Abstract
Generating a broadly protective influenza vaccine is critical to global health. Understanding how immune memory influences influenza immunity is central to this goal. We undertook an in-depth study of the B cell response to the pandemic 2009 H1N1 vaccine over consecutive years. Analysis of monoclonal Abs generated from vaccine-induced plasmablasts demonstrated that individuals with low preexisting serological titers to the vaccinating strain generated a broadly reactive, HA stalk-biased, response. Higher preexisting serum antibody levels correlated with a strain-specific HA head-dominated response. We demonstrate that this HA head immunodominance encompasses poor accessibility of the HA stalk epitopes. Further, we show polyreactivity of HA stalk-reactive antibodies that could cause counterselection of these cells. Thus, preexisting memory against HA head epitopes predominate, inhibiting a broadly protective response against the HA stalk upon revaccination with similar strains. Consideration of influenza exposure history is critical for new vaccine strategies designed to elicit broadly neutralizing antibodies.
Introduction
Influenza remains a major cause of morbidity and mortality due to both annual epidemics and potentially dangerous pandemics. The majority of neutralizing antibodies (Abs) formed in response to influenza vaccination are directed towards highly variable and mutable regions of the influenza virus hemagglutinin (HA) globular head region responsible for viral attachment to host cells. However, unlike the strain-specific HA head dominated response normally induced by seasonal drifted H1N1 strains, first exposure to the antigenically novel 2009 pandemic H1N1 strain by infection or vaccination generated a broadly protective antibody response capable of binding multiple H1N1 and H5N1 strains (1-5). These broadly protective Abs targeted the entire hemagglutinin (HA) protein but were predominantly against highly conserved HA stalk epitopes (6-8). Based on the variable gene mutation level and binding affinity of the HA stalk-specific Abs, they appear to be preexistent in the memory B cell repertoire, but their rarity suggests that they are overshadowed by the immunodominant response against less conserved epitopes on the HA head.
The discovery that divergent influenza strains can preferentially boost rare, broadly neutralizing memory B cells has led groups to design strategies for preferentially inducing these B cells (9, 10). However, a number of key issues persist regarding the feasibility of inducing broad protection by immunizing with highly novel influenza strains in humans. First, as implied by the findings described above, a predominance of B cells activated by influenza must be from memory cells. Most memory B cells have somatically mutated variable genes (11) and we have shown previously that IgG+ influenza+ plasmablasts, including those generated in response to the novel pandemic H1N1 influenza strain, have extensively mutated variable region genes (3, 12, 13). This suggests that influenza+ plasmablasts derive from memory cells that have undergone multiple rounds of somatic mutation and affinity maturation, but this has never been directly shown. Second, though serological studies have demonstrated differential responses to first exposure with the pandemic 2009 H1N1 strain, it is unclear how much the quality of the B cells directly activated by the vaccine vary from person to person and what drives that variable response. Finally, broadly protective epitopes were rarely targeted upon vaccination with annual H1N1 strains circulating prior to the 2009 pandemic (8, 12), nor with seasonal H3N2 or influenza B strains, suggesting that HA stalk and other broadly protective epitopes are subdominant. Though HA stalk-specific B cells might be activated upon first exposure to a novel influenza strain, it is important to determine if subsequent exposure will generate the same kind of response when strain-specific B cells are now more abundant. Similarly, if a novel vaccine successfully induces a predominance of broadly protective antibodies to the HA stalk, providing universal immunity to influenza, it is important to know if this response persists after natural exposure to related influenza strains.
Through an in-depth analysis of the human response to the 2009 pandemic H1N1 strain in individuals with different influenza exposure histories, we evaluated in vivo what factors drive a broadly protective HA-specific antibody response. For this, the B cell response to the pandemic 2009 H1N1 strain was evaluated upon first or second exposure in 21 individuals. To do so, we took advantage of the fact that 5-7 days after influenza vaccination, an expanded population of vaccine-induced plasmablasts appears in human peripheral blood (13). We analyzed the immunoglobulin variable regions, strain specificity and functional properties of the Abs produced by this plasmablast population at the single-cell level across multiple years, allowing us to directly evaluate the effect that immune memory has on the specificity of the current response.
We report that only individuals with low preexisting serological levels of pandemic H1N1-specific Abs generated a broadly neutralizing plasmablast response directed toward the HA stalk. Further, we demonstrate that the immune subdominance of the HA stalk is a function of both the poor accessibility to the broadly protective epitopes and inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains, and determines the likelihood of generating a broadly protective response.
Results
Memory B cells are frequent precursors to influenza-induced plasmablasts
Design of new vaccine strategies assumes that the adult influenza vaccine response is driven by activation of preexisting memory B cells. To determine directly if memory B cells are the precursors to the plasmablast influenza response we analyzed the B cell response to vaccination in adult subjects who received the influenza vaccine over two consecutive years (in 2006-to-2007, 2009-to-2010, or 2010-to-2011 detailed in table S1). Within each year, single plasmablasts were isolated by flow-cytometry at seven days post-vaccination and the variable genes were sequenced and expressed as recombinant monoclonal antibodies (mAbs) to identify influenza vaccine-specific plasmablasts, as described previously (13, 14). Monoclonal antibodies generated from each subject were tested for binding to the vaccine by ELISA. We restricted the analysis to subjects from whom we isolated at least 10 vaccine-specific mAbs in each year. In total, from 4 subjects, 103 vaccine-specific plasmablasts were identified from year 1, and 81 vaccine-specific plasmablasts were identified from year 2. Despite only sampling 10-40 vaccine positive plasmablasts per subject each year, we readily found the same plasmablast clones in consecutive years (Fig. 1A-B). For the 4 subjects studied, 46% of the plasmablasts when averaged by individual, and 43% in total (35/81), isolated in the second year were clonally related to plasmablasts isolated in the first year. Conversely, no clonally related plasmablasts of the 70 vaccine negative day-7 plasmablasts in year 2 were found in year 1 (χ2 p <0.0001). Because the vaccine positive sampling in each of the two years was limited, the likelihood of detecting a particular clone in both years is low. Thus, 43% percent is certainly a significant underestimation of the total frequency of the response derived from the reactivation of memory B cells.
Figure 1. Plasmablasts induced by influenza vaccination are often derived from memory B cells.
(A, B) Four subjects were immunized in each of two consecutive years and mAbs made from vaccine-induced plasmablasts were tested for reactivity to influenza strains present in the vaccine. Shown are the numbers of mAbs that were clonally related in both of the seasons by individual (colored black) (A) and in total (B). (C-F) Phylogenetic trees depicting the variable gene sequences rooted in the germline sequence of vaccine-positive clonotypes isolated from 3 subjects over 2 years. mAb from year one (Y1) are in blue and from year two (Y2) in red. In (F), members of the same influenza+ clone were found in memory B cells (Mem) isolated from blood collected 21 days after vaccination in year one and year two and plasmablasts (PB) from year two.
We then estimated the phylogeny of 3 influenza-binding clones in which multiple clonal members were isolated in consecutive years from the plasmablast population. For each of these three clones, the B cells from consecutive years appear to have derived from several branches of the clonotypes’ phylogeny, suggesting that plasmablasts were activated from memory cells that were maintained from the entire diversity of mutational variants in the clonotype over multiple years (Fig. 1C-E, fig. S1A). Notably, the frequency of somatic mutations within clonal lineages were the same over multiple years (fig. S1B). We also sorted HA-binding memory B cells from one subject 21 days after vaccination in both year 1 and year 2 and found memory B cells from both years clonally related to plasmablasts that responded to the year 2 vaccine (Fig. 1F, fig. S1A). Plasmablasts are short-lived, and thus are not themselves persisting from year to year. We can conclude, then, from this analysis and the high frequency of returning clonotypes over consecutive years, that a highly diverse pool of memory B cell precursors persists from year to year in humans that might be readily activated against influenza strain variants.
Epitopes targeted in response to influenza vaccination vary between individuals
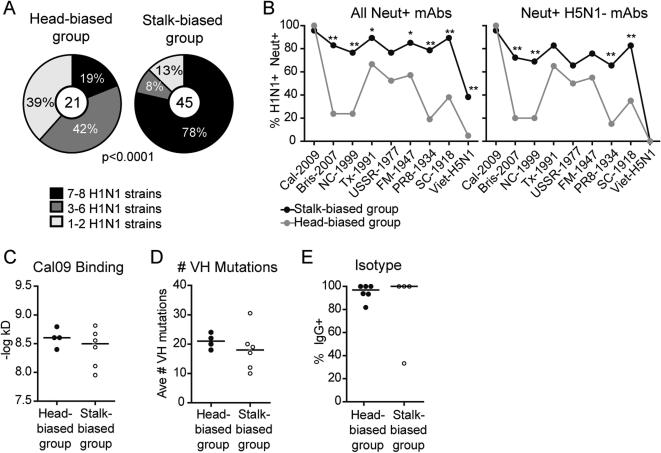
To understand the impact of the preexisting memory B cell repertoire on the influenza vaccine response, we analyzed in detail the plasmablast response to the novel, antigenically shifted 2009 pandemic H1N1 strain A/California/04/2009 (Cal09) in 10 subjects vaccinated with the 2009-10 monovalent vaccine (table S2). We identified 193 mAbs that bound whole virus Cal09 and 184 that bound recombinant HA (rHA) (80 clonally distinct) (table S3). A high percentage of rHA+ plasmablasts neutralized viral replication in all subjects, with little difference between subjects (Fig. 2A). However, there was a clear division of the subjects into two response groups, based on ability of rHA+ plasmablasts to inhibit hemagglutination, and thus bind the HA sialic acid-binding site (Fig. 2B). In 4 subjects, at least 90% of the mAbs were HAI+, while less than 60% of the mAbs in the remaining six subjects inhibited hemagglutination (Fig. 2B). These results changed little when we included only clonally distinct mAbs in the analysis, demonstrating that large clonal pools were not skewing the data (Fig. 2B). The large number of HAI negative neutralizing mAbs in 6 of the subjects suggested that many of the responding B cells in these subjects bound the HA stalk. H5N1 and H1N1 have highly divergent HA heads but contain conserved and protective epitopes in the HA stalk. We therefore measured the ability of these mAbs to bind H5N1 and compete for binding with well-characterized stalk-specific mAb sc70-1F02 (12). In this way we identified HA stalk-specific mAbs in all 6 subjects that displayed a significant HAI negative response (Fig. 2C and D). All together, whereas 99% of the neutralizing mAbs in the first 4 individuals bound the HA head, 1/3 of the neutralizing mAbs in the remaining 6 were confirmed to bind the HA stalk. Another 1/3 were HAI negative but did not bind H5N1 and/or did not compete with sc70-1F02 for binding, making the epitopes they recognize unclear (Fig. 2E). From here on these two response groups will be referred to as the HA head-biased (subjects SFV015, SFV018, SFV019, SFV020) and HA stalk-biased (subjects SFV009, 029-09, 030-09, 045-09, 047-09, 051-09) groups.
Figure 2. A subset of subjects generated HA stalk-biased plasmablast response.
(A, B) Proportion of H1N1 rHA+ mAbs in each subject capable of neutralizing viral replication (A) or inhibiting turkey red blood cell agglutination including all mAbs (B, left panel) or only clonally distinct mAbs (B, right panel). The black line separates the subjects based on level of HAI+ mAbs (C) Neutralizing but HAI negative mAbs were tested for binding to H5N1 rHA and the ability to compete for binding to Cal09 rHA with the HA stalk-binding mAb sc70-1F02. Each bar represents the neutralizing titer (PRNT50), binding affinity to H5N1, or percent inhibition for each HAI negative mAb. HA stalk-binding mAbs are represented by black bars. Only one representative member of clonally related mAbs is shown. (D-E) The subjects were divided into two groups as indicated in (B) and the proportion of neutralizing rHA-binding mAbs that bind the HA stalk in each subject (D) or within each group is shown (E). mAbs that were HAI negative but did not bind H5N1 or compete with sc70-1F02 are labeled as unknown. mAbs capable of inhibiting hemagglutination are labeled as HA head-binding. The number in the middle of the pie charts represents the total number of mAbs tested from that response group. Statistical significance of the difference between the two groups was determined using the Mann Whitney test (D) or Chi-square test (E). All data is representative of 2-3 replicates per mAb.
Not surprisingly, this difference in epitope binding also affected the level of crossreactivity of the neutralizing mAbs. When we tested the H1N1 rHA+ mAbs against 8 different rHAs from virus strains representative of H1N1 diversity in the past 100 years (fig. S2), close to 80% of the mAbs from the HA stalk-biased group bound 7-8 different rHAs while only 20% of the mAbs from the HA head-biased group crossreacted with this many rHAs (Fig. 3A). In particular, few neutralizing mAbs in the HA head-biased group bound recent seasonal strains Bris-2007 and NC-1999, or the older strain, PR8-1934 while mAbs in the HA stalk-biased group were generally able to bind all strains tested (Fig. 3B, left panel and fig. S3A). This was true even when we removed all mAbs able to bind H5N1, including the confirmed stalk-reactive mAbs, from the analysis (Fig. 3B, right panel). This demonstrates that even HA head-binding mAbs from the HA stalk-biased group were more broadly reactive. Despite these stark differences in epitope binding, we saw no difference in binding avidity to Cal09, somatic hypermutation level, or isotype usage between the two response groups (Fig. 3C-E). In summary, we found that upon first vaccination against the pandemic 2009 H1N1 strain, 4 of the 10 subjects mounted an almost solely HA head-specific B cell response that was generally not cross-reactive with the most recent seasonal H1N1 strains. The remaining 6 subjects targeted more conserved neutralizing HA epitopes including the well described HA stalk epitope.
Figure 3. A subset of subjects generated a broadly neutralizing plasmablast response.
(A, B) All rHA-binding and neutralizing clonally distinct mAbs were tested for the ability to bind rHA by ELISA from 8 different H1N1 strains. Shown is the proportion of clonally distinct mAbs in each response group capable of binding the number of rHAs listed in the legend (A) or percentage of mAbs in each group (black: stalk-biased group; gray: head-biased group) that binds each of the H1N1 strains and H5N1 as listed on the X-axis with (B, left panel) or without (B, right panel) H5N1+ mAbs included in the analysis. Statistical significance of the difference between the two groups was determined using a (A) Chi-square test or (B) Fisher's exact test (*p<0.05; **p<0.005). Exact p values can be found in supplementary material (source data). All data is representative of 2-3 replicates per mAb (C) The binding avidity of all clonally distinct H1N1+ mAbs against Cal09 whole virus was determined by ELISA. Each dot represents the median of the –log transformed apparent kD of all clonally distinct mAbs in each subject. (D) The median number of mutations in the VH gene of each clonally distinct H1N1+ mAb for each subject. (E) The percentage of H1N1+ mAbs in each subject that was IgG+. All other H1N1+ mAbs were IgA+. For (C-E) the black line represents the median value of the subjects in each response group. A Mann-Whitney test showed no statistical significance between response groups.
Reexposure to the same strain generates an HA head-specific response
If a broadly protective B cell response can be induced using novel vaccination strategies, it is important to know if such a response will be maintained upon subsequent exposures to similar influenza strains. To determine if the response to the Cal09 H1N1 strain induced an HA stalk-biased response upon subsequent exposures, we vaccinated 11 subjects with the seasonal trivalent vaccine containing Cal09 6-12 months after they had received the Cal09 vaccine for the first time (designated “revaccinated group”) (table S2). One of these 11 subjects (051) was also a part of the 2009 monovalent vaccine cohort. We identified 127 mAbs capable of binding Cal09 virus, 111 of which bound rHA (83 clonally distinct) (table S3). There was some subject-to-subject variation in the proportion of mAbs able to neutralize Cal09 and inhibit hemagglutination (Fig. 4A and B), but unlike the 2009 vaccine group, we were not able to delineate two response groups based on the proportion of HAI+ mAbs as the difference between subjects was more graded (Fig. 4B). More importantly, we only identified three mAbs (3.5% of all neutralizing mAbs), all from the same subject (039), which bound the conserved neutralizing HA stalk epitope (Fig. 4C and D). While half of the neutralizing mAbs we isolated from subject 051 after first vaccination with Cal09 bound the HA-stalk (Fig. 2C and table S3), none of the H1N1+ mAbs generated from this subject after revaccination crossreacted with H5N1 (fig. S4A). Overall, the number of neutralizing mAbs crossreacting with different H1N1 strains, including recent seasonal H1N1 strain Bris-2007, was significantly lower than the 2009 HA stalk-biased group (Fig. 4E, left panel and fig. S3B). This was true even when we removed H5N1+ (including stalk-reactive) mAbs from the analysis (Fig. 4E, right panel). Thus, in contrast to first exposure to the novel Cal09 strain, revaccination with Cal09 generated a neutralizing B cell response directed towards more strain-specific HA head epitopes. Despite these differences in epitope targeting, plasmablasts generated upon revaccination had equivalent levels of immunoglobulin somatic hypermutation, binding affinity and isotype usage as upon first vaccination (fig. S4B).
Figure 4. The plasmablast response upon revaccination is HA head-biased.
(A, B) Proportion of H1N1 rHA-binding mAbs in each subject able to neutralize viral replication in MDCK cells (A) and inhibit viral-induced hemagglutination (B). (C) Neutralizing, but HAI negative mAbs were tested for their ability to bind to H5N1 rHA and compete with sc70-1F02 for binding to Cal09 rHA is in Figure 2. Each bar represents one neutralizing HAI negative mAb, black bars represent HA stalk-binding mAbs. Only one representative of clonally related mAbs is shown. (D) Proportion of neutralizing mAbs in the revaccinated group able to bind the HA stalk. The number in the center represents the total number of neutralizing mAbs. (E) mAbs from the revaccinated group were tested for their ability to bind rHA from 8 different H1N1 strains. Shown is the proportion of neutralizing and clonally distinct mAbs from the revaccinated group (grey line) and 2009 stalk-biased group (black line) able to bind each H1N1 strain as indicated in the X-axis. The panel on the right represents the proportion of mAbs able to bind each strain after excluding H5N1+ mAbs from the analysis. Statistical significance was determined using Fisher's exact test (*p<0.05, **p<0.005). Exact p values can be found in supplementary material (source data). All data is representative of 2-3 replicates for every mAb.
Pre-vaccination serum antibody levels predict the breadth of the B cell response
Second exposure to the pandemic H1N1 strain induced a consistently HA head-biased and strain-specific response. We therefore reasoned that differences in the pre-vaccination memory repertoire between subjects may be responsible for the variable response noted upon first exposure to this strain. To analyze this, we measured pre-vaccination serological levels to Cal09 in all subjects. We found that, independently of whether vaccinated for the first or second time, there was a clear negative correlation between the pre-vaccine Cal09 HA+ serum levels and the percentage of H1N1 mAbs in each subject that bound Cal09, but not the seasonal Bris-2007 H1N1 strain, as a measure of broad reactivity (Fig. 5A). When we directly compared Cal09-specific pre-vaccine serological levels in the two 2009 response groups and the revaccinated group, we found that 2009 subjects with an HA stalk-biased response had very low Cal09 specific pre-vaccine serum levels (Fig. 5B). In contrast, the average pre-vaccine Cal09-specific serum levels were much higher in 2009 subjects with an HA head-biased response upon first vaccination, comparable to the revaccinated group (Fig. 5B).
Figure 5. Pre-vaccine serum Ab levels correlate with the specificity of the plasmablast response.
Sera collected on the day of vaccination was tested by ELISA for the level of antibodies able to bind H1N1 strains. Pre-vaccine serum was not collected from three subjects (SFV018, 030-09 and 039-10) so we were unable to include data from these subjects. (A) The percentage of H1N1+ mAbs produced from each subject able to bind Cal09 but not Bris-2007 was plotted against the Cal09 EC50 of pre-vaccine serum collected from that same individual. Each dot represents one subject vaccinated either for the first or second time with Cal09. Degree of correlation was determined using the nonparametric Spearman correlation coefficient and p value. The Cal09 EC50 is the mean of three independent measurements for each sample. (B, C) Direct comparison of Cal09 EC50 in pre-vaccine sera (B) or birth year (C) of subjects in both 2009 groups and the revaccinated group. Statistical significance was determined using the Mann-Whitney test. (D) The pre-vaccine serum antibody EC50 for 9 H1N1 strains was determined in each subject. Shown is the mean EC50 with SEM from 3 independent replicates in each vaccine group. Statistical significance of differences between vaccine groups for each H1N1 strain is detailed in Fig. S4.
In explanation for these observations, the 2009 subjects with an HA head-biased response were all born before 1965, while 5 of the 6 subjects in the HA stalk-biased group were born in the late 1970's and 1980's as were 9 of the 11 subjects revaccinated the following year (Fig. 5C). This suggested those with an HA head-biased response upon first vaccination with Cal09 had been exposed to a broader range of H1N1 strains over their lifetime before 2009. Indeed, when we measured pre-vaccination serum levels to several past H1N1 strains, we found that, on average, subjects who generated a more specific response, had high pre-vaccination levels to all strains tested while the 2009 HA stalk-biased group had low levels of serum antibodies to strains older than the A/Texas/36/1991 H1N1 strain (Fig. 5D and fig. S5). Thus, immune memory to Cal09 drives the response towards immunodominant and strain-specific HA head epitopes. This is independent of whether these memory cells were generated from previous exposure to Cal09 or to older strains containing shared epitopes with Cal09. We conclude that the rare but valuable B cell capable of binding subdominant, conserved epitopes, such as on the HA stalk, will only emerge when it is the only one available to respond to widely divergent influenza strains.
Neutralizing HA stalk-binding mAbs bind weakly to whole virus
To understand why there is a preferential activation of HA head-specific B cells if available in the memory repertoire, we compared properties of 35 HA head-binding mAbs (HAI+) and 33 HA stalk-reactive mAbs that bound rHA with similar affinity (table S3 and S4). HA stalk-reactive mAbs were identified based on the characteristics described for Figure 2. The 33 HA stalk-binding mAbs included 26 unpublished mAbs generated by our group as well as 7 mAbs published previously by our group or others (7, 12, 15-17) (table S3 and S4). The majority of HA stalk mAbs were crossreactive with Group 1 HAs only, though four were also capable of binding HA from Group 2 viruses (H3N2 and H7N9).
One reason proposed for the subdominance of the stalk HA epitopes is limited access due to steric shielding by the HA globular head and/or because of their proximity to the viral envelope, as suggested by the highly biased usage of the hydrophobic VH1-69 gene for immunoglobulins with HA stalk reactivity (8, 18-20). Although structural studies have demonstrated that the HA stalk epitopes are accessible for antibody binding (21), a direct comparison of HA stalk versus HA head epitope binding has not been done. Indeed, when we compared the ability of HA stalk-binding mAbs to bind by ELISA to recombinant HA (rHA) or whole virus, we found that they bound to rHA with, on average, a log higher avidity (Fig. 6A). In contrast, HA head-binding mAbs bound with similar affinity to rHA and whole virus. The outcome was equivalent binding affinity (apparent kD) to rHA between the HA head and stalk-reactive mAbs but significantly reduced binding of HA stalk-reactive mAbs to whole virus (Fig. 6B). The apparent cause for the reduced affinity could be demonstrated by surface plasmon resonance (SPR) that indicated a reduced association rate for the stalk reactive antibody binding to rHA, although dissociation and affinity (KD) were not different, consistent with the ELISA data (Fig. 6C). This reduction in association rate was presumably even greater for HA stalk-reactive mAbs binding to whole virus, as demonstrated by the profound difference seen by ELISA.
Figure 6. HA stalk-specific mAbs bind with lower affinity.
(A-B, D) 35 HA head-reactive mAbs, 33 sc70-1F02 competing and 16 noncompeting HA stalk-specific mAbs were tested by ELISA for binding to Cal09 rHA or whole virus. Each line in (A) and (D) represents the paired apparent kD of binding of each mAb to rHA or whole virus. In (B) each dot represents the apparent kD of one mAb with the median of each group shown by a horizontal line. Data representative of 2-3 independent experiments. Statistical significance was determined using the Wilcoxon test (A and D) or Mann-Whitney test (B). (C) Testing of a subset of HA-stalk versus HA-head reactive antibodies by surface plasmon resonance to determine, association (Ka), dissociation (Kd), and affinities (KD). Shown is mean of two independent experiments. Statistical significance was determined using an unpaired two-tailed t-test.
The reduced binding to whole virus might be true for mAbs binding all HA stalk epitopes or only for those binding to the restricted broadly-protective epitopes recognized by the sc70-1F02 HA stalk-binding mAbs as described above. To determine if this was the case, we identified another 16 HAI negative mAbs that bound whole H1N1 rHA, H5N1 rHA, and a chimeric H9 Head/H5 stalk rHA, but not to molecules with the H5 or H9 HA head alone (22) (fig. S6A). However, these mAbs were either non-neutralizing or only weakly neutralized viral replication (fig. S6B), and did not compete for binding with sc70-1F02 (fig. S6C). Thus, these antibodies bind to the HA stalk region, but to different and generally non-neutralizing epitopes and are termed non-competing HA stalk-binders from hereon. These 16 antibodies were somewhat heterogeneous in their ability to bind rHA and whole virus, but as a group, there was no significant difference in their ability to bind rHA and whole virus (Fig. 6D). Thus, it is primarily antibodies binding the critical neutralizing HA stalk epitopes that bind HA with lower affinity when it is on the virion surface.
We saw no difference in the VH CDR3 length, VH gene mutation levels, or JH usage between HA stalk or head-binding mAbs (fig. S7A-C). However, as has been described by others (20, 23, 24), VH usage of the neutralizing HA stalk-reactive mAbs was highly restricted to VH1-69 (fig. S7D), with 21/33 of these mAbs using this VH gene. Interestingly, this VH restriction was not seen with the non-competing HA stalk-binding mAbs (fig. S7D). This would suggest that the limited accessibility of the neutralizing epitopes on the HA stalk imposes molecular constraints on mAbs able to bind these sites leading to restricted VH usage of neutralizing HA stalk-binding mAbs. Thus, B cells that can bind broadly neutralizing HA stalk epitopes is limited.
Neutralizing antibodies binding HA stalk epitopes are polyreactive
Memory B cells expressing polyreactive IgG immunoglobulins make up over 25% of the healthy human B cell repertoire (25, 26). These antibodies bind with low affinity to multiple antigens via various non-covalent interactions such as hydrophobic or charge interactions (e.g., the association of basic arginine residues in antibody CDRs with the acidic phosphate backbone of DNA (27)). It is believed that these non-specific interactions augment specific binding to particular classes of epitopes, particularly those that are poorly accessible. For example, HIV-specific antibodies that bind gp140 have been shown to be polyreactive, facilitating binding to this membrane-bound viral protein (28). We therefore reasoned that the HA stalk-specific mAbs might also be polyreactive, facilitating binding to this poorly accessible epitope. Indeed, we found that the 33 HA stalk-binding mAbs tested were more reactive to dsDNA, insulin and LPS compared to HA head-reactive antibodies (Fig. 7A and B). This was true when we compared either the area under the binding curve (Fig. 7A) or determined the percentage of polyreactive mAbs by setting a threshold for positivity (Fig. 7B and fig. S8A). Interestingly, the non-competing HA stalk antibodies with little neutralization capacity were not polyreactive (Fig. 7A and B), indicating that this property is primarily important for accessing the broadly neutralizing epitopes, not the entire HA stalk.
Figure 7. HA stalk-specific are polyreactive.
(A-B) Each mAb was tested for binding by ELISA to dsDNA, LPS or Insulin. For (A) Shown is a comparison of the area under the binding curve (AUC) for each mAb tested against each antigen. The data points for the well-characterized stalk-binding mAbs CR9114 and F10 are indicated in purple and green, respectively. For (B) mAbs that had an OD above 0.5 at the highest concentration were consider positive for binding that particular antigen (fig. S8A). A mAb was considered polyreactive if it bound all three antigens. Shown is the percentage of polyreactive mAbs in each group as indicated. Statistical significance was determined using Fisher's exact test. Data representative of 2-3 independent experiments. (C) Binding of the HA stalk-reactive mAbs to HEp-2 cells by immunofluorescence at a concentration of 50ug/ml. The data points for CR9114 (purple) and F10 (green) are marked. HE-p2 binding of high-affinity anti-nuclear antibody 3H9 is shown in red as a comparison. (D) The polyreactivity of HA stalk-reactive antibodies was compared with (+) and without (−) the presence of physiological concentrations (5%) of human serum albumin. Binding of the prototypical lupus-associated autoantibody mAb 3H9 (in red) was also tested as a comparison. Values represent the average of three replicates. Statistical significance was determined using paired t-tests. (E-F) Binding to dsDNA, LPS and insulin was compared between mAbs encoded by VH1-69 that bound influenza, but not the HA stalk, or bound the HA stalk (21 mAbs/group). Shown is a comparison of the AUC (E) as shown in A or percent polyreactive (F), determined as in B. Binding curves are representative of 3 independent experiments. The AUC is the mean of 3 independent experiments.
Though the HA-stalk specific mAbs were polyreactive, they did not appear to be reactive to specific self-antigens, and thus unlikely to cause pathology. With the exception of one mAb, none of the stalk-reactive mAbs bound at a significant level to HEp-2 cells by immunofluorescence (Fig. 7C). In addition, at the peak of the anti-influenza serum response, there was no increase in serum anti-dsDNA reactivity when measured in the 4 subjects with an HA stalk-prone response for which serum was available (fig. S8B). Further, the polyreactivity could be inhibited with addition of physiological concentrations of human serum albumin, demonstrating that despite having the chemical characteristic of binding non-specifically to various antigens, physiologically these antibodies are unlikely to be self-reactive (Fig. 7D and fig. S8C). However, high affinity interactions with specific epitopes could still utilize polyreactivity to augment binding. Notably, addition of albumin had no negative effect on binding to influenza for the HA stalk-reactive antibodies (fig. S8D), nor on the binding of mAb 3H9 to dsDNA, a well-known prototypical anti-nuclear lupus-associated autoantibody (29) (Fig. 7D).
As broadly neutralizing HA stalk-reactive antibodies are predominantly encoded by VH1-69, we also wanted to determine if this particular VH gene tends to be polyreactive or if the polyreactivity is an innate feature of binding the protective stalk epitopes. To do so, we compared the polyreactivity of 21 influenza-specific, but not HA stalk-reactive mAbs encoded by VH1-69 to the 21 HA stalk-binding mAbs in our collection that were also encoded by VH1-69. The HA stalk-binding mAbs were clearly more polyreactive (Fig. 7E and F), indicating that polyreactivity is a specific characteristic of mAbs capable of binding broadly protective epitopes on the HA stalk, independently of VH usage. We hypothesize that this tendency for polyreactivity, will cause broadly neutralizing stalk-binding B cells to have a selective disadvantage due to mechanisms of immune tolerance such as anergy, further contributing to HA head immunodominance.
Discussion
We observed significant subject-to-subject variation in the first response to Cal09 vaccination. Though all 10 individuals mounted a robust, high-affinity and memory-driven response primarily directed towards Cal09 rHA, we observed a striking divergence of the B cell response into two distinct qualitative categories. Four of the individuals with higher pre-vaccine Cal09 specific serological levels generated an almost exclusively HA head-specific plasmablast response that crossreacted little to the most recent seasonal H1N1 strains. Plasmablasts from the other 6 subjects targeted primarily the HA stalk and other conserved epitopes on the HA molecule and so were more broadly reactive to diverse H1N1 strains and H5N1.
The high pre-vaccine levels seen in the 2009 HA head dominated response group could be because these subjects had been naturally exposed to the Cal09 strain in 2009 before vaccination. However, given that the H1N1 specificity of their response was quite different from the revaccinated group, this seems unlikely. Alternatively, subjects who generated a more Cal09 head response could have been exposed to a wider range of historical viral strains including those with shared epitopes in the HA head, whereas those who did not were only able to activate B cells capable of binding conserved HA stalk epitopes. In support of this, the group of subjects with a more restricted Cal09-specific response were born between 1945 and 1961 whereas 5/6 subjects in the other group were born in 1978 or later. Studies have shown a strong correlation between age and preexisting serological titers to Cal09 (30, 31). At the serological level it's also been shown that people older than 65 as a whole seem to generate proportionally more HAI+ Abs to Cal09 vaccination compared to individuals between ages 18-32 (5). Exposure or vaccination with the antigenically similar NJ1976 strain has also been linked to higher Cal09 preexisting serological levels and with generating a more HA head-specific response upon vaccination with Cal09 (30, 32). However, in our cohort, though the 4 subjects with an HA head dominated response did have higher preexisting serum levels to NJ1976, it was lower than Cal09-specific levels. In addition, one of the subjects that generated a broadly reactive response with 3/12 mAbs binding HA stalk epitopes also had high NJ1976 serum levels. It seems unlikely, therefore, that previous exposure to NJ1976 solely explains the differential response between donors. Studies point to the idea that the breadth of viral strain experience, not just exposure levels to certain strains can influence the response to Cal09 vaccination (33). Though there is correlation between age and serological levels of H1N1 strains that emerged across the 20th century, there are several discrepancies (34). In addition, it's been shown in ferrets that sequential priming with variant H1N1 strains protects better against Cal09 infection than priming with one antigenically distant strain (35). Li et al demonstrated that immunization of ferrets with A/Texas/36/1991, followed by Cal09, induced a serological response targeted at a K133 residue in the RBD whereas pre-vaccination with pre-1957 or post-1999 induced a stronger G158 response in the Sa region (36). Correlated with this, individuals born after 1996 who would have been more exposed to the Bris-2007 strain had a more Sa-dominated response to Cal09 infection (36). Overall, this suggests that the memory repertoire is informed by the diversity and sequence with which an individual has encountered influenza strains over their lifetime, and is not solely the sum of strains they have been exposed to.
The immune subdominance of the HA stalk epitope was further demonstrated when we compared the vaccine-induced plasmablast response to vaccination against the Cal09 strain in individuals vaccinated for the first time and upon revaccination the following year. In contrast to the broadly neutralizing response in a subset of subjects vaccinated in 2009, where a third of the neutralizing mAbs bound HA stalk epitopes, we only found mAbs with this specificity in one of the revaccinated subjects. We were only able to measure the vaccine-induced plasmablast response to Cal09 in subsequent years from one of the subjects with an HA-stalk biased response in 2009. This subject (051) had an HA stalk-biased response upon first vaccination in 2009, but we isolated no HA stalk-binding mAbs from this subject upon revaccination. Thus, subsequent exposure to the Cal09 strain generated a strain-specific and HA head-specific response, in which about half of the neutralizing mAbs generated upon revaccination were highly specific to this strain. This is consistent with a recent report demonstrating that serological levels of antibodies Cal09 HA head-specific antibodies progressively increased between 2010 and 2014 in vaccinated individuals, while Cal09 HA stalk- specific antibody levels remained the same (37).
We provide evidence for two mechanisms explaining this subdominance of the HA stalk-specific antibody response. First, we found that the broadly protective epitopes found on the HA stalk bound with reduced affinity on whole virions when compared to recombinant HA alone, probably due in part to reduced association rates. It is well appreciated that antibodies to the HA stalk region derive from a restricted VH repertoire, likely due to the requirement for the highly hydrophobic CDR2 of VH1-69 (38). Further restriction is evident in the CDR3 region of HA stalk-reactive antibodies where tyrosine is reported to preferentially occur (20, 39). Thus, B cells with receptors that can contact the less accessible HA stalk epitopes are predicted to occur much more rarely in the preimmune repertoire. Second, we found that the HA stalk-reactive B cells were polyreactive. A heteroligation model was previously proposed for HIV anti-gp140 antibodies in which polyreactivity would allow enhanced anti-HIV antibody interactions with sparse HIV envelope proteins (28). In a similar model, we propose that polyreactivity of the HA stalk antibodies will prolong the non-specific interaction of antibodies with HA, thus increasing the chances for dynamic interactions of one or the other antibody variable regions with the less accessible HA stalk epitopes. However, this property of polyreactivity will likely further reduce the number of B cells in an already restricted repertoire that can bind the HA stalk. It has long been appreciated that though present in the B cell repertoire, polyreactive B cells have a selective disadvantage during B cell development (40-42). Further, persistent interaction with non-specific antigens is predicted to cause these cells to become anergic and more difficult to activate (43, 44). Despite this evident polyreactivity, we found no evidence that they represented high-affinity pathological antibodies. Notably, none of mAbs had anti-nuclear staining on HEp-2 slides, nor did we observe a spike in serum anti-DNA reactivity after vaccination. Finally, the polyreactivity observed could be masked by performing the assays under more stringent conditions such as blocking with high levels of human serum protein, further suggesting that these antibodies would not cause autoreactivity in vivo. Polyreactive immunoglobulins are common in the healthy human B cell repertoire (25, 26) and likely serve important functions.
Though the correlation between preexisting serum levels to the pandemic H1N1 strain and the breadth of the B cell response to vaccination to this novel strain is consistent, this study was done with relatively few individuals. This low number of subjects was both because producing monoclonal antibodies to study the vaccine response is a very labor intensive process and because there was a narrow window of time in which we could gather blood samples from individuals first exposed to this novel H1N1 strain in 2009. In addition, though we are able to show reasons why the HA stalk-specific response may be subdominant, it is impossible to show conclusively in humans the mechanism for HA head immunodominance. Further work in animal models will be needed to be more definitive.
There are two major implications of our findings as the vaccine community designs strategies to induce a broadly protective response against influenza. One is that the immune system is highly efficient at finding and activating those rare high-affinity and specific B cells normally hidden in the memory B cell repertoire, if they are indeed present. This suggests that large numbers of these broadly reactive cells may not be necessary to ensure good protection against future pandemics. Indeed, Otterstrom and colleagues found that saturating amounts of antibody protein to HA-stalk epitopes are not required for viral neutralization (45). Second, HA head-binding B cells recognizing strain-specific epitopes more prone to variability will be preferentially activated if they exist. Thus, influenza variants designed to induce a broadly protective response will not work efficiently if they contain immunodominant epitopes other than the targeted conserved epitope to which the human population has preexisting memory. It also implies that revaccination with this same immunogen may not boost the response to the desired epitopes. One strategy which has proved effective in mouse and ferrets is sequential immunization with chimeric HA molecules containing the same HA stalk region, but widely disparate HA heads, thus inducing a targeted response to the HA stalk (46-48). While the results in these animal models with experimentally induced preexisting memory repertoires have been promising, the complex and individualistic nature of the human memory B cell repertoire may make generating a qualitatively uniform and protective response in humans a challenge.
In conclusion, influenza has evolved so that broadly protective B cells are rare and likely at a selective disadvantage. As the influenza vaccine community moves forward to develop a broadly protective vaccine, possibly targeting the HA stalk, this study illustrates that a number of obstacles will need to be overcome: expanding a rare and specialized B cell specificity, overcoming natural protection against polyreactive antibodies, and finally, maintaining highly specialized immune targeting upon subsequent exposures.
Materials and Methods
Study Design
We initiated this observational study in order to compare the vaccine-induced plasmablast response upon first vaccination with the novel pandemic H1N1 strain in 2009-10 and upon revaccination with this same strain in 2010-11 or 2011-12. To monitor the vaccine response we obtained PBMCs and sera on the day of vaccination and at the time points with the peak of the plasmablast response (day 5, 6, or 7), and serological response (between day 14-21). The vaccine response was monitored by making mAbs from the responding plasmablasts and testing their specificity and functionality by ELISA, SPR, HAIs, and MN assays. As these two groups were vaccinated in different years with the vaccine available in each year this study was unblinded and not randomized. We enrolled anyone 18 years or older who was healthy and had not yet received the yearly influenza vaccine. We obtained plasmablasts from a total of 21 subjects vaccinated with the monovalent 2009 pandemic vaccine, and 23 subjects vaccinated with the 2010-11 or 2011-12 trivalent vaccine but restricted the analysis to only individuals from which we obtained at least 4 H1N1-specific plasmablasts (10 and 11 individuals, respectively). This was the maximum number of individuals we could vaccinate and collect samples from in a given influenza vaccine season before the yearly vaccine expired and from which we could go through the labor-intensive process of producing mAbs.
Influenza vaccine subjects and PBMC Isolation
All studies had institutional review board approval and informed consent was obtained from all subjects. Healthy individuals were vaccinated with the 2009 monovalent pandemic H1N1 vaccine, or seasonal inactivated trivalent influenza vaccine as indicated in table S1 and S2. The antibodies (and coding variable genes) from subject C1 (Fig. 1) were historical and from our previously published studies (13, 14, 49), but were not analyzed for clonal overlap in consecutive years as presented herein. Five of the subjects who received the 2009 monovalent pandemic H1N1 vaccine (SFV) were recruited and vaccinated at the Emory Vaccine Center as described previously (3). An overlapping and expanded set of mAbs from that publication were independently characterized for the experiments herein. All other subjects were recruited and vaccinated at the University of Chicago. For this study we only included the subjects from whom we had obtained at a minimum of 4 influenza+ mAbs so that we could evaluate subject-to-subject variation in the vaccine response. Blood was drawn on the day of vaccination, 5-7 days later (to isolate plasmablasts), and 14-21 days later (to isolate vaccine-induced memory B cells). Peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation of whole blood through a Ficoll gradient and resuspension in PBS + 0.2% BSA for staining and sorting or viably frozen at −80°C in FCS+10%DMSO for later use. Serum separated by centrifugation of whole blood was also frozen at −80°C.
Cell sorting and production of mAbs
PBMCs were stained with CD19, CD27, CD38, CD3 and CD20 followed by FACS-sorting of CD19+ CD3− CD27hi CD38hi plasmablasts or CD19+ CD3− CD27int CD38int memory B cells on an AriaII or FACSVantage. Typically 80-90% of plasmablasts were CD20− while memory cells were CD20+. HA-binding memory B cells were sorted using fluorescently-labeled HA with a mutation in sialic-acid binding site to prevent binding to sialic acids on the B cell surface (50). Sorted cells were single-cell sorted into 96-well plates containing 10mM Tris pH8.0 and RNAse inhibitor (Promega). Immunoglobulin genes were then amplified and used to produce monoclonal antibodies from each sorted B cell as described previously (Smith et al 2009, Wrammert et al 2008). Briefly, single-cell RT-PCR and amplification of rearranged immunoglobulin VH and Vκ or Vλ genes was performed using a cocktail of primers followed by sequencing and cloning into expression vectors. Cloned heavy/light chain pairs corresponding to each sorted cell were then cotransfected into HEK 293T cells and secreted monoclonal antibodies were purified from the supernatant using Protein G sepharose. All immunoglobulin gene sequences were characterized using JOINSOLVER™ (http://joinsolver.niaid.nih.gov/) (51) or IMGT (http://www.igmt.org)(52). Phylogenetic trees of clonally related immunoglobulin VH sequences and influenza strain HAs was performed with Clustal Omega (http://www.clustal.org/omega) (53) and graphed with FigTree (http://tree.bio.ed.ac.uk/software/figtree).
Virus and reagents
CD19 (HIB19), CD38 (HIT2) and CD20 (2H7) came from Biolegend, CD27 (CLB-27/1) and CD3 (7D6) were purchased from Invitrogen. Influenza stocks were freshly grown in eggs, harvested and purified using PEG virus precipitation (BioVision Research Products, CA) according to the manufacturer's instructions and stored at −80°C. Viral hemagglutination activity units (HAU) was used to quantify virus as measured by incubating serial dilutions of virus with a 1:1 ratio of 0.5% turkey red blood cells (Lampire Biological Laboratories). 1 HAU represents the minimum viral amount capable of causing red blood cell agglutination. Recombinant HA was produced as described previously (54) (H1N1 strains) or obtained from BEI Resources (H5N1 A/Vietnam/1203/2004).
ELISAS
High-protein binding microtiter plates (Costar) were incubated overnight at 4°C with 8 HAU whole virus/well or 2ug/ml rHA, followed by washing with PBS/0.05% Tween and blocking 1hr at 37°C with PBS+20%FCS. Samples were serially diluted 3-fold 7 times, starting at 10ug/ml (monoclonal antibodies) or 1:100 dilution (sera), and incubated on the plate 1hr at 37°C followed by HRP conjugated goat anti-human IgG (Jackson Immunolabs) and development with Super AquaBlue ELISA Substrate (eBioscience). For human albumin blocking of poly-reactivity, 5% highly purified human serum albumin (>96% pure, Sigma-Aldrich) was added to the ELISA buffer. Absorbencies were measured at OD405. To standardize the results, a control antibody was included on each plate and the plate allowed to develop until this control reached an OD of 3.0. Estimated avidities (kD) (monoclonal Abs) or EC50 (sera) were determined by nonlinear regression analysis of the serially diluted samples, as calculated by GraphPad Prism. For detection of polyreactivity, plates were coated with 10ug/ml calf-thymus dsDNA (Life Technologies), 10ug/ml LPS (Sigma) or 5ug/ml recombinant human insulin (Fitzgerald) in carbonate buffer. Plates were blocked with PBS/0.05% Tween. mAbs were diluted 4-fold 4 times, starting with 1ug/ml and incubated on the plate 1hr at 37°C followed by incubation with goat anti-human IgG at 1:2000 diluted in PBS/0.05% Tween. Plates were developed with AquaBlue ELISA substrate as above. Anti-dsDNA antibody 3H9 was used as a standard, all plates were developed until this antibody reached an OD of 3.0. The area under the curve (AUC) was calculated using GraphPad Prism.
Competition ELISAs were performed as described previously (12). Briefly, plates were coated with 1ug/ml rHA, incubated with the 20ug/ml competitor HA stalk-specific mAb sc70-1F02, HA head-specific mAb EM4C04 or the mAb being tested, followed by incubation with the half-maximal binding concentration of biotinylated mAbs being tested. Plates were developed with streptavidin-HRP until samples in the absence of competitor antibody reached an OD of 1.
HAI
Monoclonal antibodies were diluted 2-fold starting at 60ug/ml (final 30ug/ml) in U-bottom 96-well plates. An equal volume of PBS containing 16 HAU of live virus/well (final 8 HAU) was added to the diluted antibodies for a total volume of 50ul/well. After 30min incubation at RT, 50ul of 0.5% Turkey red blood cells were added and an hour later the minimum antibody concentration which inhibited hemagglutination was recorded.
Neutralization Assay
4-8×105 MDCK cells were plated in 6-well plates. Virus titered to produce 50-100 plaques/well was incubated with 1:3 serial dilutions of mAbs, starting with 30ug/ml final dilution for 1hr at room temperature. The virus/mAbs mix was then added to PBS-washed cells for 45 minutes at room temperature and removed. An MEM/Agar mixture was then overlaid in each well and incubated for 48 hrs at 37°C. Viral plaques were visualized with crystal violet and counted. The PRNT50 was determined as the lowest mAb concentration at which viral plaques was 50% or less of those in the no antibody control well.
Surface plasmon resonance
Kinetic interactions of the monoclonal antibodies (IgGs) with rHA protein (A/California/04/09 rHA from BEI NR-13691) were measured on the ProteOn XPR36 (BioRad). Experiments were performed in HBS-EP running buffer. 20ug/ml of goat anti-human IgG Fc fragment specific antibody (Jackson ImmunoResearch) in acetate buffer pH5.5 was immobilized on a GLC chip at 30ul/min by amine coupling resulting in around 3000RU. 0.625-1.25ug/ml of each antibody of interest was injected in running buffer at a flow rate of 40ul/min for 60s for capture of an average of 40RUs by the immobilized anti-IgG. For kinetic measurements, rHA at five different concentrations ranging from 0.78125nM to 50nM in HBS-EP buffer was injected at flow rate of 25ul/ml with 5 min association and 4 hour dissociation times. The surface was regenerated by two injections of 0.85% phosphoric acid at 100ul/min for 18s each. This removed antibody that was captured by the anti-IgG; hence leaving the immobilized anti-IgG available for capturing another round of antibodies. For data analysis, double subtraction with interspot (blank reference) and running buffer was done. Affinities, or KD values, were calculated by aligning the data to 1:1 binding two-state model using ProteOn Manager v.3.1 (BioRad).
Statistical Analysis
All statistical analysis was performed using GraphPad Prism. Statistical significance was usually determined using two-tailed non-parametric unpaired Mann-Whitney test, paired Wilcoxon test, Chi-square or Fisher's exact test as detailed in figure legends. Non-parametric tests were used in most cases as few groups had a normal distribution as determined by GraphPad Prism using the D'Agostino and Pearson omnibus normality test. If the data did pass the normality test than paired or unpaired two-tailed t-tests were performed as detailed in the figure legends. P values equal or lower to 0.05 were considered significant. The nonparametric Spearman correlation coefficient (rs) was calculated to determine the level of correlation.
Supplementary Material
Acknowledgments
We thank Jori Reigle, Marissa Kumabe, and Elizabeth Yan for help recruiting subjects and collecting blood samples and Nai-Ying Zheng for technical help and guidance. Funding: This work was supported in parts by NIH grants 1U19AI08724 (PCW), 5U54AI057158 (PCW, RA), 5U19AI057266 (PCW, RA, JW), 1U19AI090023 (PCW, RA, JW), 1P01AI097092 (PCW, RA, PP), F32 AI93087 (SFA) and by funds provided by the Gwen Knapp Center for Lupus and Immunology Research. This work was also partly supported by CRIP (Center for Research on Influenza Pathogenesis), an NIAID funded Center of Excellence for Influenza Research and Surveillance (CEIRS, contract # HHSN266200700010C) (PCW, PP and FK). Florian Krammer was supported by an Erwin Schrödinger fellowship (J3232) from the Austrian Science Fund (FWF). Kaval Kaur was supported by a National Science Scholarship (PhD) from the Agency of Science, Technology and Research (A*STAR), Singapore.
Footnotes
Author contributions: SFA and PCW wrote the manuscript and provided intellectual oversight, SFA planned and performed experiments, YH, NTP, CHD, KK, WMT, SL, MH, XQ, JL, MSF, IYH, LIP contributed to the results, FK and PP contributed reagents, JW, RA provided patient samples.
One Sentence Summary: The quality of the B cell response to influenza vaccination is dependent on the preexisting memory B cell repertoire.
Competing interests: The authors have no competing interests.
Data and materials available: mAb sequences were deposited in Genbank with accession #___.
References
- 1.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, Garcia-Sastre A, Palese P. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, Keleta L, Silva V, Diederich S, Jones RB, Gubbay J, Pasick J, Petric M, Jean F, Allen VG, Brown EG, Rini JM, Schrader JW. Pandemic H1N1 Influenza Infection and Vaccination in Humans Induces Cross-Protective Antibodies that Target the Hemagglutinin Stem. Frontiers in immunology. 2012;3:87. doi: 10.3389/fimmu.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangster MY, Baer J, Santiago FW, Fitzgerald T, Ilyushina NA, Sundararajan A, Henn AD, Krammer F, Yang H, Luke CJ, Zand MS, Wright PF, Treanor JJ, Topham DJ, Subbarao K. B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination. Clin Vaccine Immunol. 2013;20:867–876. doi: 10.1128/CVI.00735-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corti D, Suguitan AL, Jr., Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Current opinion in virology. 2013;3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchenbaum GA, Ross TM. Eliciting broadly protective antibody responses against influenza. Current opinion in immunology. 2014;28:71–76. doi: 10.1016/j.coi.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc. 2009;4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin O, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 17.Henry Dunand CJ, Leon PE, Kaur K, Tan GS, Zheng NY, Andrews S, Huang M, Qu X, Huang Y, Salgado-Ferrer M, Ho IY, Taylor W, Hai R, Wrammert J, Ahmed R, Garcia-Sastre A, Palese P, Krammer F, Wilson PC. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J Clin Invest. 2015;125:1255–1268. doi: 10.1172/JCI74374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1 doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graves PN, Schulman JL, Young JF, Palese P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology. 1983;126:106–116. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- 20.Avnir Y, Tallarico AS, Zhu Q, Bennett AS, Connelly G, Sheehan J, Sui J, Fahmy A, Huang CY, Cadwell G, Bankston LA, McGuire AT, Stamatatos L, Wagner G, Liddington RC, Marasco WA. Molecular signatures of hemagglutinin stem-directed heterosubtypic human neutralizing antibodies against influenza A viruses. PLoS Pathog. 2014;10:e1004103. doi: 10.1371/journal.ppat.1004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris AK, Meyerson JR, Matsuoka Y, Kuybeda O, Moran A, Bliss D, Das SR, Yewdell JW, Sapiro G, Subbarao K, Subramaniam S. Structure and accessibility of HA trimers on intact 2009 H1N1 pandemic influenza virus to stem region-specific neutralizing antibodies. Proc Natl Acad Sci U S A. 2013;110:4592–4597. doi: 10.1073/pnas.1214913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. Influenza viruses expressing chimeric hemagglutinins: Globular head and stalk domains derived from different subtypes. J Virol. 2012 doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur K, Sullivan M, Wilson PC. Targeting B cell responses in universal influenza vaccine design. Trends Immunol. 2011;32:524–531. doi: 10.1016/j.it.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingwood D, McTamney PM, Yassine HM, Whittle JR, Guo X, Boyington JC, Wei CJ, Nabel GJ. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature. 2012;489:566–570. doi: 10.1038/nature11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in Human IgG(+) Memory B Cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, Jared M, Smith K, Capra JD, Wilson PC. Mature B cells class switched to IgD are autoreactive in healthy individuals. J Clin Invest. 2007;117:1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radic MZ, Weigert M. Intricacies of anti-DNA autoantibodies. J Immunol. 2004;172:3367. doi: 10.4049/jimmunol.172.6.3367. author reply 3367-3368. [DOI] [PubMed] [Google Scholar]
- 28.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, Walker BD, Ho DD, Wilson PC, Seaman MS, Eisen HN, Chakraborty AK, Hope TJ, Ravetch JV, Wardemann H, Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 31.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 32.Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J Infect Dis. 2013;207:98–105. doi: 10.1093/infdis/jis652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross TM, Lin CJ, Nowalk MP, Huang HH, Spencer SM, Shay DK, Sambhara S, Sundaram ME, Friedrich T, Sauereisen S, Bloom CE, Zimmerman RK. Influence of pre-existing hemagglutination inhibition titers against historical influenza strains on antibody response to inactivated trivalent influenza vaccine in adults 50-80 years of age. Human vaccines & immunotherapeutics. 2014;10:1195–1203. doi: 10.4161/hv.28313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter DM, Lu HR, Bloom CE, Crevar CJ, Cherry JL, Lipman DJ, Ross TM. Complex patterns of human antisera reactivity to novel 2009 H1N1 and historical H1N1 influenza strains. PLoS One. 2012;7:e39435. doi: 10.1371/journal.pone.0039435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter DM, Bloom CE, Nascimento EJ, Marques ET, Craigo JK, Cherry JL, Lipman DJ, Ross TM. Sequential seasonal H1N1 influenza virus infections protect ferrets against novel 2009 H1N1 influenza virus. J Virol. 2013;87:1400–1410. doi: 10.1128/JVI.02257-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Myers JL, Bostick DL, Sullivan CB, Madara J, Linderman SL, Liu Q, Carter DM, Wrammert J, Esposito S, Principi N, Plotkin JB, Ross TM, Ahmed R, Wilson PC, Hensley SE. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. The Journal of experimental medicine. 2013;210:1493–1500. doi: 10.1084/jem.20130212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, Chang CY, Davis CW, McCausland M, Elbein R, Edupuganti S, Spearman P, Andrews SF, Wilson PC, Garcia-Sastre A, Mulligan MJ, Mehta AK, Palese P, Ahmed R. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee PS, Wilson IA. Structural characterization of viral epitopes recognized by broadly cross-reactive antibodies. Curr Top Microbiol Immunol. 2015;386:323–341. doi: 10.1007/82_2014_413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, Sallusto F, Zhu Q, Vicenzi E, Corti D, Lanzavecchia A. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature. 2014 doi: 10.1038/nature13764. [DOI] [PubMed] [Google Scholar]
- 40.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 41.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, Meffre E. Complement receptor 2/CD21-negative human naive B cells mostly contain autoreactive unresponsive clones. Blood. 2010 doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, Garman L, Helms C, Nakken B, Smith K, Farris AD, Wilson PC. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 45.Otterstrom JJ, Brandenburg B, Koldijk MH, Juraszek J, Tang C, Mashaghi S, Kwaks T, Goudsmit J, Vogels R, Friesen RH, van Oijen AM. Relating influenza virus membrane fusion kinetics to stoichiometry of neutralizing antibodies at the single-particle level. Proc Natl Acad Sci U S A. 2014;111:E5143–5148. doi: 10.1073/pnas.1411755111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol. 2013;87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado VH, Ryder AB, Miller MS, Rose JK, Palese P, Garcia-Sastre A, Albrecht RA. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol. 2014;88:3432–3442. doi: 10.1128/JVI.03004-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol. 2014;88:2340–2343. doi: 10.1128/JVI.03183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaur K, Zheng NY, Smith K, Huang M, Li L, Pauli NT, Henry Dunand CJ, Lee JH, Morrissey M, Wu Y, Joachims ML, Munroe ME, Lau D, Qu X, Krammer F, Wrammert J, Palese P, Ahmed R, James JA, Wilson PC. High Affinity Antibodies against Influenza Characterize the Plasmablast Response in SLE Patients After Vaccination. PLoS One. 2015;10:e0125618. doi: 10.1371/journal.pone.0125618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whittle JR, Wheatley AK, Wu L, Lingwood D, Kanekiyo M, Ma SS, Narpala SR, Yassine HM, Frank GM, Yewdell JW, Ledgerwood JE, Wei CJ, McDermott AB, Graham BS, Koup RA, Nabel GJ. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J Virol. 2014;88:4047–4057. doi: 10.1128/JVI.03422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Souto-Carneiro MM, Longo NS, Russ DE, Sun HW, Lipsky PE. Characterization of the human Ig heavy chain antigen binding complementarity determining region 3 using a newly developed software algorithm, JOINSOLVER. J Immunol. 2004;172:6790–6802. doi: 10.4049/jimmunol.172.11.6790. [DOI] [PubMed] [Google Scholar]
- 52.Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37:D1006–1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular systems biology. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margine I, Palese P, Krammer F. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. Journal of visualized experiments : JoVE. 2013:e51112. doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.