Abstract
Background
Tenofovir disoproxil fumarate (TDF) pre-exposure prophylaxis (PrEP) use is associated with a small but statistically significant decline in estimated glomerular filtration rate (eGFR). We investigated the reversibility of eGFR decline among HIV-uninfected adults discontinuing PrEP.
Methods
Data were from the Partners PrEP Study, a randomized trial of daily oral TDF and emtricitabine (FTC)-TDF PrEP among African HIV-uninfected men and women with baseline creatinine clearance ≥60mL/min. Serum creatinine was measured quarterly while on study medication and at month 1 and 2 after discontinuation. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration Equation.
Results
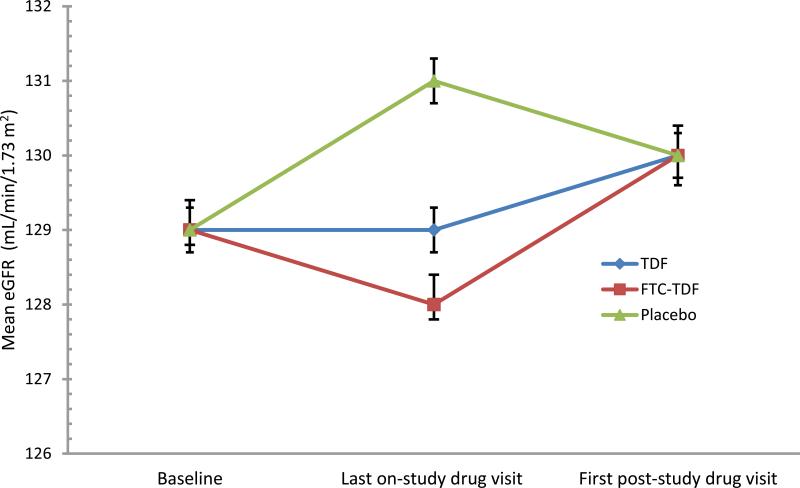
A total of 3924 individuals had a post-study drug serum creatinine measurement after the scheduled drug discontinuation (1271 for TDF, 1308 for FTC-TDF, and 1345 for placebo); 65% were male, median age was 35 (range 18–64) years. Median time on study drug was 33 (IQR 25–36) months overall, and 36 months (IQR 30–36) for TDF and FTC-TDF. Mean eGFR at the last on-treatment visit was 129 mL/min/1.73 m2 for TDF and 128 mL/min/1.73 m2 for FTC-TDF versus 131 mL/min/1.73 m2 for placebo (2-3 mL/min/1.73 m2 mean decline for PrEP versus placebo, p ≤0.01) and this difference reversed by 4 weeks after drug discontinuation (mean eGFR at the first post-drug visit: 130 mL/min/1.73 m2 in all groups). More than 96% of participants had a confirmed >75% eGFR rebound to baseline eGFR level by 8 weeks after drug discontinuation, with similar proportions across treatment groups.
Conclusions
In this large, placebo-controlled study of TDF-based PrEP, the small reduction in mean eGFR associated with PrEP reversed within weeks after discontinuation.
Keywords: eGFR decline reversibility, Tenofovir disoproxil fumarate, pre-exposure prophylaxis, eGFR recovery after TDF discontinuation
Introduction
Tenofovir disoproxil fumarate (TDF)-based pre-exposure prophylaxis (PrEP) has demonstrated protection against HIV acquisition in diverse geographical and at-risk populations1-4. The World Health Organization and the US Centers for Disease Control and Prevention recommend TDF-based PrEP as part of a comprehensive package to prevent HIV infection in high-risk individuals 5,6. Although generally well tolerated, TDF exposure has been associated with small but statistically significant decline in estimated glomerular filtration rate (eGFR) in HIV infected adults receiving TDF-containing antiretroviral regimens 7-9 and in HIV uninfected persons receiving TDF-based PrEP for HIV prevention 10,11. Among HIV infected adults, kidney function returns to baseline level in a majority who discontinue TDF, but cases of less than optimal recovery have been reported 9,12. In PrEP trials 1-4,13, renal adverse events based on serum creatinine levels and calculated creatinine clearance generally resolved with TDF discontinuation, but detailed data on the reversibility of TDF-related eGFR decline after PrEP discontinuation are limited. We assessed the reversibility of eGFR decline in HIV uninfected adults discontinuing TDF-based PrEP in the Partners PrEP Study, a randomized, double-blind, placebo-controlled trial of daily oral TDF and emtricitabine (FTC)-TDF PrEP among African heterosexual HIV serodiscordant couples (Clinicaltrials.gov number NCT00557245).1 Adherence to PrEP was very high in the Partners PrEP study (tenofovir was detected in 82% of random sample of participants) 14,15, making it an important source of evidence for changes in kidney function during and after stopping PrEP.
Methods
Study design and participants
The design, recruitment, procedures, and primary results for the Partners PrEP study are reported elsewhere 1. Briefly, between July 2008 and November 2010, 4747 heterosexual HIV serodiscordant couples were enrolled at nine sites in Kenya and Uganda. Eligible HIV uninfected participants were ≥18 years of age, did not have active hepatitis B infection, had normal renal function (defined by serum creatinine ≤1.3 mg/dL for men / ≤1.1 mg/dL for women and Cockcroft-Gault calculated creatinine clearance of ≥60 mL/min), were not receiving ongoing therapy with agents with known significant nephrotoxic potential, and did not have diabetes requiring hypoglycemic medication or clinically significant cardiac disease. Participants were randomized in a 1:1:1 ratio to one of the three study groups: TDF, FTC-TDF, or placebo. In July 2011, the study's independent Data and Safety Monitoring Board recommended early discontinuation of the placebo arm due to definitive demonstration of PrEP efficacy against HIV acquisition. Thereafter, the two active arms continued blinded follow-up to garner additional data on safety and efficacy of FTC-TDF versus TDF 16. The present analysis presents data including the additional follow-up of the two active PrEP arms with the placebo group follow-up truncated at July 2011. The study protocol, including the post-study drug visits, was approved by the University of Washington Human Subjects Review Committee and ethics review committees at each of the study sites. All participants provided written informed consent.
Participant follow-up and procedures
TDF and FTC were dosed at 300 mg daily and 200 mg daily, respectively; these doses are also the standard for treatment of HIV 17. HIV uninfected partners were followed monthly up to 36 months with HIV testing, study medication refill for 30 days, collection of the prior month's unused medication, and adverse event assessment. After completing follow-up on study drug, HIV uninfected participants completed two additional post-study drug visits four and eight weeks after study drug was discontinued at the final visit. Study medication was withheld in women who became pregnant for the duration of pregnancy and breastfeeding. Serum creatinine was measured at baseline, month 1, and quarterly thereafter up to 36 months, and at the two post-study drug visits, 4 and 8 weeks after stopping study drug, by site laboratories which participated in regular proficiency testing for quality control and quality assurance.
Study medication was temporarily withheld if a participant had an increase in serum creatinine to 1.1 times the upper limit of normal or to >1.5-fold times the baseline creatinine value, even if still within the normal range; an abnormal result needed to be confirmed with repeat testing, scheduled within 7 days of the first abnormal result, for the temporary study medication hold to be initiated. For participants with a recorded graded creatinine-related graded drug adverse event, serum creatinine was monitored weekly until the abnormality resolved or stabilized. Once held, study drug could be restarted if serum creatinine returned to within normal limits or, in a >1.5-fold increase in creatinine from baseline, decreased to within 1.3-fold of the baseline value. Permanent study drug discontinuation occurred under the following circumstances: 1) at completion of scheduled follow-up on study medication, 2) in participants who seroconverted to HIV infection, 3) in participants who experienced a confirmed grade 2 or higher serum creatinine abnormality (defined as ≥1.4 times the upper limit of serum creatinine normal or a Cockcroft-Gault calculated creatinine clearance <50 mL/min), regardless of whether serum creatinine returned to baseline upon withdrawal of the study medication.
Assessment of GFR
For the present analysis, eGFR was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation 18, although the Cockcroft-Gault equation was used for safety monitoring during the study, as described above. The CKD-EPI equation is validated in African populations and provides more accurate estimates for eGFR values in the normal range than both the Modification of Diet in Renal Disease Study and Cockcroft-Gault equations when compared to a direct measure of GFR by iohexol clearance 19,20. For the present analysis, the primary assessment measure of renal function recovery was pre-defined as mean eGFR after the last on-study drug date. The pre-defined secondary outcome of eGFR reversibility was a return to >75% of the baseline eGFR level, confirmed by repeat testing. . The cutoff of reversibility to >75% of baseline was used because it is the reciprocal of a ≥25% decline in eGFR, which is an established criterion for the diagnosis of acute kidney injury 21 and has been associated with increased morbidity and mortality 22-24.
Statistical Analysis
The primary outcome was mean eGFR after the last on-study drug date and the primary analysis included all participants who had any protocol defined post-study drug serum creatinine obtained within 12 weeks of scheduled study drug discontinuation (i.e., generally at 4 and 8 weeks visits after study drug discontinuation, and accounting for late visits that occurred at up to 12 weeks). To estimate the total effects of study drug discontinuation on mean eGFR, we used linear regression to compare mean eGFR, separately, at the last on-treatment visit and the first post-study drug visit, Kaplan-Meier method to estimate the cumulative probability, and cox proportional hazards regression to compare the time to post-drug eGFR reversibility >75% of baseline between active PrEP versus placebo group. In multivariate analyses, we adjusted for duration of drug exposure, sex, baseline age, eGFR, and indicators for BMI and systolic blood pressure. We performed a sensitivity analysis considering all persons with any creatinine measurement taken after the last on-study drug date (i.e., regardless of whether the post-study drug phase serum creatinine was obtained within 12 weeks of scheduled study discontinuation); this approach minimized potential exclusions of participants with early drug holds for creatinine-related abnormality or pregnancy and provided the opportunity for longer observation period after study drug discontinuation beyond the 8 weeks protocol defined post-study phase (i.e., participants with early study drug hold for safety or pregnancy continued follow-up in the study and could potentially be observed for up to 36 months after drug discontinuation). Analyses were conducted using SAS software (version 9.3, SAS Institute) and Stata version 12.
Results
Of the 4747 HIV uninfected persons enrolled and followed in the Partners PrEP Study, 3924 (83%) had a serum creatinine measured within 12 weeks after study drug was discontinued: 1271 in the TDF group, 1308 in the FTC-TDF group, and 1345 in placebo group. Of these 3924, 65% were male, with median age of 35 years (range, 18-64), and baseline characteristics were comparable across the three exposure groups (Table 1). Median duration of study drug exposure was 33 months overall [interquartile rage (IQR) 25 to 36]; 36 months (IQR; 30-36) for TDF and FTC-TDF, and 25 months (IQR; 18-30) for placebo. Participants were observed for a median of 8 weeks (range, 1 to 12) after study drug discontinuation; median duration to the first post-study drug creatinine measurement was 4 weeks (range, 1 to 12). An additional 436 participants (165 TDF, 137 FTC-TDF, and 134 placebo) had ≥1 creatinine measured after the last on-study drug date but not within 12 weeks (i.e., due to study drug hold or early discontinuation); thus, a total of 4360 participants were included in the sensitivity analysis. The remaining 387 participants (148 TDF, 134 FTC-TDF, and 105 placebo) did not have a post-study drug creatinine measurement, mostly due to missed visits, post-HIV seroconversion, and refusal of study procedures; they tended to be males of younger age and higher baseline eGFR compared to those included in the analysis (excluded versus included persons; p <0.05 for all comparison: age-years: 33 vs 35; male: 67% vs 62%; and baseline CKD-eGFR: 133 vs 129 mL/min/1.73 m2) but were comparable on all other baseline characteristics.
Table 1.
Enrollment characteristics according to treatment group
| Characteristic | FTC-TDF (n=1308) | TDF (n=1271) | Placebo (n=1345) |
|---|---|---|---|
| Age (years) | |||
| Mean (range) | 35 (18-64) | 35 (18-64) | 35 (18-64) |
| <25 | 9% | 9% | 9% |
| 25-34 | 43% | 44% | 43% |
| 35-44 | 33% | 33% | 34% |
| ≥45 | 15% | 14% | 14% |
| Male | 67% | 66% | 63% |
| Serum Creatinine (mg/dL) | 0.79 ± 0.15 | 0.79 ± 0.15 | 0.78 ± 0.15 |
| eGFR (mL/minute/1.73 m2) | 129 ± 17 | 129 ± 16 | 129 ± 16 |
| eGFR <90 mL/minute/1.73 m2 | 98% | 98% | 98% |
| Weight (kg) | 61 ± 9 | 61 ± 10 | 61± 10 |
| Body Mass Index | |||
| <18.5 | 6% | 5% | 4% |
| 18.5-24.9 | 78% | 79% | 79% |
| 25-29.9 | 13% | 12% | 13% |
| ≥30 | 3% | 4% | 4% |
| Systolic blood pressure ≥140mmHg | 5% | 5% | 6% |
Unless stated, statistics are mean ± standard deviations for continuous covariates and percentages for categorical covariates. For the present analysis, glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation, although the Cockcroft-Gault equation was used for safety monitoring during the study. FTC denotes emtricitabine TDF denotes tenofovir disoproxil fumarate.
Mean last on-treatment and post-study drug discontinuation eGFR
Mean eGFR at entry into the randomized trial was 129.3 mL/min/1.73 m2 for TDF, 128.8 mL/min/1.73 m2 for FTC-TDF, and 128.6 mL/min/1.73 m2 for placebo (p>0.05 for both TDF and FTC-TDF vs placebo). As reported previously 10, TDF and FTC-TDF use was associated with a small, nonprogressive decline in eGFR, and in the current analysis crude mean eGFR was 2-3 mL/min/1.73 m2 lower in the active PrEP arms (representing annual eGFR loss of ≤1 mL/min/1.73 m2 per year) compared to placebo at the last on-study drug visit: 129 mL/min/1.73 m2 for TDF and 128 mL/min/1.73 m2 for FTC-TDF versus 131 mL/min/1.73 m2 for placebo (Table 2; p<0.01 for both TDF and FTC-TDF vs placebo). By 4 weeks after study drug discontinuation, mean eGFR were similar across the three groups (Figure): 130.1 mL/min/1.73 m2 for TDF, 129.9 mL/min/1.73 m2 for FTC-TDF, and 130.1 mL/min/1.73 m2 for placebo (p>0.05 for both TDF and FTC-TDF vs placebo). Multivariate analyses adjusting for duration of drug exposure, sex, baseline age, eGFR, BMI, and systolic blood pressure yielded similar patterns (Data not shown). Notably, the proportion of persons with <90 mL/min/1.73 m2 at the last-post drug visit was similar to that observed at study entry (≤2%), and mean eGFR recovery after drug discontinuation was not modified by either having or not having a baseline eGFR <90 mL/min/1.73 m2 (p>0.05). Overall, younger age, normal BMI, female sex, baseline eGFR ≥90 mL/min/1.73 m2, and shorter duration of drug exposure were independently associated with quicker mean eGFR recovery after drug discontinuation (p <0.05 for all) with comparable rates across treatment group (p >0.05). Sensitivity analysis including all participants with ≥1 creatinine measurement after the last-on study drug date yielded qualitatively consistent patterns to the primary analysis (supplemental digital content Table).
Table 2.
Reversibility of eGFR decline after study drug discontinuation, according to treatment group
| FTC-TDF (n=1308) | TDF (n=1271) | Placebo (n=1345) | |
|---|---|---|---|
| A: Mean eGFR | |||
| Study visit | Mean eGFR (95%Cl)mL/min/1.73m2 | Mean eGFR (95%Cl) mL/min/1.73m2 | Mean eGFR (95%Cl)mL/min/1.73m2 |
| Enrollment | 128.8 (127.9, 129.7); p=0.73 | 129.3 (128.4, 130.2); p=0.28 | 128.6 (127.7, 129.5) |
| Last on-treatment* | 128.3 (127.5, 129.1); p<0.01 | 129.0 (128.2, 129.9); p<0.01 | 131.3 (130.4, 132.1) |
| First post-drug visit‡ | 130.0 (129.1, 130.8); p=0.80 | 130.1 (129.3, 130.9); p=0.99 | 130.1 (129.3, 130.9) |
| B. Proportion of participants with >75% eGFR reversibility after study drug discontinuation | |||
| Time after the last on-study drug date |
FTC-TDF (n=1308)
No. at risk; Cum. probability† (95%CI) |
TDF(n=1271)
No. at risk; Cum. probability† (95%CI) |
Placebo (n=1345)
No. at risk; Cum. probability† (95%CI) |
| 4 weeks | 1300; 70% (67, 72); p=0.70 | 1258; 71% (69, 74); p=0.52 | 1342; 70% (67, 72) |
| 8 weeks | 80; 96% (95, 97); p=0.75 | 59; 97% (96, 98); p=0.31 | 66;97% (96, 98) |
| 12 weeks | 9; 100%; p =0.37 | 6; 100%; p=0.49 | 4; 100% |
The primary analysis included all participants who had any post-study drug phase serum creatinine measurement obtained within 12 weeks of scheduled study drug discontinuation.
Median follow time on study drug was 33 months and resulting annualized eGFR decline attributable to PrEP versus placebo of mL/min/1.73m2 per year
Median duration to the first post-study drug creatinine measurement was 4 weeks (IQR 3-5), similar across treatment groups. Addition of sex, age, body mass index, and baseline eGFR to model did not have substantial effect on estimates in the primary analysis.
Cumulative probabilities of >75% eGFR recovery were generated by Kaplan Meier methods calculated over full data and evaluated at indicated times; they are not calculated from aggregates of raw numbers shown. The numbers risk (raw numbers) represent the number of participants whose eGFR had not reversed to >75% of baseline right before the indicated time point. Median time for eGFR to return >75% of baseline was 4 weeks similar across treatment groups.
P- values are for Wald tests, testing for the null hypothesis of no difference between active PrEP versus placebo.
eGFR estimated glomerular filtration rate; FTC emtricitabibe; TDF tenofovir disoproxil fumarate
Figure. Mean eGFR at the last-on study drug visit and the first post-study visit after discontinuation of study drug, according to treatment group.
Represents the primary analysis that includes persons with a protocol defined post-study drug creatinine measurement within 12 weeks of the last on-treatment visit. The plots correspond to the primary analysis in table 2A but are rounded to integers to smoothen the plots. Median time from the last on-study drug visit to the first post-study drug visit was 4 (IQR, 3 to 5) weeks, similar across the three groups. FTC denotes emtricitabine, TDF denotes tenofovir disoproxil fumarate
Frequency of eGFR reversibility to >75% of baseline levels after study drug discontinuation
Overall, eGFR retuned to >75% of baseline level in >70% of subjects by 4 weeks after discontinuation of study drug, >96% by 8 weeks, and 100% at 12 weeks in the primary analysis (i.e. limited to participants with a post-study drug creatinine measurement within 12 weeks of the last on-study drug visit), and these proportions were similar across the three treatment groups (Table 2; p>0.10 for both TDF and FTC-TDF vs placebo). Additional analysis with cut offs of 90% eGFR reversibility yielded similar patterns across treatment groups. Similarly, >98% of persons had eGFR ≥90 mL/min/1.73 m2 at last visit after drug discontinuation. In the sensitivity analysis (i.e., considering all persons with any creatinine measurement after the last on-study drug date, including participants with early creatinine abnormality related drug discontinuation, n=4360), the proportion of participants with a post-study drug eGFR >75% reversibility was >74% by 4 weeks, >91% by 8 weeks, and >95% by 12 weeks after discontinuation of study drug; median time to >75% eGFR reversibility was qualitatively similar in the three groups but statistically significantly quicker in those assigned placebo versus PrEP (PrEP vs placebo: 4 vs 3 weeks, p <0.05; supplemental digital content table). In the sensitivity analysis, there were 161 participants whose eGFR had not reversed to >75% of baseline level by 12 weeks after study drug discontinuation (54 in TDF, 66 in FTC-TDF, and 41 in placebo); of these, the final post-study drug eGFR rebounded to >75% of their baseline eGFR level in all but 4 (3 in placebo and 1 in TDF group). In the 4 participants, the final recorded eGFR was >60 mL/min/1.73 m2 (specifically, >86 mL/min/1.73 m2) in all but 1 participant, a 46 year old male in the TDF group with acute HIV seroconversion and a creatinine clearance of 36 mL/min/1.73 m2 at the last on treatment visit. Detailed description of these four participants is provided in the supplemental digital content.
Frequency of creatinine-related study drug interruption and drug re-challenge
Study drug interruption triggered by any creatinine-related abnormality was recorded in 62 (1.2%) participants: 22 for TDF, 25 for FTC-TDF, and 15 for placebo; median time of drug exposure to the first recorded creatinine-related study drug interruption was 12 months (range 1-35) in the two active PrEP groups. For 57 (92%) participants (20 TDF, 23 FTC-TDF, and 14 placebo) the creatinine abnormality resolved after temporary drug withdrawal during study follow-up; these participants were re-challenged with study medication per the protocol. In two re-challenged participants (1 each for the TDF and placebo group), the creatinine abnormality recurred on more than two occasions and study drug was not re-established per protocol. First, a 30 year old, 45 kg female in the FTC-TDF group with baseline eGFR 135 mL/min/1.73 m2 (serum creatinine: 0.7 mg/dL) had eGFR of 98 mL/min/1.73 m2 (serum creatinine: 0.9 mg/dL) and 111 mL/min/1.73 m2 at the last on-treatment (month 18) and post-study drug visit, respectively. Second, a 35 year old, 71 kg male in the placebo group with baseline eGFR 146 mL/min/1.73 m2 (serum creatinine: 0.65 mg/dL), had a >1.5 fold increase in serum creatinine compared to baseline (serum creatinine of 1.05 mg/dL) at the last on-treatment visit (month 18) that did not resolve by the end of final study follow-up visit. Throughout the post-study drug phase, the participant's eGFR was ≥90 mL/min/1.73 m2 (108 mL/min at the final visit). As previously reported,10 permanent study drug discontinuation occurred in 5 persons with ≥ grade 2 creatinine abnormalities during follow-up (Table 3): 2 each in the TDF and FTC-TDF groups and 1 in placebo. In a sixth participant, a 30 year old, 45 kg female in the TDF group, with baseline eGFR of 134 mL/min/1.73 m2 (serum creatinine 0.7 mg/dL) experienced ≥25% eGFR decline from baseline (eGFR 98 mL/min/1.73 m2, serum creatinine 0.9 mg/dL; Table 3). Overall, among participants with creatinine abnormality-related drug discontinuation, median time to eGFR recovery after drug discontinuation (i.e. eGFR return to >75% of baseline level) was 4 weeks, similar across the three treatment groups (p >0.1 for TDF and FTC-TDF vs placebo).
Table 3.
Characteristics of persons with creatinine abnormality-related permanent drug discontinuation
| Group | Sex | Age-years | Baseline eGFR mL/min/1.732 (serum Creatinine mg/dL) | Last on-treatment eGFR mL/min 1.732 (serum Creatinine mg/dL); months in the study | Last observed post-study drug eGFR mL/min 1.732 (serum Creatinine mg/dL) | Relevant history† |
|---|---|---|---|---|---|---|
| FTC-TDF | F | 55 | 119 (0.60) | 95 (0.9, a 1.5 fold increase in creatinine from baseline) at month 24 | 101 (0.75) | -No history of use of known nephrotoxic drugs within 1 month. Drug discontinuation based on creatinine clearance which was <50 mL/min) -Baseline serum phosphate: 2.7 mg/dL |
| FTC-TDF | F | 38 | 127 (0.70) | 72 (1.03) at month 15 | 122 (0.71) | -Treated for upper respiratory tract infection with cephalosporin and fluconazole in proceeding 2 weeks - Creatinine fold change from baseline had resolved by 4 weeks after drug discontinuation (serum creatinine 0.78 mg/dL, eGFR 110 mL/min/1.732) |
| TDF | F | 50 | 117 (0.70) | 55 (1.30) at month 6 | 98 (0.80) | -Pre-existing Cervical lesions with history of laparotomy and chronic use of analgesics including NSAID - eGFR was >90mL/min by 8 weeks after drug discontinuation, remained stable and non-progressive until exit |
| TDF | M | 56 | 99 (0.98) | 57 (1.53) at month 30 | 78 (1.18) | -History of recent relocation to a hot and dry region -No urine dipstick protein /glucose -eGFR was 74 mL/min by 2 weeks after drug discontinuation |
| TDF | F | 30 | 134 (0.7) | 98 (0.9) at month 18 | 110 (0.81) | Had ≥25% eGFR decline from baseline at month 18. Creatinine elevation grade was based on calculated creatinine clearance. Baseline weight=45 kg (BMI=24) |
| Placebo | F | 40 | 93 (0.9) | 41 (1.78) at month 1 | 109 (0.78) | -Baseline weight=46 kg (BMI=19) -No urine dipstick protein/ glucose - eGFR was >60 mL/min within 8 weeks after drug discontinuation |
For the present analysis, eGFR was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration Equation, although the Cockcroft-Gault equation was used for safety monitoring during the study.
eGFR denotes estimated glomerular filtration rate, FTC denotes emtricitabine TDF denotes tenofovir disoproxil fumarate
All persons had normal serum phosphate at the last on-treatment visit.
Discussion
In this longitudinal analysis among HIV-uninfected African men and women receiving and then discontinuing daily oral TDF-based pre-exposure prophylaxis, the decline in eGFR associated with TDF exposure was small and rapidly resolved after discontinuation of study medication, including in individuals having treatment-emergent creatinine elevations resulting in temporary discontinuations of study medication. Our study has many strengths: a prospective design, high adherence to PrEP (with tenofovir detected in 82% of random sample of participants) 14,15, a placebo comparison, a large sample size of men and women across a broad range of ages, and regular measurement of eGFR, and thus this study provides robust evidence on the reversibility of eGFR decline in HIV uninfected persons. PrEP is a potentially powerful biomedical intervention with the potential to significantly impact the global HIV epidemic if rolled-out to scale and taken with high adherence among key at risk populations. However, for any preventive intervention, tolerance of adverse effects in healthy persons is low compared to therapeutic interventions. We recently reported that TDF exposure was associated with a small but non-progressive eGFR decline in men and women in the Partners PrEP Study and that clinically relevant eGFR decline (≥25%) was rare and no more frequent in the active than the placebo group 10. These results were consistent with those from the iPrEx study among men who have sex with men 11. The current analysis builds on those data to report that recovery of eGFR decline after TDF discontinuation is robust in healthy persons, even in the minority of participants who developed a clinically relevant decline in eGFR on PrEP.
The intracellular mechanism by which TDF induces nephrotoxicity is not well understood, but is hypothesized to result from the direct tubulo-cytotoxicity effects mediated through mitochondrial DNA injury. In case series of HIV infected persons with potential TDF-related nephrotoxicity 25, light microscopic and ultrastructural evaluations have documented acute tubular necrosis with varying degrees of chronic tubulointerstitial scarring, which may account for the less than optimal reversibility reported in a minority of cases. However, extrapolating results from these studies among HIV infected persons to the PrEP context may be limited by the lack of a truly non-active comparator and the potential confounding effects by other risk factors for kidney injury, including HIV infection 26,27 and use of other antiretroviral medications by persons taking TDF for HIV treatment, decreased body mass, preexisting decrease in kidney function, other co-morbidities, and concomitant use of other potentially nephrotoxic drugs, which are all common in HIV infected persons 24. Nonetheless, comparatively more studies have given credence to robust resolution of TDF associated nephrotoxicity in HIV infected persons, including recovery from profound kidney injury requiring renal replacement therapy25. The US Centers for Disease Control and Prevention has issued guidelines for the delivery of PrEP in clinical settings, and guidelines for other settings have been developed or are in development. US CDC guidelines recommend renal monitoring at 3 months after starting PrEP and semi-annually thereafter 5. Our findings might suggest that renal monitoring for oral TDF-based PrEP could potentially be less frequent than in the CDC guidelines, unless there are comorbidities or longer term use than 36 months.
Although this is the largest prospective study of the reversibility of TDF-related eGFR decline in HIV uninfected adults, this study has limitations. First, the trial only enrolled persons with baseline creatinine clearance ≥ 60 mL/min, and reversibility of eGFR decline among subpopulations with lower baseline eGFR, co-morbid risk factors for kidney disease, or concurrent nephrotoxic medications should be evaluated. Second, creatinine-based GFR estimates are less accurate in persons with low creatinine generation, including those with low muscle mass, muscle wasting, or reduced animal protein intake, which may be more common in African individuals. Third, we did not routinely evaluate proteinuria and changes in proximal tubular function, another potential consequence of TDF exposure.
In conclusion, in this large secondary analysis of a placebo-controlled trial of daily oral TDF-based PrEP among HIV-uninfected African men and women with a median TDF-exposure of 36 months, declines in eGFR rapidly resolved within weeks after study drug discontinuation. These findings are encouraging for the delivery of PrEP in clinical settings.
Supplementary Material
Acknowledgement
Funding/Support: This work was supported by the Bill & Melinda Gates Foundation (OPP47674) and the US National Institutes of Health (R01MH095507 and R01DK100272). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Study medication was donated by Gilead Sciences.
Role of the Sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Partners PrEP Study Team:
University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam R. Lingappa, M. Juliana McElrath.
Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth H. Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig R. Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James D. Campbell, Jordan W. Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi, University of Washington): Nelly R. Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James D. Campbell, Jordan W. Tappero, Jonathan Wangisi. Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Laboratory Services (CLS) of the Wits Health Consortium (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
Abstract presented at the Conference on Retroviruses and Opportunistic Infections, Seattle, 2015
Author Contributions: K.K.M., C.W., and J.M.B. conceived the study and wrote the first draft of the manuscript. K.K.M. performed the statistical analysis. C.C., D.D., J.K., and A.R. contributed critical revisions to the analysis and interpretation. All authors contributed to the writing of the final draft.
Additional Contributions: We thank the HIV serodiscordant couples who participated in this study for their invaluable contributions, and the teams at the study sites and at the University of Washington for work on data and sample collection and management.
Conflict of Interest Disclosures: All authors declare no conflict of interest and no financial interests.
References
- 1.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N. Engl. J. Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N. Engl. J. Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N. Engl. J. Med. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention [April 27, 2015];Pre-exposure Prophylaxis for the Prevention of HIV Infection in the United States: A Clinical Practice Guideline. 2014 May; http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf.
- 6.World Health Organization . Guidance on oral pre-exposure prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. WHO; Geneva, Switzerland: 2012. [April 27, 2015]. http://www.who.int/hiv/pub/guidance_prep/en/. [PubMed] [Google Scholar]
- 7.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic Review and Meta-analysis: Renal Safety of Tenofovir Disoproxil Fumarate in HIV-Infected Patients. Clin. Infect. Dis. 2010;51(5):496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 8.Laprise C, Baril J-G, Dufresne S, Trottier H. Association Between Tenofovir Exposure and Reduced Kidney Function in a Cohort of HIV-Positive Patients: Results From 10 Years of Follow-up. Clin. Infect. Dis. 2013;56(4):567–575. doi: 10.1093/cid/cis937. [DOI] [PubMed] [Google Scholar]
- 9.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among hiv-1–uninfected men and women receiving emtricitabine–tenofovir disoproxil fumarate preexposure prophylaxis: A randomized clinical trial. JAMA Internal Medicine. 2015;175(2):246–254. doi: 10.1001/jamainternmed.2014.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon MM, Lama JR, Glidden DV, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014;28(6):851–859. doi: 10.1097/QAD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wever K, van Agtmael MA, Carr A. Incomplete Reversibility of Tenofovir-Related Renal Toxicity in HIV-Infected Men. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2010;55(1):78–81. doi: 10.1097/QAI.0b013e3181d05579. 10.1097/QAI.1090b1013e3181d05579. [DOI] [PubMed] [Google Scholar]
- 13.Van Damme L, Corneli A, Ahmed K, et al. Preexposure Prophylaxis for HIV Infection among African Women. N. Engl. J. Med. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnell D, Baeten JM, Bumpus NN, et al. HIV Protective Efficacy and Correlates of Tenofovir Blood Concentrations in a Clinical Trial of PrEP for HIV Prevention. J. Acquir. Immune Defic. Syndr. 2014;66(3):340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberer JE, Baeten JM, Campbell J, et al. Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa. PLoS Med. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndase P, Celum C, Campbell J, et al. Successful discontinuation of the placebo arm and provision of an effective HIV prevention product after a positive interim efficacy result: the partners PrEP study experience. J. Acquir. Immune Defic. Syndr. 2014;66(2):206–212. doi: 10.1097/QAI.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 17.Gilead Sciences [September 7, 2014];Emtricitabine/Tenofovir Disoproxil Fumarate Prescribing Information. http://www.gilead.com/~/media/Files/pdfs/medicines/hiv/truvada/truvada_pi.PDF.
- 18.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Deventer HE, Paiker JE, Katz IJ, George JA. A comparison of cystatin C- and creatinine-based prediction equations for the estimation of glomerular filtration rate in black South Africans. Nephrol. Dial. Transplant. 2011;26(5):1553–1558. doi: 10.1093/ndt/gfq621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyatt CM, Schwartz GJ, Owino Ong'or W, et al. Estimating Kidney Function in HIV-Infected Adults in Kenya: Comparison to a Direct Measure of Glomerular Filtration Rate by Iohexol Clearance. PLoS ONE. 2013;8(8):e69601. doi: 10.1371/journal.pone.0069601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int. 2010;78(5):478–485. doi: 10.1038/ki.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyatt CM, Arons RR, Klotman PE, Klotman ME. Acute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortality. AIDS. 2006;20(4):561–565. doi: 10.1097/01.aids.0000210610.52836.07. [DOI] [PubMed] [Google Scholar]
- 25.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D'Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78(11):1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 26.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18(3):541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 27.Ross MJ, Fan C, Ross MD, et al. HIV-1 Infection Initiates an Inflammatory Cascade in Human Renal Tubular Epithelial Cells. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;42(1):1–11. doi: 10.1097/01.qai.0000218353.60099.4f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.