Abstract
Background
Depot-medroxyprogesterone acetate (DMPA) is associated with HIV acquisition. We studied changes in vaginal microbiota and inflammatory milieu after DMPA initiation.
Methods
In a cohort of HIV-negative Kenyan women, we collected monthly vaginal swabs over 1 year pre- and post-DMPA. Using quantitative PCR, we compared quantities of Lactobacillus crispatus, L. jensenii, L. iners, Gardnerella vaginalis, and total bacterial load (16S rRNA gene levels). Six vaginal immune mediators were measured with ELISA. Trends in detection and quantity of bacteria were estimated by logistic and linear mixed-effects regression.
Results
From 2010-2012, 15 HIV-seronegative women initiated DMPA, contributing 85 visits (median 6 visits/woman (range 3-8)). The median time of DMPA-exposed follow-up was 8.4 months (range 1.5-11.6). Seven women (46%) had bacterial vaginosis (BV) within 70 days before DMPA start. Lactobacillus iners was detected in 13 women (87%) prior to DMPA start, but other lactobacilli were rarely detected. Gardnerella vaginalis declined by 0.21 log10 copies/swab per month after DMPA exposure (p=0.01). Total bacterial load declined by 0.08 log10 copies/swab per month of DMPA (p=0.02). Sustained declines in interleukin (IL)-6 (p=0.03), IL-8 (p=0.04) and IL-1 receptor antagonist (p<0.001) were also noted. Nine women (60%) had L. crispatus detected post-DMPA; which was significantly correlated with reduced IL-6 (p=<0.01) and IL-8 (p=0.02).
Conclusion
Initiation of DMPA led to sustained shifts in vaginal bacterial concentrations and levels of inflammatory mediators. Further studies are warranted to outline components of the vaginal microbiota influenced by DMPA use, and impact on HIV susceptibility.
Keywords: Bacterial vaginosis, BV, vaginal microbiota, depot-medroxyprogesterone actetate, DMPA, Depo-Provera, Lactobacillus, Gardnerella vaginalis, HIV-1 susceptibility, IL-6, IL-8, IL-1 receptor antagonist, IL-1ra
Introduction
Hormonal contraception, especially progesterone-based contraception such as depot-medroxyprogesterone acetate (DMPA), has been associated with human immunodeficiency virus-1 (HIV) acquisition in multiple observational cohort studies of high-risk women in sub-Saharan Africa.1-4 DMPA is the cornerstone of many family planning programs, especially in Africa, as it is requires no daily adherence, preserves women's contraceptive privacy, and can be distributed without a sophisticated health care infrastructure. Effects of DMPA on HIV susceptibility and transmission may result from behavioral, biological, or a combination of effects. Given the urgency of the HIV epidemic among women of reproductive age in sub-Saharan Africa, where 7,000 young women are infected each week, the association of DMPA and HIV acquisition requires urgent investigation and is a priority issue in women's reproductive health today.5
A potential biological explanation of the increased HIV risk observed among DMPA users is that DMPA may affect the composition of the vaginal microbiota in ways that increase HIV susceptibility. In fact, DMPA has long been observed to reduce the incidence of bacterial vaginosis (BV),6-9 a syndrome of overgrowth of anaerobic vaginal bacteria instead of Lactobacillus species-dominant microbiota typically associated with vaginal health. Reductions in BV from DMPA use would be expected to reduce HIV risk, since BV may be a modifiable risk factor for HIV acquisition,10,11 and affects at least 20-40% of women in sub-Saharan Africa,9,12 where women are disproportionately affected by HIV.13 Yet while DMPA use may lead to fewer women with BV, there are plausible mechanisms by which DMPA's effect on vaginal microbiota could lead to increased HIV acquisition risk. For example, DMPA use might change the vaginal microbiota so that BV is reduced, but does not result in a Lactobacillus species-rich vaginal bacterial community that is associated with the lowest HIV risk.14 DMPA, which binds avidly to glucocorticoid receptors, may also change the inflammatory environment surrounding vaginal bacteria.
Investigating the influence of DMPA on vaginal microbial communities could elucidate mechanisms by which DMPA use could augment mucosal HIV susceptibility.15 The objective of this study was to explore changes in key vaginal bacteria (Lactobacillus crispatus, L. jensenii, L. iners and Gardnerella vaginalis) after DMPA initiation. Due to DMPA's known immunomodulatory effects, we also studied innate immune mediators that could be impacted by DMPA.
Methods
Study population
The Mombasa Cohort prospectively follows Kenyan women at risk for HIV acquisition from transactional sex. To characterize changes in the quantities of key vaginal bacterial species before and after DMPA use, we identified HIV-seronegative women initiating DMPA between May 2010 – June 2012. All women with DMPA initiation were included if they met the following criteria: a 70-day window unexposed to DMPA; at least one specimen collected 3 months before starting DMPA; and at least 2 specimens after DMPA initiation. Up to 8 specimens were tested per woman, and specimens were tested up to 12 months after DMPA initiation. This study was approved by institutional review boards of University of Washington, Fred Hutchinson Cancer Research Center and University of Nairobi/Kenyatta National Hospital. Written informed consent was obtained from all participants.
Study procedures
Women returned for monthly visits, and completed a questionnaire at every visit assessing HIV risk behaviors and contraceptive use; visits were rescheduled if women were menstruating. Clinical examinations, STI and HIV testing were performed monthly; vaginal fluid was collected for wet mount and Gram staining. Vaginal swabs for analysis of microbiota were collected by swabbing the fornices and vaginal walls with dry Spin-Eze™ polyester push-off swabs (Fitzco, Spring Park, MN) which were frozen immediately, and cryopreserved at −70 C. Lactobacillus culture was performed. Cytokine swabs were collected by rolling a polyester swab across the vaginal wall and freezing in 1 mL of media (70% RPMI, 20% fetal calf serum and 10% dimethyl sulfoxide). Antibiotic dispensing was recorded at each visit; exposure was defined as any antibiotic dispensed in the 30 days prior to sample collection.
Laboratory methods
Chlamydia trachomatis and Neisseria gonorrhoeae were tested quarterly using molecular methods (APTIMA Combo-2, Hologic/Gen-Probe, San Diego, CA); Lactobacillus cultures were performed monthly. Vaginal saline wet mount was examined microscopically for presence of motile trichomonads and fungal elements. A drop of 10% potassium hydroxide was added to the slide and evaluated for presence of yeast buds or hyphae. HIV testing was performed by enzyme-linked immunosorbent assay (ELISA) as previously described.16 Gram stains of vaginal discharge were evaluated according to Nugent's methods, with Nugent score ≥ 7 considered a diagnosis of BV.17
For quantification of vaginal microbiota, each sample was prepared by adding 500 μL filtered saline to the dry swab in tube, which was vortex-mixed for 1 minute and spun at 14,000 xg to pellet cells. Supernatant was removed, then DNA was extracted from pelleted material using the BiOstic Bacteremia DNA Isolation Kit (Mobio, Carlsbad, CA),18 eluted in 120 μl buffer and 3 μl of DNA used for each qPCR assay. To assess contamination, sham digests from swabs without human contact were processed in parallel. DNA samples were tested for PCR inhibition as described previously,19 defined as delay in the threshold cycle by ≥2 cycles compared to no-template controls.
Using previously described techniques,20,21 qPCR assays were used to detect and quantify the vaginal bacterial species L. crispatus, L. jensenii, L. iners, and G. vaginalis, relying upon a probe-based assay with 16S rRNA gene-specific primers, and a taxon-directed hydrolysis probe (Taqman format). Total bacterial load was assessed by the qPCR detection of the 16S rRNA gene in bacteria using broad range bacterial primers.22 Core reagents were from Life Technologies (Carlsbad, CA) and master mixes contained buffer A (1 mM), deoxynucleotide triphosphates (1 mM), magnesium (3 mM), AmpErase uracil-N-glycosylase (0.05 U) and AmpliTaq Gold polymerase (0.6-0.9U) per reaction. Primers were added at 0.8 μM per reaction and final probe concentration was 150-300 nM. Assays underwent 45 cycles of amplification on the StepOnePlus thermal cycler (Life Technologies). Specificity and sensitivity were defined as described previously.20 Plasmid standards were run in duplicate from 107 to 2.5 copies, and values are reported as 16S rRNA gene copies/swab.
Commercial ELISA kits (IL-8 [Life Technologies], IL-6, Interferon-γ- inducible protein 10 (IP-10), IL-1 receptor antagonist (IL-1ra), regulated on activation, normal T-cell expressed and secreted (RANTES), secretory leukocyte protease inhibitor (SLPI) [all R&D Systems, Minneapolis, MN]) were used to determine quantities of inflammatory mediators. Vaginal swabs were thawed and immediately loaded on plates for analysis. Fifty μL were analyzed for IL-8, 100μL for all other kits. Kit standards were diluted in serial 3:1 dilutions covering the suggested linearity range, using calibrator diluents provided, and analyzed in duplicate on separate plates to create standard curves. Samples above the range of the standard curve were diluted using reagent-specific diluents and re-run. IL-6, IL-8, IP-10 and RANTES were tested without dilution, IL-1ra was tested at 1000x dilution, SLPI was tested at minimum 20x dilution. Optical density at 450 nm was measured using a SpectraMax M2 microplate reader and SoftMax Pro software (Molecular Devices, Sunnyvale, CA). All results were analyzed using a log-log plot, except IL-8, which was analyzed using four-parameter logistic regression.
Statistical analysis
We used data from before and after DMPA initiation, so each woman served as her own control. Bacterial and inflammatory mediator levels were treated as detectable (yes/no) binary variables if greater than 30% of the data were below limits of detection. Trends in detection and log10 quantity of bacteria were estimated by logistic and linear mixed-effects regression models respectively, with random intercepts and with random slopes (for linear models only) addressing right-censoring under the assumption of continued trends per subject. Due to both right and left censoring of inflammatory mediator levels, tobit models were used, with intercept-only random effects. Contraceptive method is self-reported at enrollment and each follow-up visit. DMPA start date was computed as the midpoint between the date of the last visit of DMPA-nonuse and the first visit of reported DMPA use. Analysis time is centered on this start date estimate. Bacterial levels, when not detected, were assigned half the detection limit. Tobit models were also run to assess sensitivity to this “imputation” of values below detection. Data from clinical correlates including vaginal discharge, pH and Nugent score were included from all visits pre and post DMPA, including visits where vaginal swabs were not collected.
In addition to an unadjusted model, we adjusted estimates of changes in bacteria levels and inflammatory mediators, or changes in odds of detection, on/off DMPA by including pre-specified potential confounding factors that may change in women following DMPA initiation. We investigated antibiotic use, vaginal washing, new STI, age, number of partners and sexual activity in the previous week, condom use (100% v. intermittent v. never) and parity, in that order. Adding each variable to the model cumulatively, variables that changed estimates by greater than 10% were retained in adjusted models. Antibiotic exposure in past 30 days was assessed for effect modification in all models; metronidazole exposure was assessed separately to account for its differing effects on vaginal Lactobacillus colonization.23,24
Results
Baseline characteristics
Fifteen women met inclusion criteria, contributing 85 vaginal swab samples. The median length of DMPA-unexposed follow up was 42 days (minimum [min] 7, maximum [max] 117). The median length of DMPA-exposed follow-up was 8.4 months (min-max 1.5-11.6); the median number of samples analyzed per woman was 6 (min-max 3-8). Baseline characteristics of these women are summarized in Table 1. Seven (47%) of the women had BV (Nugent score ≥7) within 70 days of their last pre-DMPA visit, and of those, 5 (33%) had BV at the visit immediately pre-DMPA. Fourteen of the women reported last menstrual periods of a median of 4.4 weeks (interquartile range [IQR] 1.7, 6.4) prior to their last pre-DMPA visit. One woman reported not menstruating for 30 weeks at baseline. No woman tested positive for Trichomonas vaginalis, Chlamydia trachomatis, or Neisseria gonorrhoeae at the pre-DMPA visit. After initiation, STIs remained rare; 2 women were diagnosed with C. trachomatis. At baseline, two women had been exposed to antibiotics in the 30 days prior to study entry. During follow-up, 4 women were exposed to antibiotics, affecting 8 specimens, and no woman had metronidazole exposure within 30 days before specimen collection.
Table 1.
Baseline characteristics of 15 women starting DMPA.
| Characteristic | Median (IQR) or N(%) |
|---|---|
| Age at pre-DMPA visit | 33 (25, 38) |
| Years of education at enrollment | 12 (6, 12) |
| Ever married at enrollment | 7 (47) |
| Workplace at enrollment: bar/restaurant/guesthouse | 9 (60) |
| Workplace at enrollment: nightclub | 3 (20) |
| Workplace at enrollment: home-based* | 1 (7) |
| Number sex partners in last week at pre-DMPA visit | 2 (1, 3) |
| Number of new sex partners in last week at pre-DMPA visit | 0 (0, 4) |
| Number of sex acts without condom in last week at pre-DMPA visit | 0 (0, 2) |
| Weeks since last menstrual period at last pre-DMPA visit¥ | 4.4 (1.7, 6.4) |
| Ever pregnant at enrollment | 14 (93) |
| Number of pregnancies among those ever pregnant at enrollment | 2.5 (1,3) |
| Reported any vaginal washing activity in past week at last pre-DMPA visit | 9 (60) |
| Any antibiotics prescribed at or before last pre-DMPA visit€ | 2 (13) |
| Self-reported abnormal discharge at any pre-DMPA visit£ | 4 (27) |
| Clinician reported abnormal discharge at last pre-DMPA visit | 6 (40) |
| Contraceptive method: condoms only at last pre-DMPA visit | 15 (100) |
| Any BV (Nugent score ≥ 7) in last 70 days at last pre-DMPA visit | 7 (47) |
| BV (Nugent score ≥ 7) at last pre-DMPA visit | 5 (33) |
| Vaginal pH at last pre-DMPA visit | 4.7 (4.4, 5.3) |
IQR= interquartile range, N=number of respondents, DMPA=depot-medroxyprogesterone acetate, BV=bacterial vaginosis
The remaining two women responded “Other” when asked about workplace.
Missing for one woman.
Missing for four women.
Zero women reported abnormal discharge at the last pre-DMPA visit.
Baseline bacteria quantities
We studied changes in 4 vaginal bacteria: Lactobacillus crispatus and Lactobacillus jensenii, two components of a healthy vaginal microbiome; Gardnerella vaginalis, an anaerobe typically linked to BV; and Lactobacillus iners, a species which is a component of both healthy and abnormal vaginal communities. Baseline bacteria quantities, measured in 16s rRNA gene copies/swab at the last pre-DMPA visit, are summarized in Table 2. Lactobacillus crispatus and L. jensenii were below detection for the majority of samples, with detection in only 2 (13%) and 1 (7%) of the 15 women at their last pre-DMPA visit sample. The majority of women had detectable levels of L. iners and G. vaginalis at baseline, with a median of 7.7 log10 copies/swab (IQR: 5.8, 8.6) and 7.8 log10 copies/swab (IQR: 6.7, 8.2) respectively.
Table 2.
Bacterial levels detected in each vaginal swab (16S rRNA gene copies/swab) at the pre-DMPA visit; and change over time in bacteria levels after DMPA initiation.
| N (%) above detection | Median (IQR), log10 scale* | Estimate (Slope post-DMPA)¥ | (95% CI) | p-value | Adjusted p-value** | ||
|---|---|---|---|---|---|---|---|
| Detect L. crispatus (OR) | 2 (13) | - | 1.20 | 0.96 | 1.49 | 0.11 | 0.18 |
| Detect L. jensenii (OR) | 1 (7) | - | 0.99 | 0.79 | 1.24 | 0.93 | 0.93 |
| Log10L. iners | 13 (87) | 7.7 (5.8, 8.6) | −0.04 | −0.2 | 0.13 | 0.66 | 0.83 |
| Log10G. vaginalis | 15 (100) | 7.8 (6.7, 8.2) | −0.21 | −0.37 | −0.05 | 0.01 | 0.04 |
| Log10 Total bacterial load (BR16s) | 15(100) | 8.7 (8.4, 9.0) | −0.08 | −0.14 | −0.01 | 0.02 | 0.04 |
N = participants, IQR = Interquartile Range, LLOD = Lower limit of detection, OR = Odds ratio, CI = Confidence interval; p-values and estimates are by linear mixed effects models with linear splines pre- and post-DMPA initiation (random intercepts and random slopes post DMPA-initiation).
Median (IQR) only reported for those with greater than 50% above detection.
Adjusted using Simes’ method.
# Nine of the 15 women (60%) had detectable L. crispatus levels at some time during follow up.
Estimated multiplicative change per month in Odds Ratio of detection or arithmetic change per month in log10 levels following estimated date of DMPA initiation, accounting for variability between subjects in slopes.
Change in bacteria over time
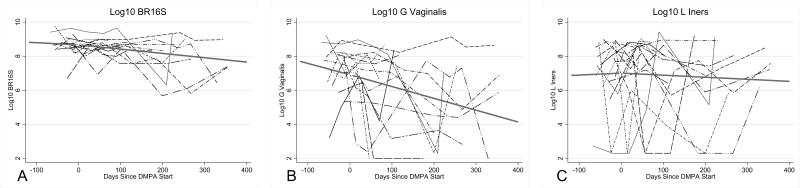
Quantities of G. vaginalis and total vaginal bacterial load declined markedly after DMPA initiation (p=0.01, p=0.02, respectively) (Table 2, Figure 1). For G. vaginalis, this translated to a nearly 2-log decline over 12 months of followup. L. iners remained highly prevalent with no significant changes observed after DMPA initiation. Among the two species rarely detected at baseline, L. crispatus and L. jensenii, we found no change in the odds of detection of either species after initiation of DMPA. There was also no change in the likelihood of detection of Lactobacillus by culture before compared to after DMPA initiation (odds ratio (OR) for Lactobacillus detection by culture for each additional month on DMPA =1.013, 95% CI: 0.877, 1.168; p=0.87).
Figure 1.
Quantities of vaginal bacteria over time, for all subjects, with depot-medroxyprogesterone acetate (DMPA)-fitted trend lines pre- and post-DMPA. A, Bacterial load by broad-range 16S rRNA gene qPCR. B, Gardnerella vaginalis. C, Lactobacillus iners. Bacterial quantities were log10-transformed. Values below the lower limit of detection were assigned a value of half the lower limit of detection. P-values and estimates were calculated using linear mixed effects models with linear splines pre- and post-DMPA initiation (random intercepts and random slopes post DMPA-initiation).
We modeled trends in bacterial species over time, adjusting for potential confounding factors. The model estimates for lactobacilli time trends were not substantially influenced by potential confounding factors. The adjusted model for G. vaginalis incorporated vaginal washing and recent antibiotic use, and continued to show that quantities decreased over time (p=0.04). These relationships were preserved after assessing for effect modification with recent antibiotic use, with no significant interaction between time trends and antibiotic use observed (data not shown). Total bacterial load also declined over time (p=0.02) and this decline was sustained after adjustment for multiple comparisons (p=0.04).
Clinical correlates with changes in vaginal microbiota
Nugent scores were compared by linear spline, stratified by BV within 70 days of the last pre-DMPA sample. There was no significant association between DMPA exposure and Nugent score, with an estimated 0.03 Nugent score units per month decrease following initiation among subjects without BV exposure pre-DMPA (change – 0.027; 95% CI: 0.12, 0.068; p-value = 0.58 and an increase among subjects with BV exposure pre-DMPA (0.018; 95% CI -0.12, 0.16; p-value = 0.80).
No clinical correlates of vaginal health were significantly associated with DMPA use, including vaginal pH, Nugent score, self-report of abnormal vaginal discharge, or clinician observation of abnormal vaginal discharge (data not shown).
Baseline inflammatory mediator levels
We measured inflammatory mediators IL-6, IL-8, IL-1ra, IP-10, and SLPI, and RANTES at the same timepoints pre- and post-DMPA initiation. Three women in the microbiota analysis did not have specimens for analysis. Of the remaining 12 women, at baseline, RANTES was not detected in 5 women and was detected below laboratory standard for the remaining 7 women. The other inflammatory mediators were detected in all women, and are described in Table 3 and supplemental Table 1.
Table 3.
Baseline levels and estimates of change over time in inflammatory mediator levels after DMPA initiation.
| Baseline level, Median (IQR), log10 scale* | Estimate (Slope post-DMPA)¥ | (95% CI) | p-value | Adjusted p-value* | ||
|---|---|---|---|---|---|---|
| Log10 IL-6 | 1.1 (0.6, 1.4) | −0.07 | −0.12 | −0.01 | 0.03 | 0.08 |
| Log10 IL-8 | 2.9 (2.5, 3.1) | −0.06 | −0.12 | −0.00 | 0.04 | 0.08 |
| Log10 IL1-ra | 2.6 (2.5, 3) | −0.04 | −0.07 | −0.02 | <0.001 | 0.001 |
| Log10 IP-10 | 1.7 (1.2, 2.1) | 0.01 | −0.03 | 0.04 | 0.71 | 0.71 |
| Detect RANTES | - | 1.07 | 0.85 | 1.35 | 0.56 | 0.67 |
| Log10 SLPI | 2.2 (1.5, 2.4) | −0.02 | −0.06 | 0.02 | 0.35 | 0.53 |
Units for IL-6, IL-8, IP-10, RANTES: pg/ml. Units for SLPI, IL1-ra: ng/ml.
adjusted for multiple comparisons using Simes’ method.
Estimated multiplicative change per month in Odds Ratio of detection or arithmetic change per month in log10 levels following estimated date of DMPA initiation, accounting for variability between subjects in slopes.
CI = Confidence Interval; p-values and estimates are by linear mixed effects models with linear splines pre- and post-DMPA initiation (random slopes post DMPA-initiation)
Changes in inflammatory mediators levels over time
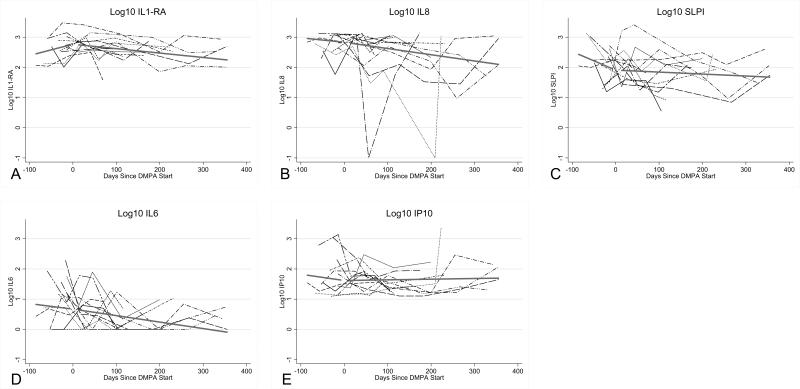
Quantities of 3 inflammatory mediators decreased significantly over time after DMPA initiation. The slope of decline was as follows: for log10 IL-6 levels, slope -0.07 (p=0.03), log10 IL-8 levels, slope -0.06 (p=0.04), log10 IL-1ra levels, slope -0.04 (p<0.001) (Table 3). We looked for possible confounding factors among our pre-specified mediators of the relationship between inflammation and DMPA use. No factors altered the strong association between DMPA initiation and decline in IL-1ra; after adjusting for vaginal washing, the relationship between IL-6 and DMPA was slightly attenuated (slope -0.06, p=0.04); although a trend persisted, DMPA no longer significantly affected IL-8 levels after adjustment for vaginal washing (slope -0.05, p=0.07). Figure 2 displays individual inflammatory mediator quantities over time.
Figure 2.
Levels of inflammatory mediators for all subjects over time with depot-medroxyprogesterone acetate (DMPA)-fitted trend lines pre- and post-DMPA. A: Interleukin (IL)-1 receptor antagonist. B, IL-8. C, secretory leukocyte protease inhibitor (SLPI). D, il-6. E, Interferon-γ-inducible protein 10 (IP-10). P-values and estimates were calculated using linear mixed effects models with linear splines pre- and post-DMPA initiation (random slopes post DMPA-initiation).
Since there was high diversity of vaginal microbiota among women studied, we examined bacterial species and immune factors to see if there were associations. We found that L. crispatus was consistently inversely associated with immune factors IL1-ra, IL-6 and IL-8, and G. vaginalis was consistently positively associated with IL-8 (Supplemental Figure 1).
Discussion
This unique prospective cohort study demonstrated that initiation of DMPA led to significant changes in vaginal microbiota. The quantity of G. vaginalis declined by almost 100-fold after initiation of DMPA, and this decline was sustained during long-term DMPA use. Total vaginal bacterial load also declined with DMPA use. There were marked decreases in pro-inflammatory mediators IL-6, IL-8 and regulatory protein IL-1ra following DMPA initiation, suggesting that either DMPA exposure or the observed microbiota shifts precipitated changes in the immune environment of the vagina.
Prior studies have found decreased incidence of BV among users of DMPA in both developed and developing country settings.7,8,25,26 Our results, showing decline in G. vaginalis, a BV-associated species, expand on this observation. Gardnerella vaginalis is nearly universally associated with BV, and the reduction in this species documented in our cohort may help to explain the decreased incidence of BV among users of DMPA. Studies that quantify vaginal microbiota daily have noted that menses coincide with transient increase in G. vaginalis, which requires iron for growth.21,27 Since DMPA induces amenorrhea with long-term use, lack of menstrual blood could provide a mechanism for long-term reductions in G. vaginalis or reduced incidence of BV.21 However, amenorrhea would not explain the relatively rapid shift seen in our study, as these women would be unlikely to experience amenorrhea in the first months of using DMPA.
Despite reductions in G. vaginalis, we did not observe increases in L. crispatus or L. jensenii among study participants. Mitchell et al reported, similarly, that among 32 US women, there was a 50% reduction in culture detection of H202-producing lactobacilli after one year of DMPA exposure.28 This further illustrates that while DMPA use may reduce BV, it does not increase Lactobacillus colonization. A Lactobacillus-dominated vaginal microbiota may be protective against HIV,29-31 but our understanding of protection offered by lactobacilli is limited by the lack of application of molecular methods to understand specifically which lactobacilli are most beneficial. Studies are underway to determine if specific anaerobic species associated with BV are linked to HIV susceptibility, which would aid in the interpretation of our observations of low Lactobacillus colonization in these women.
Our cohort had high rates of L. iners, a species that is not associated with protection; L. iners is linked with higher baseline pH levels in the vagina, disrupted microbiota, and intermediate Nugent scores.32,33 Lactobacillus iners is globally common in both healthy and dysbiotic vaginal states,12,34,35 and appears to differentially express genomic elements in vaginal dysbiotic and healthy states.36 Lactobacillus iners quantities did not change with DMPA use in our cohort. Understanding why L. iners remained unaffected by DMPA, determining whether L. iners dominates the microbiota of DMPA users, and determining the role of DMPA in hormonal regulation of L. iners gene expression would represent major advances in understanding how the predominant vaginal microbiota of African women may be influenced by reproductive hormones and contraception.
We observed significant declines over time in inflammatory mediators IL-6 and IL-8 after initiation of DMPA. Higher levels of L. crispatus were associated with lower levels of IL-6 and IL-8. Previous studies have also noted lower IL-8 levels in women with the highest levels of L. crispatus in the vagina.37 A possible mechanism for this effect was suggested by a study showing that medroxyprogesterone acetate (MPA) acts on glucocorticoid receptors in vitro to reduce pro-inflammatory gene expression in cervical epithelial cells, which include reductions in mRNA expression of the genes encoding IL-6, IL-8 and RANTES.38 This explanation for our findings makes more sense than an upstream explanation for this effect, which would suppose that a reduction in pro-inflammatory mediators is caused by fewer immune cells present. In fact, some studies have shown increased numbers of immune cells, including HIV target cells such as T cells, macrophages and CCR5+ cells, in the vaginal epithelium of women initiating DMPA,39,40 although there is little accompanying description of the vaginal microbiota. In one study, both microbiota and target cells were assessed, and CCR5+ target cells were not increased in DMPA users, while lactobacillus colonization declined among DMPA users.28 Our understanding of the inflammatory effects of DMPA will remain limited until microbiota can also be studied as a major modifier of local immunity.
Our observation of IL-1ra decline with DMPA use is intriguing. A statistically significant reduction in IL-1ra among DMPA users was also seen in a large cohort study in Africa assessing HIV acquisition, although there was no difference in IL-1ra levels among women who acquired HIV compared with women who remained HIV-negative.41 A study of US women noted that those with vaginal microbiota dominated by L. iners or G. vaginalis had substantially higher levels of IL-1ra than women with microbiota dominated by L. crispatus.42 Reduction in IL-1ra may be a reaction to a reduction in overall inflammation, or may be a specific transcriptional effect of DMPA that is not linked to other inflammatory activity.
Our study had several strengths. We used comprehensively collected longitudinal clinical data to conduct this analysis, which allowed us to observe changes in individual women. Further, our relatively long period of follow up (median 8 months) allowed for measurement of trends over time. Our study population of African urban women represents the population in which heterosexual HIV acquisition risk is highest, and in which prior studies have indicated increases in HIV acquisition among DMPA users.2 DMPA users have been noted to have increases in sexually transmitted infections (STIs) including chlamydia,6-8 possibly due to behavior changes after starting contraception. Women in our study had similar sexual behavior and condom use before and after starting DMPA, and we collected detailed data on these behaviors, allowing us to attempt to control for them. Women also received regular screening and treatment for STIs, which allowed for more focused observation of the vaginal microbiota with less interference from asymptomatic or untreated STIs. Bacterial vaginosis was treated only if women were symptomatic, as there is currently no indication for treatment of asymptomatic BV in non-pregnant women.
This study was limited by the small number of women investigated. Although we did not have a control group, our measurements pre- and post- DMPA allow each woman to serve as her own control. We studied 4 species of bacteria in the vagina, but were unable to include most BV-associated bacteria because of budgetary constraints. Information about vaginal bleeding post-DMPA was imprecise, and we were unable to examine the role of vaginal bleeding and menstruation on changes in the microbiota and inflammatory markers. Our findings in these women engaging in transactional sex in an African city may not be representative of women in other settings.
In conclusion, using DMPA appears to have caused important, lasting alterations in vaginal microbiota, and was significantly associated with a large reduction in the quantity of G. vaginalis and inflammatory mediators IL-6, IL-8 and IL-1ra. At present, there are insufficient data to make definitive statements about how these changes would influence HIV susceptibility; but these findings are intriguing, and highlight the need for further research to define quantitative changes in components of the full vaginal microbiota that could be related to HIV acquisition and transmission risk. Further investigation of microbiome and mucosal immunological changes with DMPA may help to elucidate the multifactorial influences that this hormonal contraceptive exerts on the vaginal immune environment.
Supplementary Material
Acknowledgments
Funding: This research was funded by P01-HD64915 to J.O. – Project 2, R.S.M. from NIAID, an International Pilot Award to A.C.R. through a Center for AIDS Research (CFAR) award to the University of Washington (P30AI027757) which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA). A.C.R. is supported by K23 HD071788-01A1 from NICHD. The funders had no role in study design, data collection and analysis, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conferences: This research was presented as Poster #861 and at a themed oral discussion at Conference on Retroviruses and Opportunistic Infections (CROI) Feb 23-26, 2015 in Seattle, Washington.
Competing Interests: D. N. F. and T. L. F. have developed intellectual property related to the use of PCR for the diagnosis of bacterial vaginosis (BV). Refer to US patent 7625704 on use of PCR for diagnosis of BV. R.S.M. receives research funding and donated STD testing kits from Hologic/Gen-Probe Incorporated. R.S.M. has received payment for invited talks and donation of study product for a clinical trial of BV from Embil Pharmaceutical Company. No other coauthors report any competing interests to disclose.
References
- 1.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21(13):1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 3.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24(11):1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polis CB, Phillips SJ, Curtis KM, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90(4):360–390. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 5.PEPFAR . PEPFAR 3.0: Controlling the Epidemic: Delivering on the Promise of an AIDS-free Generation. Washington, DC: 2014. [Google Scholar]
- 6.Baeten JM, Nyange PM, Richardson BA, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185(2):380–385. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 7.Pettifor A, Delany S, Kleinschmidt I, Miller WC, Atashili J, Rees H. Use of injectable progestin contraception and risk of STI among South African women. Contraception. 2009;80(6):555–560. doi: 10.1016/j.contraception.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClelland RS, Richardson BA, Graham SM, et al. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sex Transm Dis. 2008;35(6):617–623. doi: 10.1097/OLQ.0b013e31816907fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jespers V, Crucitti T, Menten J, et al. Prevalence and Correlates of Bacterial Vaginosis in Different Sub-Populations of Women in Sub-Saharan Africa: A Cross-Sectional Study. PLoS One. 2014;9(10):e109670. doi: 10.1371/journal.pone.0109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22(12):1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr., Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192(8):1372–1380. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 12.van de Wijgert JH, Borgdorff H, Verhelst R, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One. 2014;9(8):e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukusi EA, Cohen CR, Meier AS, et al. Bacterial vaginosis: risk factors among Kenyan women and their male partners. Sex Transm Dis. 2006;33(6):361–367. doi: 10.1097/01.olq.0000200551.07573.df. [DOI] [PubMed] [Google Scholar]
- 14.Martin HL, Jr., Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178(4):1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 15.Achilles SL, Hillier SL. The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS. 2013;27(Suppl 1):S5–15. doi: 10.1097/QAD.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham SM, Jalalian-Lechak Z, Shafi J, et al. Antiretroviral treatment interruptions predict female genital shedding of genotypically resistant HIV-1 RNA. J Acquir Immune Defic Syndr. 2012;60(5):511–518. doi: 10.1097/QAI.0b013e31825bd703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007;45(10):3270–3276. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khot PD, Ko DL, Hackman RC, Fredricks DN. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infect Dis. 2008;8:73. doi: 10.1186/1471-2334-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J Clin Microbiol. 2009;47(3):721–726. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baeten JM, Hassan WM, Chohan V, et al. Prospective study of correlates of vaginal Lactobacillus colonisation among high-risk HIV-1 seronegative women. Sex Transm Infect. 2009;85(5):348–353. doi: 10.1136/sti.2008.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197(10):1361–1368. doi: 10.1086/587490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rifkin SB, Smith MR, Brotman RM, Gindi RM, Erbelding EJ. Hormonal contraception and risk of bacterial vaginosis diagnosis in an observational study of women attending STD clinics in Baltimore, MD. Contraception. 2009;80(1):63–67. doi: 10.1016/j.contraception.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis. 2007;34(12):954–959. [PubMed] [Google Scholar]
- 27.Santiago GL, Tency I, Verstraelen H, et al. Longitudinal qPCR study of the dynamics of L. crispatus, L. iners, A. vaginae, (sialidase positive) G. vaginalis, and P. bivia in the vagina. PLoS One. 2012;7(9):e45281. doi: 10.1371/journal.pone.0045281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell CM, McLemore L, Westerberg K, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis. 2014;210(4):651–655. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180(6):1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 30.van de Wijgert JH, Morrison CS, Cornelisse PG, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr. 2008;48(2):203–210. doi: 10.1097/QAI.0b013e3181743936. [DOI] [PubMed] [Google Scholar]
- 31.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12(13):1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Mirmonsef P, Gilbert D, Zariffard MR, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65(3):190–195. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol. 2009;9:116. doi: 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hummelen R, Fernandes AD, Macklaim JM, et al. Deep sequencing of the vaginal microbiota of women with HIV. PLoS One. 2010;5(8):e12078. doi: 10.1371/journal.pone.0012078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Backer E, Verhelst R, Verstraelen H, et al. Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners. BMC Microbiol. 2007;7:115. doi: 10.1186/1471-2180-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macklaim JM, Gloor GB, Anukam KC, Cribby S, Reid G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4688–4695. doi: 10.1073/pnas.1000086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirmonsef P, Gilbert D, Zariffard MR, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2010;65(3):190–195. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govender Y, Avenant C, Verhoog NJ, et al. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS One. 2014;9(5):e96497. doi: 10.1371/journal.pone.0096497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandra N, Thurman AR, Anderson S, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses. 2013;29(3):592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ildgruben AK, Sjoberg IM, Hammarstrom ML. Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet Gynecol. 2003;102(3):571–582. doi: 10.1016/s0029-7844(03)00618-5. [DOI] [PubMed] [Google Scholar]
- 41.Morrison C, Fichorova RN, Mauck C, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr. 2014;66(2):109–117. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 42.Orfanelli T, Jayaram A, Doulaveris G, Forney LJ, Ledger WJ, Witkin SS. Human epididymis protein 4 and secretory leukocyte protease inhibitor in vaginal fluid: relation to vaginal components and bacterial composition. Reprod Sci. 2014;21(4):538–542. doi: 10.1177/1933719113503416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.