Abstract
Quantification of alternative splicing to detect the abundance of differentially spliced isoforms of a gene in total RNA can be accomplished via RT-PCR using both quantitative real-time and semi-quantitative PCR methods. These methods require careful PCR primer design to ensure specific detection of particular splice isoforms. We also describe analysis of alternative splicing using a splicing “minigene” in mammalian cell tissue culture to facilitate investigation of the regulation of alternative splicing of a particular exon of interest.
Keywords: Alternative splicing, RNA, RT-PCR, Variable exon, Minigene, Splicing factors, Splicing regulation
1 Introduction
Alternative splicing is a key cellular process whereby particular combinations of exons in a nascently transcribed pre-mRNA are either included or excluded to generate different mature mRNA splice isoform transcripts, resulting in multiple protein isoforms encoded by a single gene. It is estimated that nearly all proteincoding genes in the human genome are alternatively spliced, providing an essential source of protein diversity [1, 2]. Differentially spliced isoforms may exert distinct biological functions, therefore accurate quantification of the relative amounts of splice isoforms of a gene is essential to explore the role of alternative splicing in biological processes as well as disease [3]. The pre-mRNA of an alternative spliced gene contains both constitutive exons and variable exons interspaced by introns that are ultimately spliced out and excluded from the final mRNA transcript. In this chapter, we describe the characterization of exon-skipping, the most common form of alternative splicing, where one or more variable exons are either included or skipped from the final transcript to make up the array of spliced isoforms of a gene [4].
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) is one of the most convenient methods for studying RNA transcripts and can generally be used with total RNA extracted from any biological source. Quantitative PCR (qPCR) following RT is often the method of choice given its extreme sensitivity of detection and accurate comparison of amplification of PCR products during the exponential phase of the PCR reaction [5]. Semiquantitative methods are less useful for precise quantification of the relative abundance of splice isoforms, however, combined with electrophoresis, these methods allow for the direct visualization and comparison of splice isoform abundance based on size disparity between differentially spliced transcripts.
Quantification of levels of different splice isoforms is especially useful in studying the regulation of alternative splicing. Regulation of alternative splicing is accomplished by the recognition of cis-acting sequences in the pre-mRNA, usually located in the variable exonic or adjacent intronic sequences, by trans-acting RNA-binding proteins known as splicing factors [6]. In this protocol, we make use of a splicing “minigene” construct to study the regulation of alternative splicing of a variable exon within an alternatively spliced gene [7]. This minigene then offers a versatile tool for studying the regulation of splicing of a particular exon as it can be expressed in cell-culture along with potential splicing factors that could influence variable exon inclusion or skipping.
2 Materials
2.1 RNA Source Material
Alternative splicing can be examined using RNA from any source. In this protocol we use RNA extracted from mammalian cells grown in tissue-culture.
2.2 RNA Extraction and RT
E.Z.N.A.® Total RNA Isolation Kit (Omega Bio-Tek).
GoScript™ Reverse Transcriptase Reagents (Promega).
2.3 qPCR
GoTaq® Green Master Mix (Promega).
Primers for specific splice isoform detection and a housekeeping gene. As an example, primers for the detection of CD44 splice isoforms and housekeeping gene TBP are included in Table 1.
Table 1.
qPCR Primers for the analysis of CD44 alternative splicing
| Primer name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| CD44v with v5/v6 exons | GTAGACAGAAATGGCACCAC | CAGCTGTCCCTGTTGTCGAA |
| CD44s | TACTGATGATGACGTGAGCA | GAATGTGTCTTGGTCTCTGGT |
| Human TBP | GGAGAGTTCTGGGATTGTAC | CTTATCCTCATGATTACCGCAG |
2.4 Semiquantitative PCR
HotStarTaq Plus DNA Polymerase Reagents (Qiagen).
Primers for detecting minigene splice isoforms. As an example, primers for the detection of a CD44 variable exon 8 (v8) minigene are included in Table 2.
0.5× TBE buffer (45 mM Tris, 45 mM boric acid, 1 mM EDTA) for electrophoresis.
1.5 % agarose gel prepared with 0.5× Tris–borate–EDTA (TBE) buffer and 0.5 µg/mL ethidium bromide.
Table 2.
Primers for the analysis of CD44 v8 minigene alternative splicing
| Prime rname | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| qPCR v8 minigene inclusion | CAATGACAACGCTGGCACAA | CCAGCGGATAGAATGGCGCCG |
| qPCRv8 minigene skipping | GAGGGATCCGGTTCCTGCCCC | CAGTTGTGCCACTTGTGGGT |
| Semi-quantitative PCR v8 | GAGGGATCCGGTTCCTGCCCC | CCAGCGGATAGAATGGCGCCG |
2.5 Co-transfection of Splicing Minigene and Splicing Factors
Transfectable mammalian cell line, such as HEK293T (ATCC® CRL-3216™).
Gibco® Dulbecco’s Modified Eagle Medium High Glucose (plain media as well as media supplemented with l-glutamine and 10 % fetal bovine serum).
Lipofectamine 2000 Reagent (Invitrogen).
Mammalian expression plasmids encoding splicing minigene and splicing factors of interest. As an example, we use a CD44 v8 minigene plasmid, splicing factor hnRNPM plasmid, and pcDNA3 control plasmid for co-transfection.
2.6 Equipment
Thermo Fisher Scientific NanoDrop 1000 Spectrophotometer for quantification of RNA.
A thermal cycler, such as the Bio-Rad DNA Engine Tetrad® 2, for Reverse Transcriptase reaction and semi-quantitative PCR.
A real-time thermal cycler such as the Roche Lightcycler® 480 II System with associated LightCycler software for qPCR.
Horizontal electrophoresis apparatus.
A UV transilluminator with camera such as the Bio-Rad Gel Doc XR System with Quantity One 1-D Analysis Software for visualization and densitometric analysis of ethidium-bromide stained DNA.
A tabletop centrifuge such as the Eppendorf Centrifuge 5424.
3 Methods
Prepare all reactions with molecular grade nuclease-free water to prevent RNA degradation.
3.1 RNA Extraction and Purification
Total RNA from mammalian cells in tissue culture is extracted using the E.Z.N.A.® Total RNA Isolation Kit (Omega BioTek) by following the product manual (see Note 1). For RNA isolation via this kit, cells can be directly lysed using the provided lysis buffer.
As per the manufacturer protocol, first collect cells in 350 µL RNA lysis buffer.
Add 350 µL of 70 % ethanol to the lysate and mix thoroughly. Then transfer the sample to the RNA purification column.
Centrifuge at 10,000 × g for 1 min and discard flow-through.
Wash the column once with 500 µL of RNA wash buffer I and twice with RNA wash buffer II, centrifuging at 10,000 × g for 1 min between each wash and discarding flow-through.
Remove residual RNA wash buffer II from the column by centrifugation at maximum speed for 2 min.
Transfer the column into a new 1.5 mL tube, add 50 µL nuclease-free water at the center of the column matrix, and elute RNA by centrifugation at the maximum speed for 1 min.
Determine RNA quantity using a UV spectrometer (such as NanoDrop). High quality RNA should have a 260 nm/280 nm absorbance ratio of approximately 2.0. RNA should be stored at −80 °C for long-term storage.
3.2 RT Reaction
In a reaction totaling 20 µL, combine 250–1000 ng of total RNA with 0.05 µg random hexamer primers, 50 pmol MgCl2, 10 pmol dNTPs, 2 µL 5× GoScript™ Buffer, and 1 µL of GoScript™ Reverse Transcriptase (see Note 2). Mix by vortex and briefly centrifuge.
Incubate reaction mixture 25 °C for 5 min for primer annealing, 42 °C for 60 min for the RT reaction, and 70 °C for 5 min to inactivate the RT enzyme. These incubations are best performed in a programmable thermocycler. Completed reactions may now be frozen at −20 °C for long-term storage.
3.3 Primer Design for qPCR
-
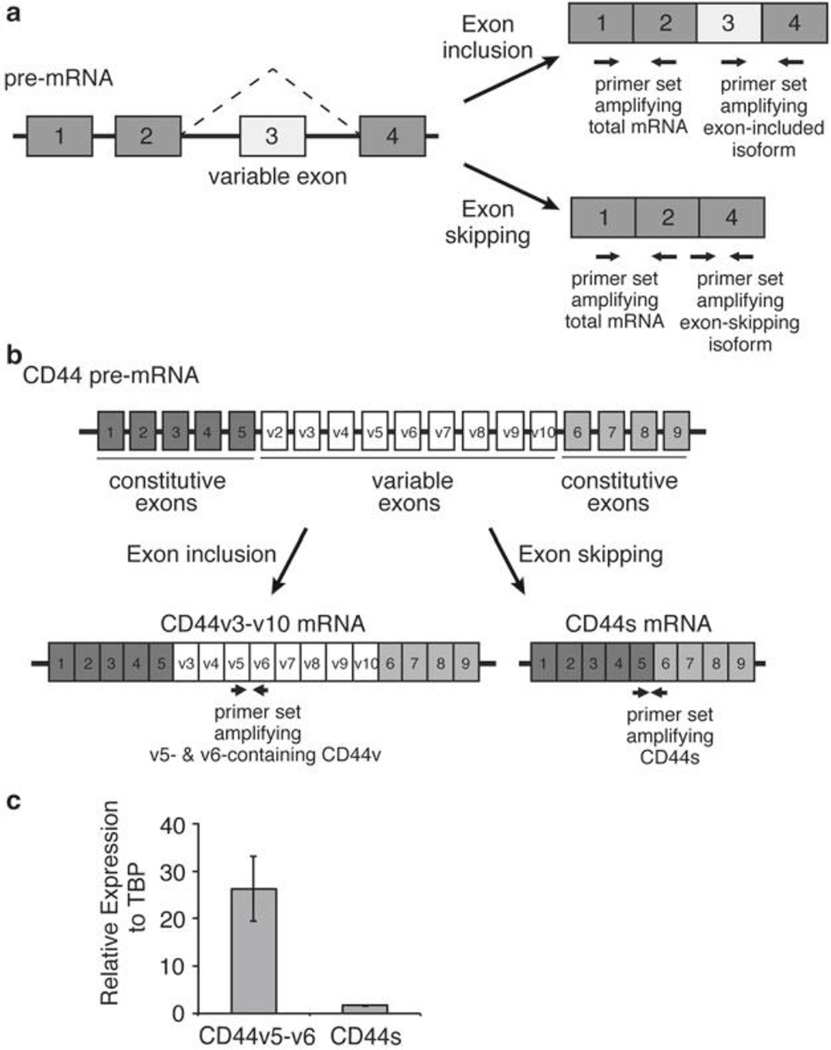
In this protocol we provide an example to detect the most common form of alternative splicing, exon skipping. In a hypothetical four-exon pre-mRNA (Fig. 1a), exon 3 is a variable exon that is either included in the mature mRNA to generate a longer isoform or skipped to generate a shorter isoform. Three primers are designed to differentiate one splice isoform from another.
A forward primer lies within the variable exon 3 with a reverse primer in the immediately downstream constitutive exon 4. These primers allow the selective amplification of the longer isoform with variable exon 3 included. To detect the shorter isoform with the variable exon 3 skipped, a forward primer is designed that spans the junction between exon 2 and 4.
The junction between exon 2 and 4 is disrupted with exon 3 inclusion. Thus, together with the corresponding reverse primer located in constitutive exon 4, this primer set only amplifies mRNA isoforms where exon 3 is skipped. When multiple consecutive variable exons exist in the gene of interest, primers can be designed in two different variable exons for detection of specific variable exon-containing isoforms (Fig. 1b).
It is useful to design qPCR primers that can detect all isoforms of the mRNA of interest. In this way the total expression of the gene as well as the expression of individual isoforms can be compared. In the hypothetical four-exon pre-mRNA (Fig. 1a), exons 1 and 2 are constitutive exons immediately adjacent to one another without any variable exons in between. By designing a forward primer in exon 1 and a reverse primer in exon 2, all mature mRNA isoforms generated from this pre-mRNA can be detected via PCR.
Primers should also be designed to amplify a reference “house-keeping” gene that is expected to be uniformly expressed in all samples. In this protocol we use primers amplifying a segment of human TATA-binding protein (TBP) (Table 1).
Primers should be designed with an ideal melting temperature (Tm) of approximately 55 °C. The primer length is generally 18–22 nucleotides. Tm of each primer can be estimated using online tools such as the oligoanalyzer of Integrative DNA Technologies (http://www.idtdna.com/analyzer/applications/oligoanalyzer). The amplicon for each primer pair should be between 80 and 150 base pairs.
As an example, we have depicted primer design to detect alternatively spliced isoforms of the CD44 gene that encodes a family of CD44 transmembrane proteins (Fig. 1b, Table 1). CD44 contains nine constitutive exons and nine variable exons, and in our example primers have been designed to detect isoforms containing CD44 variable exons 5–6 (CD44v5–6) and isoforms in which all variable exons have been skipped (CD44s).
Fig. 1.
Characterization of alternative splicing by qRT-PCR. (a) A schematic illustrating a hypothetical four-exon gene with a variable exon 3 that undergoes exon-inclusion or exon-skipping. Three primer sets for qPCR indicated by the paired arrows are designed to amplify the total mRNA, the variable exon 3 inclusion isoform, or the exon-skipping isoform. (b) A schematic depicting the exon structure of the transmembrane protein CD44, with the nine constitutive and nine variable exons labeled. CD44v3-v10 and CD44s are predominant splice isoforms and are depicted. Paired arrows indicate primer sets to amplify CD44v v5-v6 containing isoforms and the CD44s isoform which lacks all variable exons. (c) Representative qRT-PCR results using primers in Table 1 to show the relative expression of CD44v5/v6 and CD44s, normalized to TBP, in an immortalized human mammary epithelial cell line (HMLE)
3.4 Quantification of Splicing via qPCR
Prepare qPCR reactions in a total volume of 20 µL by combining 0.1–1.0 µL of cDNA generated from the RT reaction with 10 µL GoTaq® Green Master Mix and 5 pmol each of forward and reverse primers. Be sure to include reactions for the reference gene.
Perform triplicate qPCR reactions with 40–50 cycles of the following steps: 95 °C denaturation for 10 s, 58 °C annealing for 10 s, 60 °C extension for 30 s. Include a thermal denaturing step to generate dissociation curves that can be used to verify specific amplification of PCR products (see Note 3).
After the PCR is completed, the amplified signal from each reaction is presented as a threshold cycle (Ct) determined using an arbitrary detection threshold, usually set by the qPCR software to reside within the exponential range of PCR amplification. Relative quantification of splice isoforms is accomplished via comparison to expression of a reference gene such as TBP. Relative quantification assumes that the PCR amplification efficiency for each primer pair is perfect, resulting in doubling of PCR amplicons with every cycle. Since the reference gene expression remains constant between experimental samples, the quantity of mRNA for each splice isoform relative to the reference gene is calculated using the following formula: 2−∆Ct, where ∆Ct = (Ct splice isoform − Ct reference mRNA). As an example, quantification of two splice isoforms of CD44 was conducted via qPCR using RNA extracted from the immortalized human mammary epithelial cell line HMLE (Fig. 1c). The primers used are in Table 1.
Relative levels of splice isoforms of the same gene can also be expressed as a ratio of one splice isoform to another, for example a variable exon inclusion/variable exon skipping ratio. In such a scenario, a reference gene is not required because splice isoforms of the same gene from the same sample are compared. The ratio of splice isoforms is calculated using the following formula: 2−∆Ct, where ∆Ct = (Ct inclusion splice isoform mRNA − Ct skipping splice isoform mRNA in the same sample). Relative comparison of this ratio between different experimental samples is accomplished via the formula: 2−∆Ct(experiment)/2−∆Ct(control), where ∆Ct(experiment) = (Ct inclusion splice isoform mRNA in experimental sample − Ct skipping splice isoform mRNA in experimental sample) and ∆Ct(control) = (Ct inclusion splice isoform mRNA in control sample − Ct skipping splice isoform mRNA in control sample). In this way the fold change in inclusion/skipping ratio compared to the control sample is obtained.
3.5 Primer Design for Detection of Splice Isoforms via Semiquantitative PCR
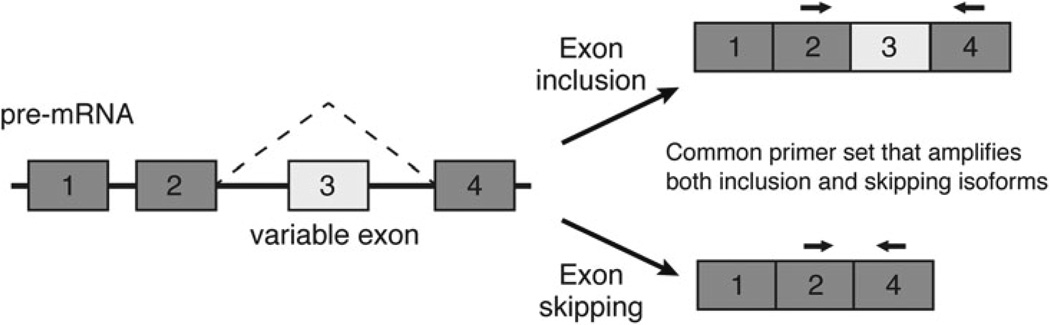
Through careful primer and PCR design, differences in splice isoform expression in cDNA generated from total RNA can be visualized using agarose gel electrophoresis. Returning to a hypothetical example of a four-exon pre-mRNA, a forward primer in constitutive exon 2 and a reverse primer in constitutive exon 4 will amplify differently sized PCR amplicons depending on whether or not the template includes variable exon 3 (Fig. 2). Using semi-quantitative PCR, these primers facilitate the amplification, separation via electrophoresis, and approximate quantification of different splice isoforms. Primers should be designed with an ideal Tm of 55 °C, and ideal amplicons should be no more than 300 base pairs long (see Note 4).
Fig. 2.
Semi-quantitative PCR for examination of exon skipping. A schematic is shown depicting a hypothetical four-exon gene with variable exon 3 undergoing alternative splicing. The paired arrows indicate primer sets that flank the variable exon 3 and thus amplify both variable exon-inclusion and skipping isoforms from a cDNA template. The different splice isoforms can be distinguished by size using semi-quantitative PCR and electrophoresis
3.6 Performing and Analyzing Semi-quantitative PCR
In a 20 µL reaction, combine 0.1–1.0 µL cDNA with 2 µL 10× PCR Buffer (Qiagen), 4 µL 5× Q-Solution (Qiagen), 0.5 µL HotStarTaq DNA Polymerase (Qiagen), and 5 pmol each of forward and reverse primers.
Complete PCR reaction using the following “hot start” program: 95 °C denaturation for 5 min, approximately 30 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, and lastly 72 °C for 10 min. Ensure that PCR is completed in the exponential range (see Note 5).
Visualize PCR reactions via horizontal gel electrophoresis using a 1.5 % agarose-TBE gel prepared with 0.5 µg/mL ethidium bromide (see Note 6). Detection of two distinct bands differing in size by exactly the length of the variable exon indicates a successful PCR amplification. The approximate quantity of each splice isoform present in the original RNA sample can be inferred using densitometric analysis by comparing the fluorescence intensity of the inclusion and skipping PCR amplicons using a UV transilluminator with camera and image intensity quantitation software (such as the BioRad Gel Doc XR system with Quantity One 1-D Analysis Software). Brighter intensity of the band is equivalent to higher expression of the splice isoform in the original RNA.
3.7 Generating a Splicing Minigene to Analyze Regulation of Alternative Splicing of a Variable Exon
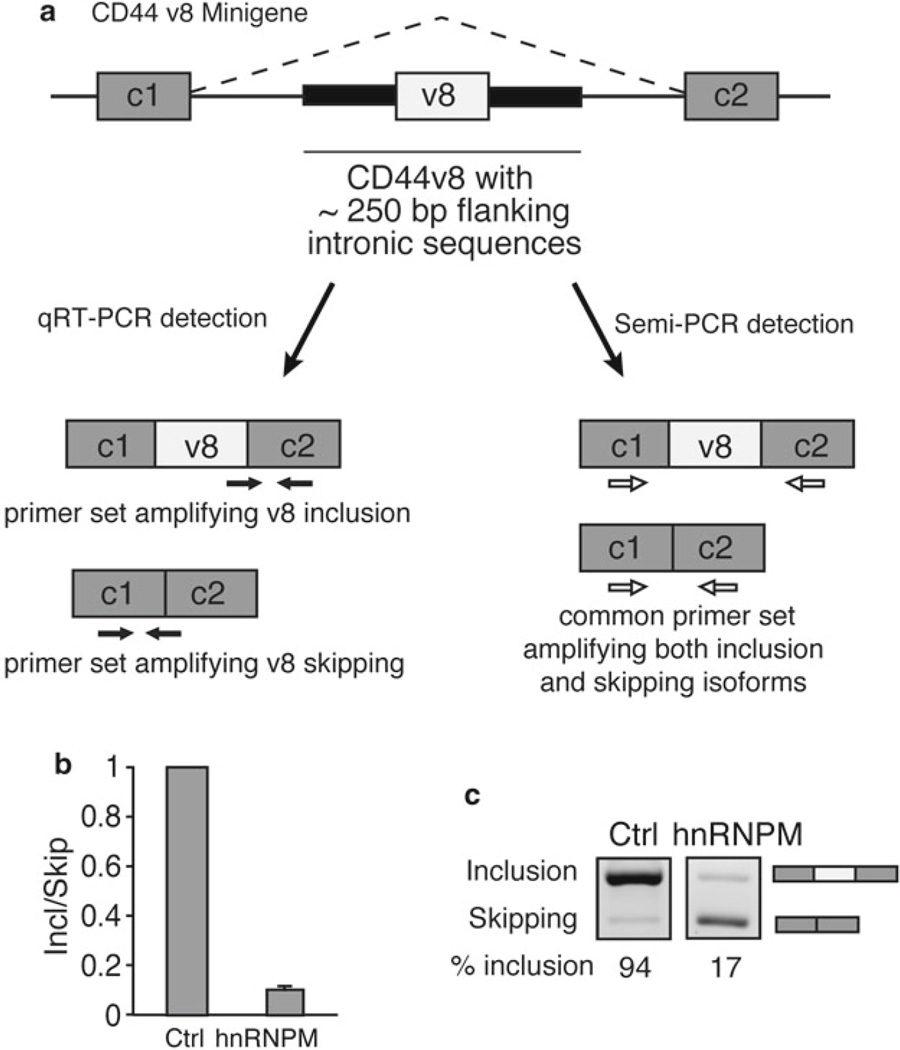
Splicing minigenes are useful tools to interrogate the regulation of splicing of a variable exon of interest. A splicing minigene is an expression plasmid consisting of a variable exon of interest flanked by two constitutive exons. It is important to include the immediately upstream and downstream intronic sequences flanking the variable exon where potential cis-acting sequences important for determining the inclusion or skipping of the exon may be located [7] (see Note 7). As an example, we have included a CD44 v8 minigene with variable exon 8 (v8) of CD44 bounded by the adjacent 250 base pairs of its upstream and downstream introns. This sequence is flanked by two constitutive exons from the human insulin gene with functioning 5′ and 3′ splice sites. Primers for detection of exon inclusion and exon skipping were designed in accordance with the principles mentioned in Subheadings 3.3 and 3.5 (Fig. 3a, Table 2).
Fig. 3.
A splicing minigene assay for characterization of exon skipping. (a) A schematic of the CD44 v8 minigene is shown. The CD44 v8 variable exon flanked by approximately 250 bp of upstream and downstream intronic sequence is cloned between constitutive exons c1 and c2. Exons are shown as boxes and introns as lines. Primer sets in black are used for qRT-PCR reactions. The primer set in white is designed for semi-quantitative methods. (b) qRT-PCR data after co-transfection of 100 ng CD44 v8 minigene with 400 ng of splicing factor hnRNPM using v8 inclusion and skipping primers in Table 2. A relative Inclusion/Skipping ratio (Incl/Skip) is plotted indicating that the Incl/Skip ratio decreases when hnRNPM is transfected. (c) Semi-quantitative PCR image showing decreased percent inclusion (% inclusion) after transfection with 400 ng hnRNPM using semiquantitative v8 primers in Table 2. Both images were from the same gel with uniform exposure time. % inclusion was calculated using densitometric image analysis software to divide the pixel intensity of the inclusion band by the combined pixel intensity of both inclusion and exclusion bands. Figure 3b and c was modified from Fig. 4 in our previous publication [8] under Creative Commons License CC-BY-NC 4.0
3.8 Investigating the Regulation of Alternative Splicing via Co-transfection of a Splicing Minigene and Splicing Factors
As the splicing minigene contains native cis-acting splicing regulatory sequences within or near the variable exon, trans-acting RNA binding proteins are capable of modulating inclusion or skipping of the variable exon in the minigene in a transfectable cell model. As an example, we describe co-transfection of the CD44 v8 minigene plasmid and a plasmid expressing splicing factor hnRNPM in the HEK293T mammalian cell line. In this case, hnRNPM promotes exon-skipping of the CD44 v8 minigene [8] (Fig. 3b, c).
In a tissue culture hood using aseptic technique, plate 2.25 × 105 HEK293T cells in 24-well tissue culture plates using DMEM containing 10 % FBS (see Subheading 2).
24 h after seeding cells, at which time the cells are approximately 90 % confluent, conduct transfections using Lipofectamine 2000 (Invitrogen) (see Note 8). Per well of a 24-well plate, combine 1.5 µL Lipofectamine 2000 with 50 µL plain DMEM (see Note 9) and incubate for 5 min at room temperature.
Combine Lipofectamine and media with transfecting plasmids diluted in 50 µL plain DMEM and mix well. As an example, transfect all wells with 100 ng CD44 v8 minigene, one well with a control plasmid such as pcDNA3, and other wells with splicing factors of interest such as hnRNPM. Each well should be transfected with a total of 800 ng of plasmid, so add in the remaining DNA with a control vector such as pcDNA3 (for example, a well could be transfected with 100 ng CD44 v8 minigene, 400 ng hnRNPM, and 300 ng pcDNA3). Incubate mixture for 20 min at room temperature.
During the above incubation, replace media on HEK293T cells with fresh DMEM containing 10 % FBS.
Add lipofectamine–transfectant mixture dropwise slowly over cells and incubate at 37 °C for 24 h (see Note 10).
Collect cells using RNA extraction protocol detailed in Subheading 3.1.
As an example, qRT-PCR data (Fig. 3b) and semi-quantitative RT-PCR data (Fig. 3c) from the CD44 v8 splicing minigene experiments were collected using primers in Table 2 [8] (see Note 11).
Acknowledgments
This work was supported by research grants from the US National Institutes of Health (R01GM110146) and the American Cancer Society (RSG-09-252-01-RMC) to C.C.
Footnotes
Using the E.Z.N.A.® Total RNA Isolation Kit (Omega BioTek), genomic DNA contamination is minimal. In addition, as described in Subheading 3, the primers are designed in different exons. This would eliminate PCR products amplified from genomic DNA which contains long intronic sequences. Therefore, the RNA may be used directly for RT-PCR analysis. If genomic DNA contamination is a concern, the manufacturer provides instructions for an on-column DNase treatment protocol. Also, during the elution step, we elute with RNAsefree H2O. If DEPC-treated H2O is used as an eluent, it can interfere with quantification of RNA via UV spectrometry [9].
Depending on the expression level of the gene of interest and the amount of different splice isoforms in the mRNA, the total input RNA into the Reverse Transcriptase reaction can be adjusted. Usually 250 ng of total RNA is sufficient, however up to 1 µg of RNA may be added to the reaction to increase the cDNA concentration without saturating the Reverse Transcriptase during cDNA synthesis.
The thermal denaturing step in qPCR is critical to determine the specific amplification of the target PCR product. The thermal denaturation curve for each pair of primers per reaction should show a single isolated peak with a uniform melting temperature. If multiple peaks with different melting temperatures are observed, then nonspecific amplification is occurring and the PCR reaction must be optimized before qPCR results can be analyzed [5]. Redesigning primers and adjusting the annealing temperature of the reaction may be necessary. We also recommend electrophoresing the PCR products on an agarose gel and visualizing the product using ethidiumbromide under UV. If amplification is specific, a single band of the appropriate size should be observed.
Designing primers so that the PCR amplicons for each splice isoform are relatively small (i.e., 300 base pairs or less) ensures that both the large and small amplicons are amplified with similar efficiency during the PCR reaction.
Accuracy of semi-quantitative PCR for visualizing the relative amounts of splice isoforms is dependent on the number of PCR cycles. The relative quantities of splice isoforms in a sample of mRNA remain proportional during the exponential phase of a PCR reaction, but this proportional relationship is less accurate in later PCR cycles once the reaction reaches the linear and plateau phases as PCR reagents and the polymerase are exhausted [5]. This is the reason qPCR offers more accurate quantification of expression compared to semi-quantitative PCR. It is important to perform a series of PCR cycles to identify the range of cycles where the proportional intensity of the different splice isoform bands remains constant [10]. This range also depends on the expression level of the gene in the original RNA sample. It is also recommended to conduct 32P-labeled PCR by adding 32P-labeled dCTP to the PCR reaction to increase the sensitivity of detecting bands on a gel. In 32P-labeled PCR, the total PCR cycle number can be reduced to approximately 20 cycles to ensure that results are captured during the exponential phase of the reaction.
To improve the resolution of PCR product bands, it may be necessary to increase to the concentration of the agarose gel to 2 %. Polyacrylamide gel electrophoresis (6 %) may also be used to resolve even smaller PCR products or resolve splice isoform bands that differ by a small size.
Splicing minigenes using constitutive exons not derived from the gene of interest offer a convenient tool for cloning different variable exons of interest into the same minigene construct. However, the actual exons flanking the variable exon may also be included in the minigene to better approximate the genomic cis-acting sequences present for native splicing of the gene. These cis-elements are located in both the variable exon of interest as well as adjacent introns [6].
When using the transfection reagent Lipofectamine 2000 (Invitrogen), it is essential that cells be at a high confluency of at least 90 %. This is to ensure that cells are still dividing to maximize uptake of transfected DNA while minimizing the impact of cell death that can occur during transfection.
Do not use DMEM containing serum when preparing transfection mixtures with the transfection reagent and transfected DNA. Serum proteins can interfere with the formation of the DNA-lipid transfection complexes and reduce transfection efficiency.
Although 24 h is usually sufficient to observe efficient expression of transfected DNA, cells can be incubated for up to 48 h to obtain even higher transgene expression.
To confirm protein transgene expression, for example transfected splicing factor expression constructs, Western blot analysis using cell lysates and an antibody specific for the protein of interest can be conducted. Efficient expression should result in an intense band on Western blot compared to control.
References
- 1.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40(12):1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Cheng C. Alternative RNA splicing and cancer. Wiley Interdiscip Rev RNA. 2013;4(5):547–566. doi: 10.1002/wrna.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keren H, Lev-Maor G, Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nat Rev Genet. 2010;11(5):345–355. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 5.Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26(1):112–122. 124–125. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Burge CB. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA. 2008;14(5):802–813. doi: 10.1261/rna.876308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper TA. Use of minigene systems to dissect alternative splicing elements. Methods. 2005;37(4):331–340. doi: 10.1016/j.ymeth.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Gao XD, Lee JH, Huang H, Tan H, Ahn J, Reinke LM, Peter ME, Feng Y, Gius D, Siziopikou KP, Peng J, Xiao X, Cheng C. Cell type-restricted activity of hnRNPM promotes breast cancer metastasis via regulating alternative splicing. Genes Dev. 2014;28(11):1191–1203. doi: 10.1101/gad.241968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto T, Okabe S. Ultraviolet absorbance at 260 and 280 nm in RNA measurement is dependent on measurement solution. Int J Mol Med. 2000;5(6):657–659. doi: 10.3892/ijmm.5.6.657. [DOI] [PubMed] [Google Scholar]
- 10.Horner RM. Relative RT-PCR: determining the linear range of amplification and optimizing the primers:competimers ratio. CSH Protoc. 2006;2006(1) doi: 10.1101/pdb.prot4109. [DOI] [PubMed] [Google Scholar]