Abstract
Background
After flexor tendon (FT) injury and repair, adhesion formation is a substantial concern as it can result in loss of motion and functional disability. Mmp9 is a gelatinase that contributes to degradation of extracellular matrix and is expressed during FT healing. Mmp9-/- mice have accelerated remodeling of adhesions during FT healing, relative to wild type mice. The purpose of this study was to investigate whether Ro 32-3555, an Mmp9 inhibitor, can improve FT healing by limiting adhesion formation or enhancing remodeling of scar tissue during murine FT healing.
Methods
Flexor digitorum longus laceration and repair was performed in female C57BL/6J mice. Mice were treated with vehicle or the Mmp9 inhibitor Ro 32-3555 for 8 days. Analysis was performed for digit range of motion and gliding function, biomechanics, gene expression, and Mmp9 activity.
Results
An Mmp9 activity assay as well as zymography confirmed suppression of Mmp9 activity in mice treated with Ro 32-3555. There was no significant difference in tendon gliding or range of motion between vehicle and Ro 32-3555 treated mice. There was also no difference in tendon biomechanical properties between the two groups.
Conclusions
Local inhibition of Mmp9 gelatinolytic activity at the FT repair site is insufficient to alter adhesion formation, remodeling of adhesions, and mechanical properties of healing murine flexor tendons.
Introduction
Tendons transmit forces from muscle to bone to generate movement. In the hand, flexor tendons (FTs) are enclosed in sheaths and pulleys which facilitate smooth tendon gliding [1]. When lacerated, FTs require surgical repair. Unfortunately, scarring between the tendon and adjacent tissue (adhesions) often interferes with gliding, leading to impaired finger flexion and hand function [2-4]. Although advances in surgical technique, suture material, and rehabilitation have improved outcomes following repair, there is a need for biological and pharmacological interventions to reduce scar formation during healing.
Matrix metalloproteinases (Mmps) are a family of endopeptidases that cleave most of the extracellular matrix (ECM) [5]. In addition to normal tissue remodeling, Mmps are involved in tendon healing [6-10]. Therefore, modulation of Mmp activity may be a potential therapeutic target. Mmp9 is a gelatinase that digests denatured collagen [5]. Increased Mmp9 activity has been observed in inflammatory diseases including rheumatoid arthritis, inflammatory bowel disease, and cancer [11-13]. It has been implicated in the setting of scarring and fibrosis several tissues, including the tendon [14-18]. Excessive scar tissue leads to adhesions, which complicate many FT repairs [1, 19]. Our lab is interested how modulation of Mmp9 activity affects adhesion formation and tendon gliding after injury and repair.
In a murine model of FT repair, Mmp9 expression is increased at the repair site during healing [20]. Further experiments have shown that global knockout of Mmp9 results in earlier remodeling of adhesions after FT laceration and repair. In addition, Mmp9 deletion does not compromise the strength of the repair [21]. It is important to preserve tendon strength because rupture of the repair site is also a notable concern during the healing process [19]. Therefore, Mmp9 is a promising target to reduce adhesion formation without impairing the strength during FT healing.
The purpose of this study was to investigate whether pharmacological inhibition of Mmp9 activity results in earlier remodeling of adhesions. Ro 32-3555 is a competitive gelatinase inhibitor that binds to Mmp9 in place of the substrate, collagen. It has been used in animal studies as a potential treatment for osteoarthritis, rheumatoid arthritis, and meningitis [22-24], however, effects on FT healing are unknown. In the present study, the inhibitor was administered after laceration and repair of the mouse flexor digitorum longus (FDL) tendon. The effects of inhibiting Mmp9 activity on adhesion formation, biomechanical properties, tissue morphology, and gene expression were assessed.
Materials and Methods
Animal Ethics
All animal procedures were completed at the University of Rochester and were approved by the University Committee on Animal Research (UCAR protocol # 2014-004). This study followed the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Animal Procedures: Flexor Tendon Repair Surgery
Eight-10 week old female C57B6l/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were housed at up to 5 mice per cage with ad lib food and water and a 12-hour light-dark cycle. Briefly, the distal FDL was isolated in the right hind-paw. The tendon was lacerated and repaired with 8-0 nylon suture (Ethicon Inc., Summerville, NJ) using a modified Kessler technique [20, 21]. The skin incisions were closed using 5-0 nylon suture (Ethicon Inc., Summerville, NJ). To protect the distal repair, the FDL was released at the proximal myotendinous junction. Mice were randomly assigned to vehicle or Mmp9 inhibitor treatment (Supplemental Table 1). The Mmp9 activity inhibitor Ro 32-3555 (R&D Systems, Minneapolis, MN) was diluted in normal saline and administered via i.p. injection (10mg/kg) [22]. Mice received a dose every 24 hours starting immediately following surgery and continuing through day 8 post-surgery. The vehicle treated group received daily i.p. injections of saline.
Mouse Mmp9 Activity Assay
Repaired FDL tendons were harvested from the distal tarsal tunnel to the digital bifurcation on day 7 post-surgery. Pooled tendons (n=3 per treatment per time-point) were homogenized by in Tris-HCL buffer containing 0.1% Triton-X-100, and assessed using the Mouse Mmp9 Activity Assay (QuickZyme Bioscience, The Netherlands). Samples and standards were added to a microplate pre-coated with Mmp9 capture antibody. Pro-Mmp9 was activated with p-Aminophenylmercuric acetate (APMA). Detection enzyme and substrate were added to each well. Absorbance at 405nm was read at 0 and 6 hours using Synergy Mx Monochromator-based Microplate Reader (BioTek Instruments, Winooski, VT). A standard curve was used to determine the concentration of Mmp9 in each sample (ng/ml).
Gelatin Zymography
Mice were sacrificed at day 7. Tendons (n=3 per treatment per time-point) were homogenized using a 6870 Freezer/Mill (SPEX Sample Prep, Metuchen, NJ) and suspended in zymography lysis buffer [25]. A BCA protein assay kit (Life Technologies, Grand Island, NY) was used to quantify protein. An equal amount of protein was diluted 2:1 with Tris-Glycine SDS sample buffer. Samples were added to a 12% Zymogram Protein Gel, which was run in Tris-Glycine SDS Running Buffer. The gel was incubated in Zymogram Renaturing buffer for 30 min, transferred to Zymogram Developing buffer, and incubated overnight at 37 °C. The gel was stained with SimplyBlue SafeStain and imaged with a Molecular Imager Gel Doc XR (Bio-Rad Technologies, Hercules, California).
Adhesion Testing: Gliding Coefficient and Range of Motion
Mice were sacrificed from 7-35 days post repair (n=8-14 mice per group per time-point) to assess changes in gliding function as previously described [26]. Briefly, the right (repair) and left (contralateral controls) hind limbs were harvested just below the knee. The FDL tendon was fixed between two pieces of tape using ethyl-2-cyanoacrylate. The tibia was secured in a clamp, and toes were passively extended to establish a neutral position. Incremental weight (0-19g) was applied to the tendon and a digital photograph was taken for each weight. Using ImageJ software (http://rsb.info.nih.gov/ij/), two independent observers measured the metatarsal phalangeal (MTP) flexion angle for each image. Angles were normalized to neutral and plotted versus applied load. Using Prism Graphpad 6.0a (GraphPad Software, Inc., San Diego, CA), the data was fit to a single-phase exponential equation. Gliding resistance (α) was determined using non-linear regression. There is an inverse relationship between gliding resistance and MTP range of motion [26].
Biomechanical Testing
After adhesion testing, the FDL tendon was released from the tarsal tunnel and the calcaneus was removed. Tensile testing utilized an Instron 8841 DynaMight™ axial servohydraulic testing system (Instron Corporation, Norwood, MA). The proximal FDL and distal bones of the digits were secured in clamps. The tendon was placed under tension at a rate of 30 mm/min until failure. Force and displacement data were used to calculate stiffness and maximum load at failure.
RNA Extraction and RT-PCR
Repaired FDL tendons were harvested at days 3, 7, 10, 14, 21, and 28 (n=3 per treatment group per time-point). Tendons were homogenized in Trizol reagent and RNA was extracted by the URMC Genomics Core. 500ng of RNA per sample was transcribed into cDNA using the iScript kit (Bio-Rad Technologies, Hercules, CA). Real-time PCR was run with cDNA, PerfeCTa SYBR Green SuperMix (Quanta Biosciences, Gaithersburg, MD) and gene specific primers for Col1a1), Mmp-2, and β-actin. Data were normalized to β-actin and expression in day 3 vehicle treated repairs.
Histology
Animals were sacrificed at days 10, 14, 21, and 28 post-repair (n=4 per treatment group per time-point). Hind-limbs were placed in 10% neutral buffered formalin and fixed for 72 hours. 14% EDTA (pH 7.2) was used to decalcify the samples for 14 days at room temperature. The samples were processed through a sucrose gradient and embedded in OCT compound (Sakura Finetek Inc., Torrance, CA). Serial eight-micron sagittal sections were cut through the FDL tendon plane and stained with Alcian blue/Hematoxylin and Orange G.
Statistical Analysis
Gliding, range of motion, biomechanics, and PCR data were analyzed using a two way analysis of Variance (ANOVA) followed by Bonferroni's multiple comparisons with a significance level of α=0.05. An un-paired t-test was used to evaluate Mmp9 activity assay results.
Results
Systemic treatment with Ro 32-3555 suppresses Mmp9 activity in healing flexor tendons
To determine whether systemic treatment with the gelatinase inhibitor Ro 32-3555 suppresses local Mmp9 activity at the FT repair site, an Mmp9 activity assay was conducted at day 7 post-surgery. Compared to vehicle treatment, Ro 32-3555 treated repairs showed a significant 48% decrease in Mmp9 activity, relative to vehicle treated tendon repairs (Vehicle: 0.83 ± 0.01ng/ml, Ro 32-3555: 0.40 ± 0.05ng/ml, p=0.001) (Fig. 1A). Gelatin zymography was also used to evaluate Mmp9 activity at day 7. Decreased Mmp9 activity, as assessed by decreased digestion of the gelatin substrate (white bands) was observed in Ro 32-3555 treated tendons relative to vehicle controls on day seven (Fig. 1B). Taken together, these data suggest that Ro 32-3555 effectively inhibits Mmp9 activity at the FT repair site.
Figure 1. Systemic Treatment with Ro 32-3555 Decreases Mmp-9 Activity at the Tendon Repair Site.
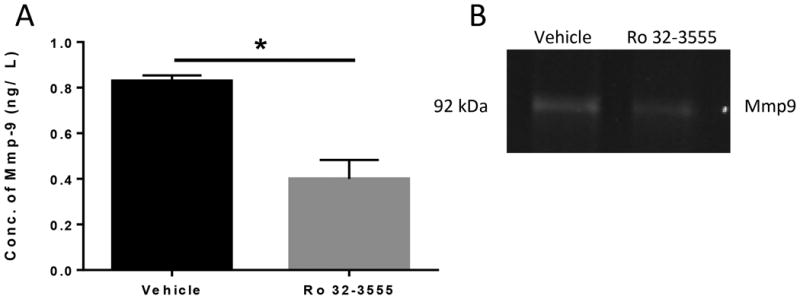
[A]: Repaired tendons were harvested 7 days post repair. A colorimetric assay was used to measure Mmp9 activity in vehicle and Ro 32-3555 treated groups. There is a 48% decrease in Mmp9 activity in the Ro 32-3555 treated group compared to vehicle. (*) indicates p<0.0001. [B]: Zymography was used to visualize Mmp9 activity in repaired tendons harvested at 7 days post-surgery. There is a decrease in band intensity in the Ro 32-3555 treated group compared to vehicle, indicating a decrease in Mmp9 activity.
Systemic Mmp9 inhibition does not alter adhesion formation during flexor tendon healing
Gliding resistance and MTP ROM were used to assess adhesion formation, with higher gliding resistance and lower MTP ROM indicative of increased adhesion formation. Vehicle-treated tendon repairs demonstrated the expected increase in Gliding Resistance (Fig. 2A) and decreased MTP ROM (Fig. 2B) with peak adhesion formation at day 14 post-surgery. No differences in Gliding Resistance or MTP ROM were observed between vehicle and Ro 32-3555 treated tendon repairs at any time-point. At 28 days, Ro 32-3555 had decreased gliding resistance relative to vehicle treated (vehicle: 22.4 ± 4.9 Arbitrary Units (A.U.), Ro 32-3555: 14.2 ± 1.4 A.U, p=0.16), and increased MTP ROM (Vehicle: 43.1 ± 3.5 Degrees, Ro 32-355: 52.4 ± 2.9 Degrees p=0.056), however, these differences were not statistically significant.
Figure 2. Systemic Treatment with Ro 32-3555 does not Impact Gliding Resistance or Range of Motion During Flexor Tendon Healing.
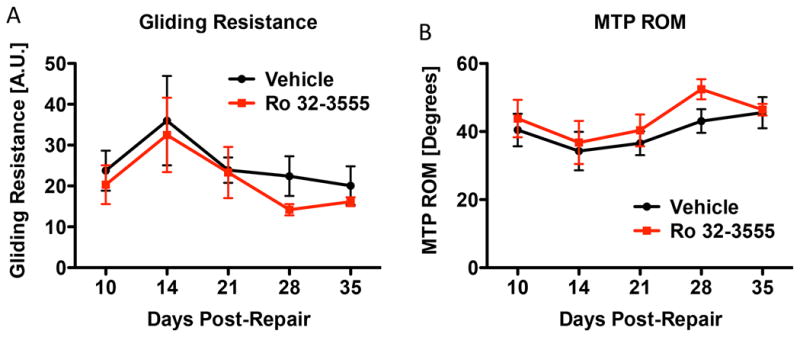
[A]: Mice were sacrificed at days 10, 14, 21, 28, and 35. Tendons were tested for gliding resistance (which represents the work required to flex the digits) by measuring the change in the angle of the MTP joint during incremental loading of the FDL. There is no significant difference in gliding resistance between the vehicle (black line) and Ro 32-3555 treated groups (red line) at any time-point. p>0.05 [B]: Hind paws were also tested for MTP range of motion, which represents the change in MTP flexion angle from the neutral position to 19g of load (heaviest load applied). There are no significant differences in MTP ROM between the vehicle and Ro 32-3555 treated groups at any time-point. p>0.05
Systemic Mmp9 inhibition does not affect the biomechanical strength of flexor tendon repairs
Tensile testing was used to assess whether Ro 32-3555 treatment alters the biomechanical strength of repairs. Maximum load at failure progressively increased over time in vehicle and Ro 32-3555 treated groups. By day 35, strength of repairs had increased by 171.8% (vehicle treated) and 212.1% (Ro 32-3555 treated), compared to treatment matched repairs at day 10. No differences in Max load at failure were observed between vehicle and Ro 32-3555 treated tendons at any time-point (Fig. 3A). Stiffness also increased over time in both groups. By day 35, stiffness had increased by 255.0% (vehicle treated) and 228.9% (inhibitor treated) compared to treatment matched repairs at day 10. There were no differences in stiffness between vehicle and Ro 32-3555 treated tendons at any time-point (Fig. 3B).
Figure 3. Ro 32-3555 Treatment does not Impact the Biomechanical Properties of Repaired FDL Tendons.
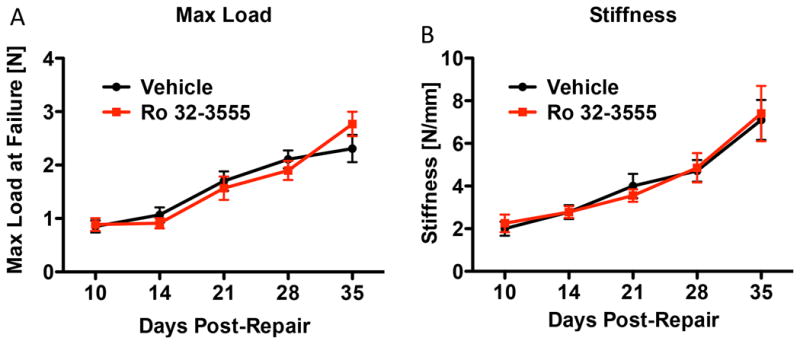
[A] FDL tendons were tested for biomechanics at 10, 14, 21, 28, and 35 days post repair. A tensile force was applied to the tendon and a force displacement curve was generated. Maximum load represents the highest force the FDL is able to withstand before failure. Maximum load increases over time for both the vehicle (black line) and the Ro 32-3555 (red line) treated groups. However, there is no difference between the groups at any time-point. p>0.05. [B] Stiffness was calculated from the slope of the linear portion of the force-displacement curve. Ro 32-3555 treatment does not affect the stiffness of the repair at any time-point. p>0.05.
Systemic Mmp9 inhibition does not alter tendon morphology during healing
Histology of the hind-paws was done to visualize the healing tendon and surrounding granulation tissue. At day 10, granulation tissue is visible above and below the repair site in both groups. By day 14, granulation tissue spans the repair site and increased cellularity can be observed within the tendon ends. At day 21, tendon thickness has visibly increased, and a disorganized network of collagen fibers bridges the repair. By day 28, the tendon ends are no longer distinguishable from each other and are held together by a more organized collagen network. Consistent with adhesion testing data, there are no apparent differences in cellularity or tendon morphology between the vehicle and Ro 32-3555 treatment groups at any time-point (Fig. 4).
Figure 4. Ro 32-3555 Treatment does not Change Tendon Morphology during the Repair Process.
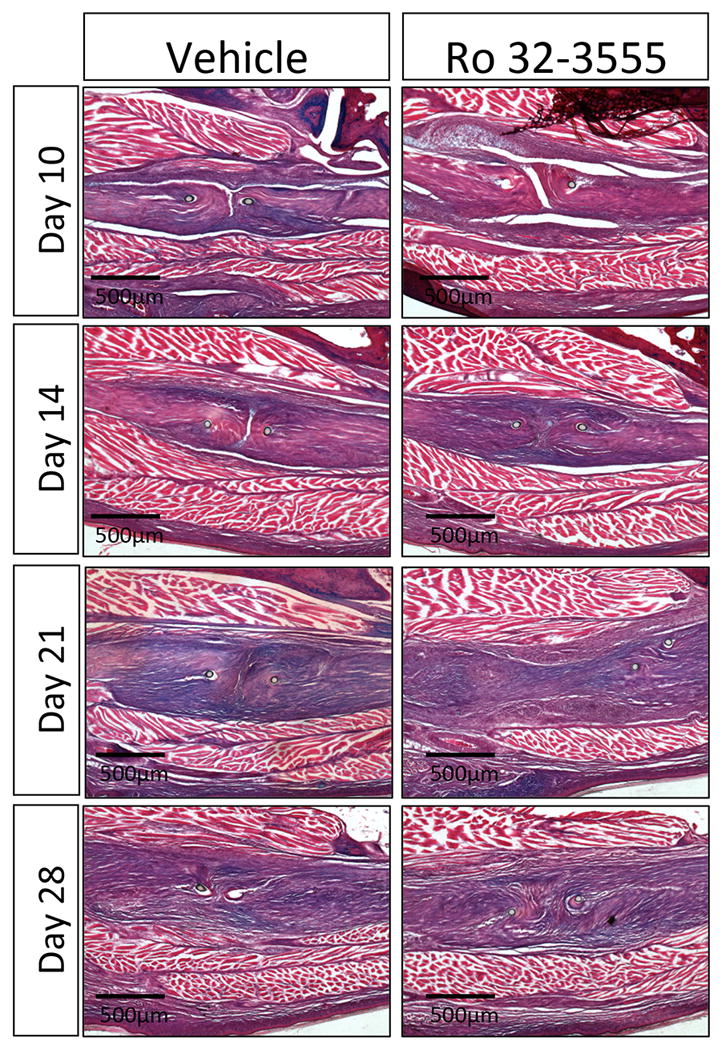
Histologic analysis of the repair site was completed at days 10, 14, 21, and 28. A similar pattern of healing was observed in both groups, beginning with granulation tissue surrounding the repair at day 10 and bridging the repair site at day 14. By day 21 there is a disorganized collagen matrix joining the two tendon ends that has becomes more organized on day 28.
Mmp9 inhibition alters Col1a1 and Mmp2 expression during flexor tendon healing
Collagen I is the main extracellular matrix component of tendon, and expression of Col1a1 is associated with restoration of tissue integrity. In vehicle treated repairs, Col1a1 expression peaked at day 21, with a 39.9-fold increase relative to day three. FDL tendons from the Ro 32-3555 treatment group had significantly less Col1a1 mRNA at day 21 compared to vehicle-treated repairs at the same time-point (p<0.0001) (Fig. 5A).
Figure 5. Ro 32-3555 Treated Mice Have Decreased Col1a1 Expression at Day 21 and Increased Mmp2 Expression at Day 28.
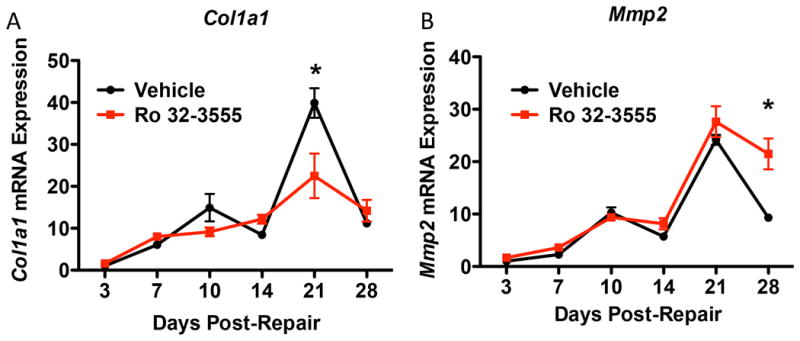
[A] RT-PCR analysis was used to assess Col1a1 expression in vehicle treated mice (black line) and Ro 32-3555 treated mice (red line) at days 3, 7, 10, 14, 21, and 28. Col1a1 expression peaked at day 21 for both groups (vehicle: 39.9-fold increase, Ro 32-3555: 22.5-fold increase, both relative to day three vehicle treatment). However, vehicle treated mice have significantly higher expression of Col1a1 at day 21 (p<0.0001). [B] Mmp2 expression was also measured in both groups, and peaks on day 21 post-repair (vehicle: 24.2-fold increase, Ro 32-3555: 27.6-fold increase, both relative to day three vehicle).
Mmp2 expression is associated with matrix remodeling during FT healing [20]. In vehicle treated mice, Mmp2 expression peaks on day 21 post-repair (24.2-fold increase relative to day three). Mmp2 expression also peaks on day 21 in Ro 32-3555 treated mice (27.6-fold increase relative to day three vehicle treatment), with no differences observed between vehicle and Ro 32-3555 treated repairs at this time. By day 28 Mmp2 expression in vehicle treated mice remains elevated (9.33-fold increase relative to day three), however, Mmp2 expression is significantly decreased relative to Ro 32-3555 treated mice which demonstrate a significant 21.5-fold increase, relative to day three repairs (p<0.0001).
Discussion
In this study, a murine model of FT repair was used to investigate whether systemic inhibition of Mmp9 activity would accelerate remodeling of fibrous adhesions. We have previously demonstrated that Mmp9-/- mice have earlier resolution of adhesions during FT healing, relative to wild type (WT) mice [21]. Additionally, WT mice that receive a bone marrow transplant from Mmp9-/- mice also demonstrate enhanced remodeling of adhesions, suggesting that bone marrow derived Mmp9 is a potential target to limit scarring [21]. Towards a more translational approach, we utilized an inhibitor of Mmp9 activity to test the hypothesis that suppression of Mmp9 activity improves adhesion remodeling.
Systemic treatment with the Mmp9 inhibitor Ro 32-3555 resulted in a significant decrease in Mmp9 activity at the repair site. However, gliding and biomechanical properties were not significantly altered. There are several potential explanations for why a change in Mmp9 activity did not result in a phenotypic change in healing. First, Ro 32-3555 did not eliminate all enzyme activity as occurs in Mmp9-/- mice. There may be a threshold of activity reduction required to observe a measurable change in phenotype. The dose administered in this study was based on previous animal experiments using Ro 32-3555, where 10mg/kg/day was sufficient to produce approximately 40% inhibition of cartilage degradation [22]. However, doses of up to 150 mg/kg/day have been used [22, 24], and increasing the dose may bring out differences in healing that were not apparent from these experiments.
Because collagen remodeling is an important part the tendon repair process [18], and because absence of Mmp9 improves tendon gliding after repair [21], a reasonable hypothesis is that the role of Mmp9 in adhesion formation relates to its effects on collagen catabolism. Mmp9 is expressed during the early stages of tendon healing [20, 27]. This correlates with the time when damaged collagen is degraded to allow for synthesis of a new provisional matrix via formation of granulation tissue [18, 27]. The role of Mmp9 in collagen digestion is further supported by the observation that Mmp9-/- mice heal with a smaller zone of injury than WT mice (in other words, there is less catabolism of the native tendon tissue) [21]. In addition, Bedi et al. found decreased collagen degradation following local delivery of an Mmp9 inhibitor to a healing rotator cuff in a rat model [6]. It is important to recognize that Mmp9 has many additional functions that could be contributing to the phenotype observed in the genetic knockout. Mmp9 has been linked to signaling pathways that affect apoptosis, angiogenesis, ECM synthesis, cell migration, cell differentiation, and inflammation [16, 28-30]. While some of these effects are mediated through collagen remodeling (including release of biologically active molecules), others are mediated by Mmp9 activity on non-ECM substrates including TNF-α, TGF-β, and IL-8 [16]. Because some of these signaling pathways may require only a small amount of Mmp9 activity, partial inhibition may be insufficient to alter these signaling pathways. This may account for the difference in phenotype observed with Mmp9-/- mice versus pharmacological inhibition of Mmp9.
The diversity of Mmp9 substrates and functions is illustrated by genetic expression changes revealed by PCR analysis. Col1a1 expression is significantly lower at day 21 in the Ro 32-3555 treated repairs relative to vehicle. Mmp9 contributes to collagen catabolism through both gelatinase activity and signaling that influences ECM synthesis [5, 29]. Therefore, it is reasonable to hypothesize that inhibition of Mmp9 not only reduces collagen degradation, but also influences collagen synthesis, leading to the difference in expression.
Another potential source of variability between the Mmp9-/- mice and inhibitor models is that Ro 32-3555 is not specific to Mmp9. It has overlapping activity with the other gelatinase, Mmp2 [22]. Mmp2 activity is increased following tendon injury [7, 31]. During FT healing in mice, Mmp2 is expressed later in the repair process than Mmp9 [20]. Peak Mmp2 expression correlates with the remodeling phase when tendon gliding is improving [20]. This suggests that Mmp2 may have a positive role in healing. Experiments with Mmp inhibition during spinal cord healing in mice also suggest a beneficial role for Mmp2. Mmp inhibition during the first three days after acute injury promotes long-term functional recovery, but this benefit is lost when treatment is extended [32, 33]. Furthermore, Mmp2-/- mice have impaired functional recovery and more extensive scar formation compared to controls [33]. These observations led us to limit Ro 32-3555 treatment so that when the remodeling phase begins and Mmp2 expression peaks, inhibitor treatment has been stopped. However, by day 10, Mmp2 expression in vehicle treated mice had increased 10-fold compared to day 3, suggesting that some Mmp2 inhibition is occurring during treatment. Therefore, a compensatory increase may explain why Mmp2 expression in Ro 32-3555 treated mice is higher relative to vehicle on day 28. This is consistent with the finding that Mmp9-/- mice have increased and earlier expression of Mmp2 compared to WT mice; a significant increase in Mmp2 was observed in Mmp9-/- mice at 14 days, while Mmp2 expression was not significantly increased until day 21 in WT. Moreover, Mmp2 mRNA expression levels in Mmp9-/- mice remained significantly elevated (4.6-fold) relative to WT at 21 days [21].
While these data demonstrate that partial suppression of Mmp9 activity is insufficient to accelerate remodeling of adhesion during FT healing, several limitations must be considered. First, we have utilized an inhibitor (Ro 32-3555) that is not specific for Mmp9, but rather has overlapping effects on Mmp2. Second, we have used a single dosage of Ro 32-3555. By increasing the dose, a phenotype may emerge, or it may reaffirm that Ro 32-3555 treatment does not significantly impact adhesion formation.
In summary, this study investigated the effect of Mmp9 inhibition on adhesion formation and remodeling during FT healing. Results show that despite a decrease in Mmp9 activity, Ro 32-3555 treatment does not significantly affect gliding function. While future studies may identify an optimal dosing regimen, our current findings suggest that suppression of Mmp9 activity alone is insufficient to enhance matrix remodeling and healing.
Supplementary Material
Number of animals per treatment group, per time-point, per experimental parameter.
Acknowledgments
We would like to thank the Histology, Biochemistry and Molecular Imaging (HBMI) core for technical assistance with histology, the Biomechanics and Multimodal Tissue Imaging (BMTI) Core for technical assistance with the biomechanical testing, and the University of Rochester Genomics Research Center for assistance with RNA extraction.
This work was supported, in part, by a Pilot Award from the Plastic Surgery Foundation/ American Academy of Hand Surgery (to AEL) and P30AR061307. CAO was supported by The University of Rochester School of Medicine Office for Medical Education Year-out Research Fellowship. MBG was supported by the University of Rochester CTSA award number TL1 TR000096 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions: Study design: WCH, RJO, AEL; Data collection, analysis, and interpretation: CAO, MBG, WCH, RJO, AEL Drafting manuscript: CAO, AEL; Revising manuscript content: CAO, MBG, WCH, RJO, AEL; Approving final version of manuscript: CAO, MBG, WCH, RJO, AEL; CAO and AEL take responsibility for the integrity of the data analysis.
References
- 1.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33(1):102–12. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Beredjiklian PK. Biologic aspects of flexor tendon laceration and repair. J Bone Joint Surg Am. 2003;85-A(3):539–50. doi: 10.2106/00004623-200303000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Aydin A, Topalan M, Mezdegi A, et al. [Single-stage flexor tendoplasty in the treatment of flexor tendon injuries] Acta Orthop Traumatol Turc. 2004;38(1):54–9. [PubMed] [Google Scholar]
- 4.Caulfield RH, Maleki-Tabrizi A, Patel H, et al. Comparison of zones 1 to 4 flexor tendon repairs using absorbable and unabsorbable four-strand core sutures. J Hand Surg Eur Vol. 2008;33(4):412–7. doi: 10.1177/1753193408090758. [DOI] [PubMed] [Google Scholar]
- 5.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 6.Bedi A, Kovacevic D, Hettrich C, et al. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(3):384–91. doi: 10.1016/j.jse.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Choi HR, Kondo S, Hirose K, et al. Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res. 2002;20(5):927–33. doi: 10.1016/S0736-0266(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 8.Guerra Fda R, Vieira CP, Almeida MS, et al. LLLT improves tendon healing through increase of MMP activity and collagen synthesis. Lasers Med Sci. 2013;28(5):1281–8. doi: 10.1007/s10103-012-1236-7. [DOI] [PubMed] [Google Scholar]
- 9.Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. Appl Physiol (1985) 2013;115(6):884–91. doi: 10.1152/japplphysiol.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32(5):1223–9. doi: 10.1177/0363546503262200. [DOI] [PubMed] [Google Scholar]
- 11.Gruber BL, Sorbi D, French DL, et al. Markedly elevated serum MMP-9 (gelatinase B) levels in rheumatoid arthritis: a potentially useful laboratory marker. Clin Immunol Immunopathol. 1996;78(2):161–71. doi: 10.1006/clin.1996.0025. [DOI] [PubMed] [Google Scholar]
- 12.Ahrens D, Koch AE, Pope RM, Stein-Picarella M, Niedbala MJ. Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum. 1996;39(9):1576–87. doi: 10.1002/art.1780390919. [DOI] [PubMed] [Google Scholar]
- 13.Marshall DC, Lyman SK, McCauley S, et al. Selective Allosteric Inhibition of MMP9 Is Efficacious in Preclinical Models of Ulcerative Colitis and Colorectal Cancer. PLoS One. 2015;10(5):e0127063. doi: 10.1371/journal.pone.0127063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racca MA, Novoa PA, Rodriguez I, et al. Renal dysfunction and intragraft proMMP9 activity in renal transplant recipients with interstitial fibrosis and tubular atrophy. Transpl Int. 2015;28(1):71–8. doi: 10.1111/tri.12445. [DOI] [PubMed] [Google Scholar]
- 15.Hsu JY, Bourguignon LY, Adams CM, et al. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci. 2008;28(50):13467–77. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31(6):599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- 17.Chiao YA, Ramirez TA, Zamilpa R, et al. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res. 2012;96(3):444–55. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 19.Dy CJ, Daluiski A, Do HT, et al. The epidemiology of reoperation after flexor tendon repair. J Hand Surg Am. 2012;37(5):919–24. doi: 10.1016/j.jhsa.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Loiselle AE, Bragdon GA, Jacobson JA, et al. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J Orthop Res. 2009;27(6):833–40. doi: 10.1002/jor.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loiselle AE, Frisch BJ, Wolenski M, et al. Bone marrow-derived matrix metalloproteinase-9 is associated with fibrous adhesion formation after murine flexor tendon injury. PLoS One. 2012;7(7):e40602. doi: 10.1371/journal.pone.0040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis EJ, Bishop J, Bottomley KM, et al. Ro 32-3555, an orally active collagenase inhibitor, prevents cartilage breakdown in vitro and in vivo. Br J Pharmacol. 1997;121(3):540–6. doi: 10.1038/sj.bjp.0701150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brewster M, Lewis EJ, Wilson KL, Greenham AK, Bottomley KM. Ro 32-3555, an orally active collagenase selective inhibitor, prevents structural damage in the STR/ORT mouse model of osteoarthritis. Arthritis Rheum. 1998;41(9):1639–44. doi: 10.1002/1529-0131(199809)41:9<1639::AID-ART15>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Liechti FD, Grandgirard D, Leppert D, Leib SL. Matrix metalloproteinase inhibition lowers mortality and brain injury in experimental pneumococcal meningitis. Infect Immun. 2014;82(4):1710–8. doi: 10.1128/IAI.00073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farhat Y. Lysis of Collagen Gels for Zymography. [cited 2015 6/5] [Google Scholar]
- 26.Hasslund S, Jacobson JA, Dadali T, et al. Adhesions in a murine flexor tendon graft model: Autograft versus allograft reconstruction. J Orthop Res. 2008;26(6):824–33. doi: 10.1002/jor.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshiro W, Lou J, Xing X, Tu Y, Manske PR. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg Am. 2003;28(5):814–23. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 28.Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130(17):4123–33. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803(1):39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Yu YY, Lieu S, et al. MMP9 regulates the cellular response to inflammation after skeletal injury. Bone. 2013;52(1):111–9. doi: 10.1016/j.bone.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21(2):185–95. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 32.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526–35. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu JY, McKeon R, Goussev S, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26(39):9841–50. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of animals per treatment group, per time-point, per experimental parameter.


