-hospital mortality via a non-linear, positive trend. Age modified the effect of cTnI on mortality.
Level of Evidence
level III retrospective study, Prognostic and Epidemiological
Keywords: traumatic brain injury, cardiac dysfunction, cardiac troponin I, in-hospital mortality
Background
Traumatic brain injury (TBI) is the leading cause of death after injury in the United States, affecting more than 1.4 million people annually. (1,2) Survivors often suffer from long-term disabilities that are estimated to be $9.2 billion in lifetime medical costs and $51.2 billion in lifetime productivity losses. (3) In an effort to improve management of patients with severe traumatic brain injury (sTBI) and minimize its socioeconomic burdens, research has primarily focused on identification, prevention, and treatment of secondary injuries including hypotension, (4) hypoxia, (5), intracranial hypertension, (6–8) and cerebral hypoperfusion. (7–9) While a multitude of unique, often interrelated systemic complications have been identified to alter morbidity and mortality, (10) the effects of sTBI on cardiac function has been poorly documented.
Neurocardiac axis has been well-defined in various non-traumatic head injuries including subarachnoid hemorrhage (SAH),(11–15) acute stroke, (16,17) intracerebral hemorrhage, (18,19) seizure, (20) and Guillain-Barre syndrome. (21) Increased risks of complications and mortality are found to be associated with a classic triad of transient left ventricular wall motion abnormality, electrocardiography changes, and elevation in cardiac enzymes in the absence of coronary artery disease. (22) Stress cardiomyopathy as a result of primary neurological condition is labelled as neurogenic stunned myocardium. While the etiology is not well understood, several pathophysiological mechanisms have been proposed including multi-vessel coronary artery spasm, (23) microvascular dysfunction, (24) and catecholamine storm. (22,25,26)
So far, the effect of cardiac dysfunction following sTBI has not been well established. It is unknown what effects these heart-brain interactions may have on morbidity and mortality of patients with sTBI, and conversely, if cardiac dysfunction can be a novel predictor of clinical outcome. Using cardiac troponin I (cTnI) as a marker for myocardial damage, our study investigated the association between myocardial injury and all cause in-hospital mortality in patients with sTBI and the effect of age on this association, and we hypothesize that a positive trend effect exists between the above factors.
Methods
Population selection and outcome definition
This retrospective study was performed at the R Adams Cowley Shock Trauma Center in Baltimore, Maryland after approval by the University Of Maryland School Of Medicine Human Research Protection Office. We reviewed all patients with TBI directly admitted from the scene of injury between June 2007 and January 2014. Patients were identified through a prospectively maintained Trauma Registry. Routine clinical characteristics were collected including sex, race, age, injury type, Injury Severity Score (ISS), Abbreviated Injury Score (AIS), admission Glasgow Coma Score (GCS), length of Intensive Care Unit (ICU) stay, length of hospital stay, discharge GCS, discharge disposition, preexisting cardiac diseases (cardiac dysrhythmia, congestive heart failure, and other cardiac diseases) and all cTnI measurements within the first 24 hours. Per institution protocol, troponins are sent on patients with non-specific ischemic EKG changes, possible blunt chest trauma, or age > 65 years.
Inclusion criteria specified adult patients of ≥18 years of age that have received a diagnosis of TBI verified by computed tomography or magnetic resonance imaging. Head AIS ≥3 and GCS ≤8 was classified as sTBI. Exclusion criteria precluded patients with significant extra-cranial injuries defined as AIS ≥3 to any other body regions and patients without cTnI measurements within 24 hours of admission. Troponin values were stratified into three categories: undetectable (< 0.06 ng/mL) and two levels of detectable categories that included mildly elevated (0.06 – < 0.21 ng/mL) and severely elevated (> 0.21 ng/mL). Median value of 0.21 ng/mL was chosen to yield equal sample sizes in the two elevated cTnI groups. Clinical outcome was designated as all cause in-hospital mortality. Survival days were calculated from hospital admission to death or discharge from hospital. Outcome measurements were analyzed as binary, either survived or expired. Dichotomization of age was selected at 65 years.
Statistical analysis
Clinical characteristics were presented as number of patients and percentage for discrete variables or as mean and standard deviation, median and interquartile range (IQR) for continuous variables where appropriate. Discrete variables were compared using chi-square test or Fisher’s exact test, and continuous variables were compared using one-way ANOVA test for means or Wilcoxon rank-sum test for mean-rank comparison. We used the direct adjusted survival curves based on a stratified Cox model. (27) Differences in survival rates among the three categories were compared using Wald Chi-square test and survivors were censored at the time of discharge. Cox proportional hazard model was applied to calculate hazard ratios (HR) and their corresponding 95% confidence intervals (CI). Two Cox models were developed based on the peak cTnI of each patient and serial cTnI values available for each patient as time-dependent variables. Restricted cubic spline method was used to describe the non-linear relationship between peak cTnI, as a continuous variable, and in-hospital mortality. Categorical cTnI values were treated as continuous parameters in the model to calculate p-trends. All calculations described above were adjusted for age, gender, injury type (including MVC, fall, other blunt trauma, penetrating injuries, and other injury types), ISS, admission GCS, surgical intervention, and preexisting cardiac diseases.
Area under the receiver operating characteristic (AUC) curve was calculated to assess the predictive power of admission GCS and TRISS alone vs. in combination with cTnI; fitness was evaluated by the likelihood-ratio test. To evaluate selection bias, troponin values were imputed for 276 patients with isolated sTBI without cTnI data using MI procedure in SAS. (28) A multivariable regression method was used to predicted log(cTnI), compensated for skewed distribution, with max 4.04 and min −3.90 for consistency with available data and predictors including age, gender, injury type, admission GCS, and pre-existing cardiac history. All hypothesis testing was two-tailed, and any p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc. Cary, NC).
Results
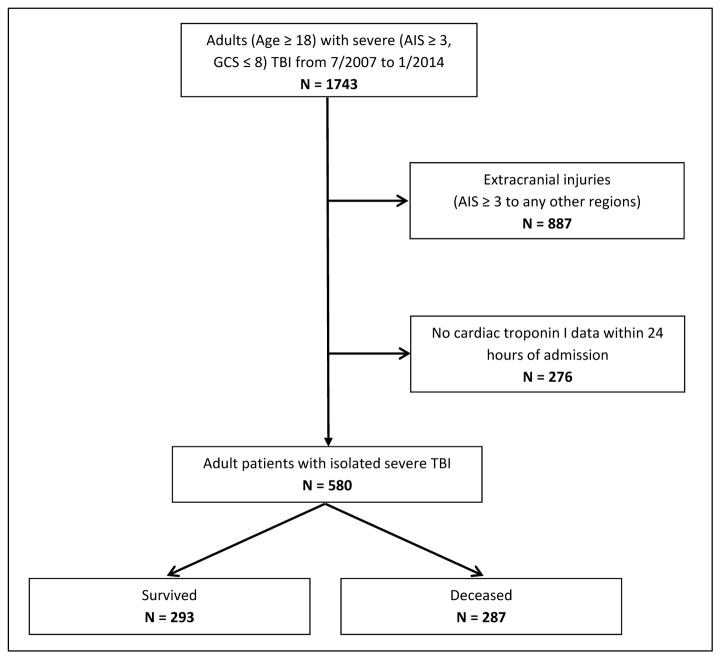
A total of 1743 adult patients with sTBI (AIS ≥ 3 and GCS ≤8) were admitted during the study period. Exclusion criteria eliminated 887 patients who suffered significant (AIS ≥ 3) extracranial injuries to the face, neck, thorax, abdomen, or extremities and 276 patients who lacked cTnI data within the first 24 hours of admission. A total of 580 patients were included for analysis (Figure 1). Eligible patients were 56.3 ± 21.1 years of age, largely male (70.8%) who suffered blunt trauma (89.8%). Injury severity was indicated by the median ISS of 25 (IQR: 17 – 26) and median admission GCS of 4 (IQR: 3 – 6). Most common injuries were cerebral hematoma (74.4%), followed by SAH (61.2%), cerebral contusion (50.2%), cerebral edema (50.2%), skull fracture (40.2%), and intraventricular hemorrhage (IVH) (26.0%). History of cardiac diseases was relatively uncommon at 8.1%, while 31.2% of the study population had surgical intervention after admission. Detectable cTnI was present 30.9% of the patients within 24 hours of admission.
Figure 1.
Flow diagram of screening protocol; a total of 580 adult patients with isolated sTBI were identified with cTnI data.
All cause in-hospital mortality
Mortality during index hospitalization was 49.5% (287 patients), of which 64% were men (Table 1). Mortality was significantly associated with > 65 years of age, injury type, male sex, higher ISS, lower admission GCS, reduced ICU and hospital stay, and preexisting cardiac diseases (all p < 0.001). These patients also had higher incidences of SAH, IVH, cerebral hematoma, cerebral contusion, cerebral edema, and surgical intervention. The deceased patients had significantly higher rate of detectable cTnI than survivors (41.8% vs. 20.1%, p < 0.001), and the reverse held true for undetectable cTnI (58.2% vs. 79.9%, p < 0.001). When separated by cTnI categories, significant differences were found in mean age, injury type, admission GCS, mortality, and specific injuries such as cerebral hematoma, cerebral contusion, and cerebral edema (all p < 0.05). Otherwise the three strata shared similar characteristics (Table 2).
Table 1.
Selected characteristics of the study population
| Characteristics* | Total (580) | Survived (293) | Expired (287) | P-value |
|---|---|---|---|---|
| Age (y) | 56.3 ± 21.1 | 49.4 ± 19.7 | 63.3 ± 20.2 | < 0.001 |
| ≤ 65 y | 366 (63.1) | 227 (77.5) | 139 (48.4) | |
| > 65 y | 214 (36.9) | 66 (22.5) | 148 (51.6) | < 0.001 |
| Sex | ||||
| Male | 410 (70.8) | 227 (77.5) | 183 (64.0) | |
| Female | 169 (29.2) | 66 (22.5) | 103 (36.0) | <0.001 |
| Injury type | ||||
| MVC | 60 (10.3) | 47 (16.0) | 13 (4.5) | |
| Fall | 210 (36.2) | 101 (34.5) | 109 (38.0) | |
| Other blunt | 251 (43.3) | 131 (44.7) | 120 (41.8) | |
| Penetrating | 43 (7.4) | 5 (1.7) | 38 (13.2) | |
| Other | 16 (2.8) | 9 (3.1) | 7 (2.4) | < 0.001 |
| ISS | 25 (17, 26) | 21 (16, 26) | 26 (25, 29) | < 0.001 |
| Admission GCS score | 4 (3, 6) | 6 (3, 7) | 3 (3, 5) | < 0.001 |
| 3–4 | 325 (56.0) | 116 (39.6) | 209 (72.8) | |
| 5–8 | 255 (44.0) | 177 (60.4) | 78 (27.2) | <0.001 |
| Coded AIS injuries | ||||
| Subarachnoid hemorrhage | 355 (61.2) | 180 (61.4) | 175 (61.0) | 0.910 |
| Intraventricular hemorrhage | 151 (26.0) | 58 (19.8) | 93 (32.4) | <0.001 |
| Cerebral hematoma | 433 (74.7) | 208 (71.0) | 225 (78.4) | 0.040 |
| Cerebral contusion | 291 (50.2) | 177 (60.4) | 114 (39.7) | <0.001 |
| Cerebral edema | 291 (50.2) | 119 (40.6) | 172 (59.9) | < 0.001 |
| Skull fracture | 233 (40.2) | 120 (41.0) | 113 (39.4) | 0.698 |
| Length of ICU stay (d) | 2.7 (0.3, 9.6) | 7.6 (2.4, 13.4) | 0.9 (0.0, 3.0) | <0.001 |
| Length of hospital stay (d) | 4.2 (1.3, 11.7) | 10.3 (4.7, 17.6) | 1.4 (0.4, 3.6) | < 0.001 |
| Cardiac troponin I | ||||
| Normal (<0.06 ng/mL) | 401 (69.1) | 234 (79.9) | 167 (58.2) | |
| Elevated (≥ 0.06 ng/mL) | 179 (30.9) | 59 (20.1) | 120 (41.8) | < 0.001 |
| Surgical intervention | 181 (31.2) | 128 (43.7) | 53 (18.5) | < 0.001 |
| Preexisting cardiac diseases | 47 (8.1) | 16 (5.5) | 31 (10.8) | 0.02 |
Data presented as n (%), mean ± standard deviation, or median (interquartile range) as appropriate.
Table 2.
Selected characteristics of the study population by cTnI levels
| Characteristics* | < 0.06 ng/mL (401) | ≥ 0.06 ng/mL (87) | ≥ 0.21 ng/mL (92) | P-value |
|---|---|---|---|---|
| Age (y) | 55.5±21.1 | 61.7 ± 20.5 | 54.8 ± 21.2 | 0.034 |
| ≤ 65 y | 257 (64.1) | 48 (55.2) | 61 (66.3) | |
| > 65 y | 144 (35.9) | 39 (44.8) | 31 (33.7) | 0.232 |
| Sex | ||||
| Male | 108 (27.0) | 29 (33.3) | 32 (34.8) | |
| Female | 292 (73.0) | 58 (66.7) | 60 (65.2) | 0.218 |
| Injury type | ||||
| MVC | 47 (11.7) | 10 (11.5) | 3 (3.3) | |
| Fall | 148 (36.9) | 34 (39.1) | 28 (30.4) | |
| Other blunt | 176 (43.9) | 34 (39.1) | 41 (44.6) | |
| Penetrating | 23 (5.7) | 7 (8.0) | 13 (14.1) | |
| Other | 7 (1.8) | 2 (2.3) | 7 (7.6) | 0.003 |
| ISS | 25 (17, 26) | 25 (20, 26) | 25 (20, 26) | 0.369 |
| Admission GCS score | 4 (3, 7) | 3 (3, 6) | 3 (3, 5.5) | < 0.001 |
| 3–4 | 202 (50.4) | 58 (66.7) | 65 (70.6) | |
| 5–8 | 199 (49.6) | 29 (33.3) | 27 (29.4) | <0.001 |
| Coded AIS injuries | ||||
| Subarachnoid hemorrhage | 240 (59.8) | 54 (62.1) | 61 (66.3) | 0.511 |
| Intraventricular hemorrhage | 97 (24.2) | 24 (27.6) | 30 (32.6) | 0.237 |
| Cerebral hematoma | 293 (73.1) | 75 (86.2) | 65 (70.7) | 0.024 |
| Cerebral contusion | 216 (53.9) | 35 (40.2) | 40 (43.5) | 0.026 |
| Cerebral edema | 183 (45.6) | 48 (55.2) | 60 (65.2) | 0.002 |
| Skull fracture | 164 (40.9) | 36 (41.4) | 33 (35.9) | 0.654 |
| Length of ICU stay (d) | 2.9 (0.2, 9.4) | 1.7 (0.0, 10.0) | 2.5 (0.5, 8.7) | 0.532 |
| Length of hospital stay (d) | 4.7 (1.5, 11.8) | 2.2 (0.5, 11.6) | 2.9 (1.1, 10.8) | 0.106 |
| Surgical intervention | 124 (30.9) | 25 (28.7) | 32 (34.8) | 0.667 |
| Preexisting cardiac diseases | 27 (6.7) | 11 (12.6) | 9 (9.8) | 0.152 |
| Mortality | 167 (41.6) | 59 (67.8) | 61 (66.3) | <0.001 |
Data presented as n (%), mean ± standard deviation, or median (interquartile range) as appropriate.
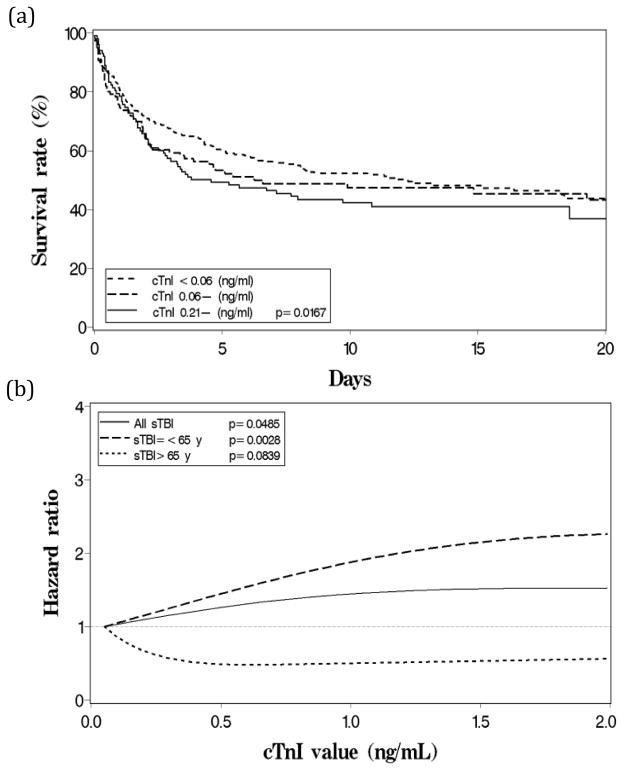
At the median survival time of 4.19 days (IQR: 1.27 – 11.69), survival rates of the three cTnI categories were 63.9%, 56.4%, and 50.1% in ascending cTnI order (Figure 2a). The differences among survival curves were statistically significant (p < 0.0001). When adjusted for potential confounders (Table 3), those in the severely elevated cTnI category (> 0.21 ng/mL) had 1.39 times (95% CI: 1.04 – 1.88) higher risk of mortality compared to patients with undetectable cTnI (< 0.06 ng/mL). The trend was dose-dependent across the three cTnI categories and statistically significant (p-trend = 0.0167). Comparable results were derived from the peak cTnI Cox model and the time-dependent cTnI Cox model that included serial cTnI values (maximum n = 6) of each patient (p-trend = 0.0162 and p-trend = 0.0307, respectively).
Figure 2.
All cause in-hospital mortality following isolated sTBI by cTnI levels. (a) Cumulative survival rates of cTnI categories from Cox model. (b) Risk of mortality, described by hazard ratio derived from Cox model, when peak cTnI increases as a continuous variable.
Table 3.
Association of cTnI and in-hospital mortality by Cox regression
| Survived (n) | Expired (n) | HR (95% CI)* | HR (95% CI)† | |
|---|---|---|---|---|
| All patients (n = 580) | ||||
| Troponin I (ng/mL) | ||||
| < 0.06 | 234 | 167 | 1.0 | 1.0 |
| 0.06 - | 28 | 59 | 1.29 (0.95–1.76) | 1.20 (0.89–1.62) |
| 0.21 - | 31 | 61 | 1.39 (1.04–1.88) | 1.39 (1.01–1.89) |
| p-trend | 0.0167 | 0.0307 | ||
| Age ≤ 65 y (n = 366) | ||||
| Troponin I (ng/mL) | ||||
| < 0.06 | 191 | 66 | 1.0 | 1.0 |
| 0.06 - | 19 | 29 | 2.54 (1.63–3.96) | 2.18 (1.40–3.41) |
| 0.21 - | 17 | 44 | 2.28 (1.53–3.40) | 2.05 (1.36–3.09) |
| p-trend | < 0.0001 | < 0.0001 | ||
| Age > 65 y (n = 214) | ||||
| Troponin I (ng/mL) | ||||
| < 0.06 | 43 | 101 | 1.0 | 1.0 |
| 0.06 - | 9 | 30 | 0.76 (0.49–1.18) | 0.74 (0.49–1.12) |
| 0.21 - | 14 | 17 | 0.67 (0.40–1.13) | 0.77 (0.43–1.38) |
| p-trend | 0.0826 | 0.1688 | ||
| p-interaction | < 0.0001 | 0.0090 | ||
Analysis of peak cTnI value as a single exposure; adjusted for age, gender, injury type (including MVC, fall, other blunt trauma, penetrating injuries, and other injury types), ISS, admission GCS score, surgical intervention, and preexisting cardiac diseases.
Analysis using repeated measures of cTnI levels as time-dependent exposures; adjusted for all potential cofounders mentioned above.
Age stratification
Age stratification revealed 366 patients (63.1%) ≤65 years of age. Among this age group, patients with severely elevated cTnI had 2.28 times (95% CI: 1.53 – 3.40) higher risk of mortality compared to patients with undetectable cTnI. The trend was dose-dependent (p-trend < 0.0001). However, cTnI failed to reflect the risk of mortality in patients > 65 years of age (p-trend = 0.0826). Age-modification effect was statistically significant (p-interaction < 0.0001). Results were again comparable between the two Cox models. Plot of continuous peak cTnI reaffirmed this association and age-modification: mortality risk increased in a linear fashion when cTnI < 1.5 ng/mL, among patients ≤65 years (Figure 2b).
Predictive value of troponin
AUC of admission GCS and TRISS were 0.6366 (95% CI: 0.6060–0.6672) and 0.6830 (95% CI: 0.6524–0.7136) in the present study population of 580 adult patients with isolated sTBI, respectively (Table 4). In combination with cTnI, predictive value of the two models increased by 3.47% (p = 0.0011) and 2.19% (p = 0.0004) compared with each factor individually. Among patients ≤65 years, AUC significantly increased by 7.52% for admission GCS with cTnI and 5.93% for TRISS with cTnI.
Table 4.
Predictive value of GCS and TRISS w/wo cTnI
| AUC (95% CI) | Δ AUC | P-value* | |
|---|---|---|---|
| All patients (n = 580) | |||
| GCS | 0.6366 (0.6060–0.6671) | ||
| GCS + cTnI | 0.6713 (0.6362–0.7064) | 0.0347 | 0.0011 |
| TRISS | 0.6830 (0.6524–0.7136) | ||
| TRISS + cTnI | 0.7049 (0.6712–0.7486) | 0.0219 | 0.0004 |
| Age ≤ 65 y (n = 366) | |||
| GCS | 0.6647 (0.6202–0.7092) | ||
| GCS + cTnI | 0.7399 (0.6891–0.7907) | 0.0752 | < 0.0001 |
| TRISS | 0.6861 (0.6436–0.7286) | ||
| TRISS + cTnI | 0.7454 (0.6976–0.7932) | 0.0593 | < 0.0001 |
| Age > 65 y (n = 214) | |||
| GCS | 0.6390 (0.5961–0.6819) | ||
| GCS + cTnI | 0.6622 (0.6124–0.7120) | 0.0232 | 0.0381 |
| TRISS | 0.6265 (0.5844–0.6686) | ||
| TRISS + cTnI | 0.6357 (0.5879–0.6835) | 0.0092 | 0.2708 |
Calculated by likelihood-ratio test
Discussion
In this single-site retrospective study, we found increased risk of all cause in-hospital mortality in patients with detectable cTnI following isolated sTBI following a non-linear, positive trend association that is independent of major confounders such as age, gender, type of injury, injury severity, and preexisting cardiac history. In age stratification, cTnI elevation is particularly sensitive predictor of risk of in-hospitality mortality in patients ≤65 years of age, however insignificant among those > 65 years. Peak cTnI and serial cTnI analysis yielded similar results. To our knowledge, this is the first study to document a direct trend association between cTnI and mortality risks following isolated sTBI and the effect of age on this association.
Heralded as the “gold standard” for acute myocardial damage, troponin is a highly sensitive and specific biomarker. In addition to acute coronary syndrome, troponin elevation has also been observed following a variety of non-coronary conditions such as pulmonary embolism, pulmonary hypertension, sepsis, and chronic renal failure and non-traumatic head injuries such as SAH, IVH, acute stroke, seizure, and Guillain-Barre syndrome. In these settings, particularly SAH, elevated cTnI was associated with increased risk of cardiopulmonary and cerebrovascular complications, as well as increased mortality and worse functional outcomes. (29,30) Pathophysiological mechanism of neurogenic stunned myocardium is poorly understood however. The prevailing theory is related to a systemic catecholamine surge driven by the central neuroendocrine axis which massively increases sympathetic outflow and activates the adrenal glands. Damage to the insular and hypothalamus also initiates a complex cascade of events, including activation followed by dysfunction of the autonomic nervous system and an acute inflammatory response, which may incur major adverse effects on the heart. (11)
Similar phenomenon was thought to occur following TBI. Several studies were able to quantify an increase in sympathetic activity after sTBI by measuring plasma and urinary catecholamine levels. (31–33) Furthermore, beta-blocker therapy has shown to confer a survival advantage to sTBI patients with elevated cardiac enzymes. (34,35) Despite growing evidence that suggest a potential link between cardiac dysfunction and TBI, only two studies have investigated the clinical outcomes of patients with cardiac dysfunction and TBI. Prathep et al reported abnormal echocardiogram, including reduced left ventricular rejection fraction and regional wall motion abnormality, was present in 22.3% of patients with isolated TBI and was independently associated with all cause in-hospital mortality. (36) Similarly, Salim et al found an elevated admission cTnI was associated with higher mortality in a population of 420 patients with isolated sTBI and peak troponin was an independent risk factor for mortality. (37) Our results substantiated previous findings while unveiled the positive trend effect of cTnI elevation on all cause inhospital mortality. Current study is also the first study to included serial cTnI when available in addition to peak cTnI, both analysis yielded comparable results.
Advanced age is among the best independent predictors of worse outcome after TBI. (38–40) The Centers for Disease Control and Prevention reported TBI-related deaths were highest among persons greater than 65 years and the age-adjusted rate of hospitalization more than doubles that of the general population. (41) In one large prospective study, Hukkelhoven et al concluded that with each 10-year incremental increase in age, odds of an unfavorable outcome after TBI increased by 40 to 50%.(39) Furthermore, a number of studies have documented higher frequency of baseline troponin elevation with age in absence of acute coronary syndrome or other acute illness known to cause troponin elevation. (42,43) Evidence mentioned above lend support to our finding that age significantly modified the association between cTnI and risk of mortality following sTBI. One possible explanation may be that older patients with TBI had elevated baseline troponin due to normal aging and/or underlying chronic comorbidities, which hampered the predictive value of cTnI. One study documented higher positive predictor value and negative predictive value of troponin in patients less than 65 years than older patients with respect to acute coronary syndrome. (44) Irrespective of the cause, our data suggested that cTnI elevation is only clinically significant among patients with sTBI ≤65 years of age.
GCS has become one of the most widely used clinical measure of severity of injury in patients with sTBI. A number of studies have confirmed its inter- and intra-rater reliability. (45,46) Likewise, trauma injury severity scoring systems such as TRISS have consistently shown to accurately predict the probability of survival in blunt trauma patients and provide reasonable assessment of survival in patients with penetrating injuries. (47,48) Our results proved congruous with prior validations of admission GCS and TRISS; indeed either score alone can provide appropriate prognostication of adult patients with isolated sTBI. However, cTnI significantly enhanced the predictive value of both admission GCS and TRISS. The effects were particularly robust in those ≤65 years. The results implied an invaluable place for cTnI in accurate prognostication of all cause in-hospital mortality in patients with isolated sTBI.
There are several important limitations to consider, including those typical of a single-site retrospective and uncontrolled comparison study. First, a portion of patients with isolated sTBI (n = 276, 32.2%) lacked cTnI data within 24 hours of admission, likely deemed unnecessary by providers or fail to meet protocolized inclusion criteria for troponin order, which may represent a selection bias in our study. In an attempt to rectify the bias, we compared selected characteristics of the study population with and without cTnI data and found no significant differences in ISS score, admission GCS score, and mortality (Supplemental Table 1). In addition, results that incorporated imputed cTnI data, total of 856 patients, were consistent with the results presented in our paper (Supplemental Table 2). Above results suggest that, irrespective of the selection bias, the association between cTnI and risk of mortality prevails. Secondly, cardiac studies on troponin suggested that peak cTnI may be reached > 24 hours after admission, particularly in the elderly. (49) By using only the cTnI measurements within the first 24 hours, we may have potentially missed the peak cTnI of the patient > 65 years which may have contributed to our finding that cTnI is not a reliable predictor in this population. However, age-stratified troponin progression has not been directly studied and may be investigated in future studies. Lastly, data on radiographic and electrocardiography were limited to confirm the diagnosis of neurogenic stunned myocardium as the cause of cTnI elevation, and data on beta-blockade usage and extracranial complications were unavailable, which may confound troponin elevations. While unlikely, non-cardiac causes of cTnI elevation cannot be ruled-out. As is, no causal relationship can be drawn and the mechanism of troponin elevation remains unclear. However, in despite of above limitation, prognostic value of cTnI in patients with isolated sTBI remains clear.
In summary, our study validated cTnI as a novel biomarker and independent predictor of all cause in-hospital mortality in patients with isolated sTBI. The association between cTnI level and mortality risk followed a non-linear curve that was most sensitive when cTnI < 1.5 ng/mL. Furthermore, age stratification revealed cTnI to be highly sensitive predictor of mortality in patients ≤65 years, but the same association cannot be found in patients > 65 years. These findings have several implications. First, cTnI assay should be considered in patients with sTBI even in absence high suspicion of cardiac injury (e.g. cardiac history or direct trauma to the chest). Second, cTnI is a sufficiently prognostic biomarker of mortality in patients with sTBI. Third, cTnI elevation in patients with sTBI must be treated with caution, particularly if the patient is less than 65 years of age. Finally, implications of cTnI elevation in patients greater than 65 years of age may be downplayed. Present results questioned the merit of cTnI in this population and suggested that an alternative method should be sought to assess the cardiac function of patients greater than 65 years of age who suffered sTBI. Our study provided important insights to the heart-brain interactions following sTBI and possible schemes for subsequent optimization of management of these patients.
Supplementary Material
Acknowledgments
Source of Funding
This study is supported in part by the NIA Short-Term Training Program on Aging Grant T35AG036679 (G.B. Carey, P.I.) to the University of Maryland School of Medicine.
We would to thank Betsy Kramer, RN for providing trauma registry information as well as the STAR-ORC personnel for providing guidance and statistical support.
Footnotes
Conflicts of Interest
No competing financial interests exist.
Preliminary results were presented at the 10th Annual Academic Surgical Congress (ASC20150192).
Author Contributions
Stephen Cai: study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revisions; Brandon Bonds: study concept and design, acquisition of data, analysis and interpretation of data, critical revisions; Peter Hu: analysis and interpretation of data, critical revisions; Deborah Stein: study concept and design, critical revisions.
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14(6):602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23(6):394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- 4.Berry C, Ley EJ, Bukur M, Malinoski D, Margulies DR, Mirocha J, Salim A. Redefining hypotension in traumatic brain injury. Injury. 2012;43(11):1833–7. doi: 10.1016/j.injury.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Stein DM, Lindell AL, Murdock KR, Kufera JA, Menaker J, Bochicchio GV, Aarabi B, Scalea TM. Use of serum biomarkers to predict cerebral hypoxia after severe traumatic brain injury. J Neurotrauma. 2012;29(6):1140–9. doi: 10.1089/neu.2011.2149. [DOI] [PubMed] [Google Scholar]
- 6.Stein DM, Brenner M, Hu PF, Yang S, Hall EC, Stansbury LG, Menaker J, Scalea TM. Timing of intracranial hypertension following severe traumatic brain injury. Neurocrit Care. 2013;18(3):332–40. doi: 10.1007/s12028-013-9832-3. [DOI] [PubMed] [Google Scholar]
- 7.Stein DM, Lindell A, Murdock KR, Kufera JA, Menaker J, Keledjian K, Bochicchio GV, Aarabi B, Scalea TM. Relationship of serum and cerebrospinal fluid biomarkers with intracranial hypertension and cerebral hypoperfusion after severe traumatic brain injury. J Trauma. 2011;70(5):1096–103. doi: 10.1097/TA.0b013e318216930d. [DOI] [PubMed] [Google Scholar]
- 8.Stein DM, Hu PF, Brenner M, Sheth KN, Liu KH, Xiong W, Aarabi B, Scalea TM. Brief episodes of intracranial hypertension and cerebral hypoperfusion are associated with poor functional outcome after severe traumatic brain injury. J Trauma. 2011;71(2):364–73. doi: 10.1097/TA.0b013e31822820da. discussion 373–4. [DOI] [PubMed] [Google Scholar]
- 9.DeWitt DS, Prough DS. Ameliorating cerebral hypoperfusion after traumatic brain injury. Crit Care Med. 1999;27(11):2592–3. doi: 10.1097/00003246-199911000-00056. [DOI] [PubMed] [Google Scholar]
- 10.Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33(3):654–60. doi: 10.1097/01.ccm.0000155911.01844.54. [DOI] [PubMed] [Google Scholar]
- 11.Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, Fitzsimmons BF, Connolly ES, Mayer SA. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112(18):2851–6. doi: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- 12.Provencio JJ. Subarachnoid hemorrhage: a model for heart-brain interactions. Cleve Clin J Med. 2007;74(Suppl 1):S86–90. doi: 10.3949/ccjm.74.suppl_1.s86. [DOI] [PubMed] [Google Scholar]
- 13.Martorano PP, Bini G, Tanara L, Sinkovets L, Pelaia P, Pietropaoli P. Subarachnoid hemorrhage and the heart. Minerva Anestesiol. 1998;64(5):231–233. [PubMed] [Google Scholar]
- 14.Jeon IC, Chang CH, Choi BY, Kim MS, Kim SW, Kim SH. Cardiac troponin I elevation in patients with aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2009;46(2):99–102. doi: 10.3340/jkns.2009.46.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hravnak M, Frangiskakis JM, Crago EA, Chang Y, Tanabe M, Gorcsan J, 3rd, Horowitz MB. Elevated cardiac troponin I and relationship to persistence of electrocardiographic and echocardiographic abnormalities after aneurysmal subarachnoid hemorrhage. Stroke. 2009;40(11):3478–84. doi: 10.1161/STROKEAHA.109.556753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen H, Johannesen HH, Christensen AF, Bendtzen K, Boysen G. Serum cardiac troponin I in acute stroke is related to serum cortisol and TNF-alpha. C Cerebrovasc Dis. 2004;18(3):194–9. doi: 10.1159/000079941. [DOI] [PubMed] [Google Scholar]
- 17.Helmers C. Cardiac sequelae of acute stroke. Stroke. 1983;14(5):828–9. doi: 10.1161/01.str.14.5.828. [DOI] [PubMed] [Google Scholar]
- 18.Putaala J, Lehto M, Meretoja A, Silvennoinen K, Curtze S, Kääriäinen J, Koivunen RJ, Kaste M, Tatlisumak T, Strbian D. In-hospital cardiac complications after intracerebral hemorrhage. Int J Stroke. 2014;9(6):741–6. doi: 10.1111/ijs.12180. [DOI] [PubMed] [Google Scholar]
- 19.Inamasu J, Ito K, Sugimoto K, Watanabe E, Kato Y, Hirose Y. Cardiac wall motion abnormality associated with spontaneous intracerebral hemorrhage. Int J Cardiol. 2013;168(2):1667–9. doi: 10.1016/j.ijcard.2013.03.096. [DOI] [PubMed] [Google Scholar]
- 20.Sieweke N, Allendörfer J, Franzen W, Feustel A, Reichenberger F, Pabst W, Krämer HH, Kaps M, Tanislav C. Cardiac Troponin I elevation after epileptic seizure. BMC Neurol. 2012;12:58. doi: 10.1186/1471-2377-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein R, Mayer SA, Magnano A. Neurogenic stunned myocardium in Guillain-Barre syndrome. Neurology. 2000;54(3):759–62. doi: 10.1212/wnl.54.3.759. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen H, Zaroff JG. Neurogenic stunned myocardium. Curr Neurol Neurosci Rep. 2009;9(6):486–91. doi: 10.1007/s11910-009-0071-0. [DOI] [PubMed] [Google Scholar]
- 23.Chang PC, Lee SH, Hung HF, Kaun P, Cheng JJ. Transient ST elevation and left ventricular asynergy associated with normal coronary artery and Tc-99m PYP Myocardial Infarct Scan in subarachnoid hemorrhage. Int J Cardiol. 1998;63(2):189–92. doi: 10.1016/s0167-5273(97)00293-3. [DOI] [PubMed] [Google Scholar]
- 24.Zaroff JG, Rordorf GA, Titus JS, Newell JB, Nowak NJ, Torchiana DF, Aretz HT, Picard MH, Macdonald RL. Regional myocardial perfusion after experimental subarachnoid hemorrhage. Stroke. 2000;31(5):1136–43. doi: 10.1161/01.str.31.5.1136. [DOI] [PubMed] [Google Scholar]
- 25.Lee VH, Oh JK, Mulvagh SL, Wijdicks EF. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5(3):243–9. doi: 10.1385/NCC:5:3:243. [DOI] [PubMed] [Google Scholar]
- 26.Naredi S, Lambert G, Edén E, Zäll S, Runnerstam M, Rydenhag B, Friberg P. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000;31(4):901–6. doi: 10.1161/01.str.31.4.901. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 28.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–42. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 29.De’Ath HD, Rourke C, Davenport R, Manson J, Renfrew I, Uppal R, Davies LC, Brohi K. Clinical and biomarker profile of trauma-induced secondary cardiac injury. Br J Surg. 2012;99(6):789–97. doi: 10.1002/bjs.8728. [DOI] [PubMed] [Google Scholar]
- 30.Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142(9):786–91. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 31.Woolf PD, Hamill RW, Lee LA, Cox C, McDonald JV. The predictive value of catecholamines in assessing outcome in traumatic brain injury. J Neurosurg. 1987;66(6):875–82. doi: 10.3171/jns.1987.66.6.0875. [DOI] [PubMed] [Google Scholar]
- 32.Hamill RW, Woolf PD, McDonald JV, Lee LA, Kelly M. Catecholamines predict outcome in traumatic brain injury. Ann Neurol. 1987;21(5):438–43. doi: 10.1002/ana.410210504. [DOI] [PubMed] [Google Scholar]
- 33.Clifton GL, Ziegler MG, Grossman RG. Circulating catecholamines and sympathetic activity after head injury. Neurosurgery. 1981;8(1):10–4. doi: 10.1227/00006123-198101000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Alali AS, McCredie VA, Golan E, Shah PS, Nathens AB. Beta blockers for acute traumatic brain injury: a systematic review and meta-analysis. Neurocrit Care. 2014;20(3):514–23. doi: 10.1007/s12028-013-9903-5. [DOI] [PubMed] [Google Scholar]
- 35.Cotton BA, Snodgrass KB, Fleming SB, Carpenter RO, Kemp CD, Arbogast PG, Morris JA., Jr Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma. 2007;62(1):26–33. doi: 10.1097/TA.0b013e31802d02d0. discussion 33–5. [DOI] [PubMed] [Google Scholar]
- 36.Prathep S, Sharma D, Hallman M, Joffe A, Krishnamoorthy V, Mackensen GB, Vavilala MS. Preliminary report on cardiac dysfunction after isolated traumatic brain injury. Crit Care Med. 2014;42(1):142–7. doi: 10.1097/CCM.0b013e318298a890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salim A, Hadjizacharia P, Brown C, Inaba K, Teixeira PG, Chan L, Rhee P, Demetriades D. Significance of troponin elevation after severe traumatic brain injury. J Trauma. 2008;64(1):46–52. doi: 10.1097/TA.0b013e31815eb15a. [DOI] [PubMed] [Google Scholar]
- 38.Flanagan SR, Hibbard MR, Gordon WA. The impact of age on traumatic brain injury. Phys Med Rehabil Clin N Am. 2005;16(1):163–77. doi: 10.1016/j.pmr.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall LF, Murray GD, Maas AI. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99(4):666–73. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 40.Mosenthal AC, Lavery RF, Addis M, Kaul S, Ross S, Marburger R, Deitch EA, Livingston DH. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma. 2002;52(5):907–11. doi: 10.1097/00005373-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Coronado VG, Thomas KE, Sattin RW, Johnson RL. The CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. 2005;20(3):215–28. doi: 10.1097/00001199-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Webb IG, Yam ST, Cooke R, Aitken A, Larsen PD, Harding SA. Elevated baseline cardiac troponin levels in the elderly - another variable to consider? Heart Lung Circ. 2015;24(2):142–8. doi: 10.1016/j.hlc.2014.07.071. [DOI] [PubMed] [Google Scholar]
- 43.Normann J, Mueller M, Biener M, Vafaie M, Katus HA, Giannitsis E. Effect of older age on diagnostic and prognostic performance of high-sensitivity troponin T in patients presenting to an emergency department. Am Heart J. 2012;164(5):698–705. doi: 10.1016/j.ahj.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Noeller TP, Meldon SW, Peacock WF, Emerman CL, McErlean ES, Vanlente F, Nissen SE. Troponin T in elders with suspected acute coronary syndromes. Am J Emerg Med. 2003;21(4):293–7. doi: 10.1016/s0735-6757(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 45.Menegazzi JJ1, Davis EA, Sucov AN, Paris PM. Reliability of the Glasgow Coma Scale when used by emergency physicians and paramedics. J Trauma. 1993;34(1):46–8. doi: 10.1097/00005373-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Teasdale G, Knill-Jones R, van der Sande J. Observer variability in assessing impaired consciousness and coma. J Neurol Neurosurg Psychiatry. 1978;41(7):603–10. doi: 10.1136/jnnp.41.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis DP, Serrano JA, Vilke GM, Sise MJ, Kennedy F, Eastman AB, Velky T, Hoyt DB. The predictive value of field versus arrival Glasgow Coma Scale score and TRISS calculations in moderate-to-severe traumatic brain injury. J Trauma. 2006;60(5):985–90. doi: 10.1097/01.ta.0000205860.96209.1c. [DOI] [PubMed] [Google Scholar]
- 48.Millham FH, LaMorte WW. Factors associated with mortality in trauma: re-evaluation of the TRISS method using the National Trauma Data Bank. J Trauma. 2004;56(5):1090–6. doi: 10.1097/01.ta.0000119689.81910.06. [DOI] [PubMed] [Google Scholar]
- 49.Myint PK, Kwok CS, Bachmann MO, Stirling S, Shepstone L, Zaman MJ. Prognostic value of troponins in acute coronary syndrome depends upon patient age. Heart. 2014;100(20):1583–90. doi: 10.1136/heartjnl-2014-305533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.