Summary
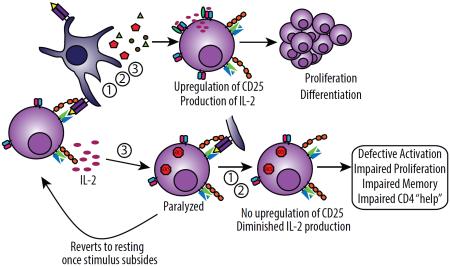
Primary T cell activation involves the integration of three distinct signals delivered in sequence: 1)antigen recognition, 2)costimulation, and 3)cytokine-mediated differentiation and expansion. Strong immunostimulatory events such as immunotherapy or infection induce profound cytokine release causing “bystander” T cell activation, thereby increasing the potential for autoreactivity and need for control. We show that during strong stimulation, a profound suppression of primary CD4+ T cell-mediated immune responses ensued and was observed across preclinical models and patients undergoing high-dose interleukin-2 (IL-2) therapy. This suppression targeted naïve CD4+ but not CD8+ T cells and was mediated through transient suppressor of cytokine signaling-3 (SOCS3) inhibition of the STAT5b transcription factor signaling pathway. These events resulted in complete paralysis of primary CD4+ T cell activation affecting memory generation, induction of autoimmunity, as well as impaired viral clearance. These data highlight the critical regulation of naïve CD4+ T cells during inflammatory conditions.
Introduction
Primary T cell activation is tightly regulated and requires three signals in sequence: “Signal 1” where T cell receptor (TCR) recognition of cognate antigen in the context of major histocompatibility complex (MHC) restriction occurs, “Signal 2” involving binding of costimulatory molecules, and “Signal 3” where cytokine “instructions” direct and amplify T cell differentiation and expansion. Lack of costimulation (Signal 2) following recognition of antigen (Signal 1) has been well-demonstrated to result in anergy and/or tolerance (Jenkins and Schwartz, 1987; Schwartz, 2003). Few studies have investigated the consequences of “out of order” signaling with regard to Signal 3 on naïve T cells which can readily occur under both acute and chronic inflammatory conditions. Naïve T cells tend to be refractory to cytokine signaling in the absence of TCR engagement (Geginat et al., 2001) in part due to decreased cytokine receptor expression compared to their resting, antigen-experienced, memory counterparts. While memory T cells undergo robust proliferation and activation marker upregulation in response to Signal 3 alone with various cytokines (ie., IL-2, IL-4, IL-6, IL-7, IL-12, IL-15, etc), naïve T cells typically only proliferate in response to interleukin-7 (IL-7) alone (Kimura et al., 2013). Despite their lack of proliferation, some studies have revealed that naïve T cells, even in the absence of TCR engagement, can show signs of responsiveness to cytokines through phosphorylation of signaling molecules (Perona-Wright et al., 2010) or upregulation of certain activation markers(Tough et al., 1999), although the ramifications from such signaling on subsequent responses remain poorly understood. Some studies suggest that local cytokine exposure during infection may play a polarizing role on naïve CD4+ T cells towards a certain T helper (Th) subset upon subsequent co-infection with a secondary pathogen (Perona-Wright et al., 2010). Other, studies suggest that elevated cytokine concentrations may play a role in propagating the exhaustion that occurs during chronic infections (Teijaro et al., 2013; Wilson et al., 2013).
Immune stimulatory therapies are being increasingly successfully applied as cancer treatments. We have previously illustrated the induction of bystander memory CD8+ T cells and their important role in tumor clearance in an antigen-nonspecific fashion during treatment with systemic immunostimulatory antibodies and cytokine-based immunotherapies (Tietze et al., 2012). Here we have investigated the effects of systemic immune stimulation on subsequent T cell receptor (TCR) mediated responses. In concerted fashion, and concomitant with the expansion of lytic bystander memory CD8+ T cells, we observe a profound loss in the ability of treated mice to mount primary T cell responses following systemic immunotherapy which centered on the lack of CD4 responsivenss to TCR engagement. This complete loss of primary T cell function in vivo also corresponded with acute thymic involution. The in vivo consequences of CD4+ T cell paralysis were considerable, leading to impaired vaccination responses and viral clearance from lack of CD4+ T cell help. This paralysis of naïve CD4+ T cells was transient, resolving within two weeks of cessation of immunotherapy, and was mediated, in part, through suppressor of cytokine signaling-3 (SOCS3) inhibition of Janus kinase (JAK)-STAT signaling pathways. Taken together, these studies have profound implications for cytokine-based therapies for cancer and provide insight into the functional mechanisms that can be exploited as possible interventions for the induction of impaired T cell immunity observed during periods of strong immune stimulation that occur not only during cancer immunotherapy but during sepsis and chronic viral infection as well.
Results
Cytokine pre-exposure selectively impairs both murine and human primary CD4+ T cell immunity
High cytokine environments predominate locally during inflammation as well as during periods of strong immune stimulation that can occur during sepsis or cancer immunotherapy. We and others have demonstrated the induction and functional significance of cytokine-induced, antigen-nonspecific “bystander activation” of memory CD8+ T cells in murine models (Tietze et al., 2012) (Figure 1a) and human patients undergoing immunostimulatory therapies for cancer (Tietze et al., 2012) (Figure 1b) as well as their similar phenotype and role during viral infections(Chu et al., 2013; Tietze et al., 2013). These expanded memory CD8+ T cells were directly activated by high cytokine concentrations instead of antigen. Thus they failed to upregulate markers consistent with TCR engagement, such as CD25, yet expressed other effector molecules such as NKG2D which confer the ability to recognize and attack targets in an antigen non-specific, NK-like fashion. We sought to investigate the effects of systemic immune stimulation, essentially out of sequence Signal 3, on subsequent primary TCR-mediated responses by the naïve T cell pool. The phenotypes of memory and/or activated (CD44high) and naïve (CD44low) T cells in control and anti-CD40 and IL-2 immunotherapy treated mice are extensively described in Figure S1. While there were clear differences in memory and activated populations, naïve T cell phenotypes, in both CD4+ and CD8+ T cells, were indistinguishable between control and immunotherapy-treated groups.
Figure 1. Impaired CD4 responses to antigen following systemic inflammatory stimuli.
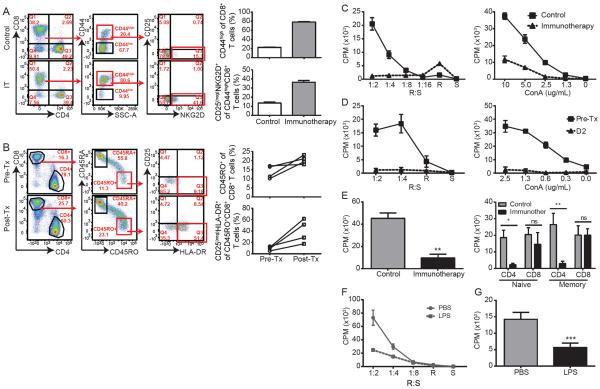
(A–B) Representative dot plots from (A) murine splenotypes or (B) human PBMCs pre and post treatment with immunotherapy for murine cells or systemic high dose IL-2 for human samples. Quantification of percent memory phenotype and percent bystander are shown for each. (C) Splenocytes or (D) Flow cytometry-sorted T cell populations from mice that were treated as controls or with systemic immunotherapy were harvested at day 12 of immunotherapy and stimulated ex vivo with irradiated allogeneic splenocytes (MLR) or concanavalin (Con)-A at indicated doses. Data are shown as counts per minute (CPM). (E) PBMCs from metastatic melanoma patients undergoing systemic high dose IL-2 (600,000 IU/kg iv bolus every 8hrs for up to 14 maximum doses) were collected prior to IL-2 treatment or on day 2 following IL-2 initiation and subjected to MLR or ConA stimulation at indicated doses. (F) Whole splenocytes or (G) sorted naïve CD4+ T cells from LPS-treated mice on day 2 and subjected to MLR. (Data are representative of 2–5 independent experiments with 2-3 mice per group for murine studies or 4–9 human samples for human studies. Statistical analysis was performed using Two-Way ANOVA with Bonferroni's post-test or Student's t Test where appropriate. *p<0.05, **p<0.01, ***p<0.001. See also supplemental figures S1–S4.
Given the profound state of memory CD8+ T cell hyperactivation and the differential effects on CD4+ and CD8+ T cells that occured following systemic immunotherapy, we assessed how primary T cell responses to TCR-mediated stimulation may be affected. To evaluate this, we tested mixed lymphocyte reaction (MLR) responsiveness at the peak of immunotherapy on splenocytes. A profound decrease (p<0.001) in proliferative capacity (Figure 1c) of the recovered T cells from mice receiving immunotherapy occurred. Markedly diminished T cell proliferation in response to the mitogen Concanavalin A (ConA) (Figure 1c), anti-CD3, and anti-CD3 and anti-CD28 (Figure S2a–b) was also observed following combination immunotherapy or with either agent (anti-CD40 or IL-2) singly (Figure S2c). This marked T cell hyporesponsiveness was observed following systemic lipopolysaccharide (LPS) administration in vivo to mimic sepsis (Figure 1f and Figure S2d). Blocking inhibitory pathways such as CTLA-4 (Figure S2e), depleting regulatory T cells (Figure S2f), adrenalectomizing to reduce glucocorticoid-mediated inhibition (Fig S2g), or even in the absence of interferon(IFN)-γ signaling (Figure S2h–i), which we have previously shown to rescue CD4+ T cells from activation induced cell death (AICD) during immunotherapy (Berner et al., 2007), did not reverse the primary T cell hyporesponsiveness. To confirm the translatability of this phenomenon, we assessed peripheral blood mononuclear cells (PBMCs) from human patients with advanced metastatic melanoma both prior to and while undergoing high-dose IL-2 therapy. Importantly, paralleling the murine data, profound T cell impairment was also observed using in these patients (Figure 1d) confirming that primary response blunting occurred in both mice and humans. These results demonstrate that global T cell activation in which bystander memory expansion occurs comes at the expense of primary T cell responsiveness such that even strong stimuli such as allogeneic responses or mitogen responses are become impaired in both mouse and humans.
To confirm whether the lack of responsiveness was due to an intrinsic T cell mechanism or the result of external factors contributed by suppressive cell types and/or varying cell frequencies, we performed the same proliferation assays on sorted T cell populations. Sorting of splenic memory (CD44high) or naïve (CD62L+CD44low) T cell populations revealed that the impairment primarily affected naïve (p<0.05) and memory (p<0.01) CD4+ T cells (Figure 1e and Figure 1g) but not CD8+ T cells after in vivo immunotherapy or LPS. This effect was also mimicked in flow based proliferation assays using BrdU incorporation as a measure of proliferation (Figure S3a). In addition to deficiencies in proliferation, CD4+ T cells from immunotherapy-treated mice also produced less IFN-γ than CD8+ T cells (Figure S3b). Importantly, this was not due to alternative polarization patterns as CD4+ T cells from immunotherapy-treated mice also exhibited minimal skewing towards any Th (Th1, Th2, Th17, Tfh, and Treg were assessed by transcription factors) population upon restimulation (Figure S3c). Furthermore, the minimal polarization that was present was diminished compared to what was observed in control treated mice (Figure S3c). Consistent with the minimal expression of transcription factors, expression of IFN-γ, IL-4, nor IL-17 could be detected in these cells (data not shown) and IL-2 expression was significantly diminished compared to controls (Figure S3d). Importantly, supplementation of MLRs utilizing sorted naïve CD4+ T cells as responders with exogenous IL-2, although it slightly improved the proliferative response, did not fully restore the hyporesponsiveness induced by strong immune stimulation (Figure S3e).
Because it has previously been shown that there can be a period of stimulation refractoriness following activation that can be partially accounted for by down modulation of CD3, we evaluated cell size and CD3 expression of memory and naïve T cells during immunotherapy. Naïve CD4 and CD8+ T cells from immunotherapy treated mice and humans undergoing high dose IL-2 were comparable in size (FSC), granularity (SSC), CD3 expression, and CD4/8 co-receptor expression compared to control treated counterparts or patients prior to immunotherapy (Figure S4). In contrast, memory phenotype (CD44high) CD4 and CD8+ T cells displayed differences in size and CD3 expression that may account for the decrease in responsiveness in sorted memory CD4+ T cells. Furthermore, we have previously demonstrated that memory CD4+ T cells undergo apoptosis following immunotherapy in mice through a mechanism of activation induced cell death (AICD) (Berner et al., 2007), thus we hypothesized that some of the underlying mechanisms may play a role in the lack of responsiveness in the memory CD4 subset. The direct effect on the naïve CD4+ T cell subset was unexpected and contrary to current paradigms in which naïve T cells are thought to exhibit only minimal responses to cytokine exposure in the absence of TCR binding to cognate antigen. Thus, we focused on the mechanisms underlying the impairment in the naïve CD4+ T cell population.
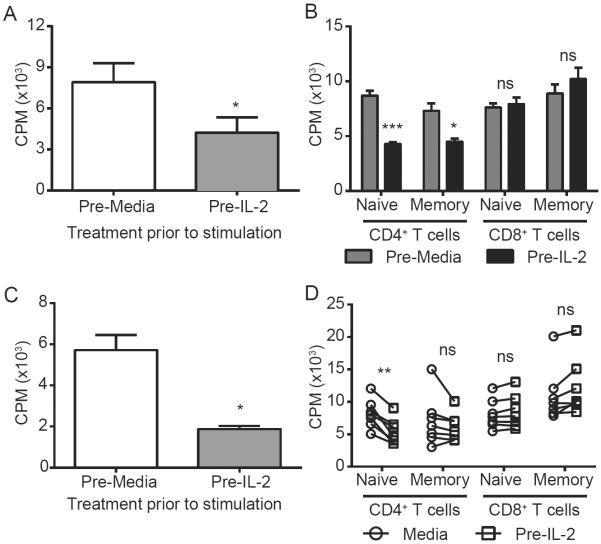
Both the systemic immunotherapy and sepsis models are hallmarked by elevated systemic pro-inflammatory cytokines (Figure S5a–b). It has previously been shown that exposure to high dose cytokine can directly impair murine T cell activation in vitro (Zhao et al., 2010). We therefore investigated the direct role of cytokines in inducing hyporesponsiveness to TCR-mediated stimuli following strong immune stimulation by comparing murine (Figure 2a–b) and human (Figure 2c–d) T cell proliferative capacity in vitro following pre-incubation with IL-2. Similar to the effects observed with T cells recovered from treated mice in vivo, pre-exposure of sorted murine or human naïve CD4, but not CD8, T cells to IL-2 in vitro also resulted in blunted responses to MLR and ConA induced proliferation further suggesting that this hyporesponsiveness is a direct physiological response to cytokine pre-exposure conserved across species.
Figure 2. Impaired CD4 responses to antigen following in vitro pre-incubation with cytokine.
Naïve CD4+ T cells were purified by magnetic separation from murine splenocytes (A–B) or from human PBMCs (C–D), pre-incubated with media or high dose IL-2, washed, and subjected to MLR (1:1 R:S) or ConA (1.25ug/mL) stimulation. Graphs with error bars indicate mean±SEM. Panel D, bars link individual responses from a given sample. Data are representative of 2-3 independent experiments 5–7 human samples for human studies. Statistical analysis was performed using Two-Way ANOVA with Bonferroni's post-test or Student's t Test where appropriate. *p<0.05, **p<0.01, ***p<0.001. See also supplemental figure S5.
Naïve CD4+ T cell impairment following cytokine exposure is transient
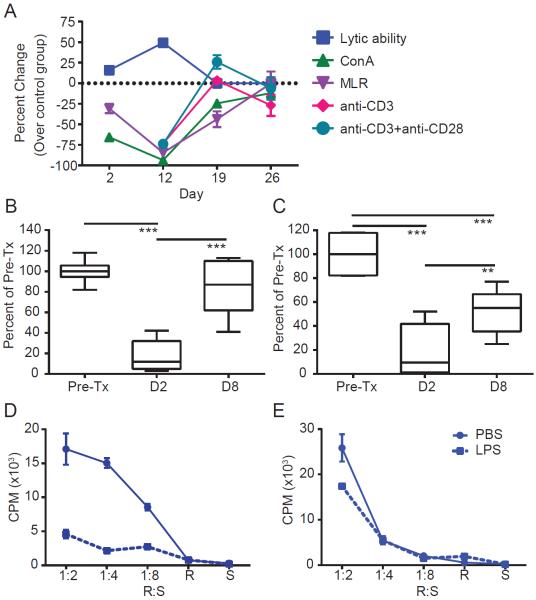
The impairment of TCR-mediated proliferation in naïve CD4+ T cells occurred early during treatment becoming apparent as early as day 2. Interestingly, the effect was transient as it resolved within 2 weeks of cessation of treatment and strongly coincided with the emergence of lytic activity in antigen nonspecific, bystander activated memory CD8+ T cells (Figure 3a). The transience of the effect appeared to be due to withdrawal of therapy as repeated treatment with a second course of the regimen resulted in prolonged impairment of primary CD4+ T cell responses as assessed by MLR (Figure S5c–e). PBMCs from human patients undergoing high dose IL-2 therapy revealed a similar transience in MLR and mitogen responsiveness (Figure 3b–c) as did our sepsis model (Figure 3d–e).
Figure 3. Impaired responses to TCR stimulation recover following cessation of stimulation.
(A) Percent change of immunotherapy treated compared to average control at indicated time points for CTL lytic ability at 50:1 E:T, MLR @ 1:2 R:S, ConA at 5ug/mL, anti-CD3 @ 10ng/mL, anti-CD3 and anti-CD28 at 10ng/50ng per mL respectively. (B–C) PBMCs from metastatic melanoma patients undergoing systemic high dose IL-2 (600,000 IU/kg bolus every 8hrs as tolerated for a maximum of 14 doses) were collected prior to IL-2 treatment or on days 2 and 8 following IL-2 initiation and subjected to MLR (1:8) or ConA (2.5ug/mL) stimulation. (D–E) Mice were administered LPS systemically and harvested at (D) day 2 and (E) day 7 at which point MLRs were performed on splenocytes. All murine graphs indicate mean±SEM. Human data is shown as box whisker plot, bars indicate min to max. Data are representative of at least 2 independent experiments. Statistical analysis was performed using Two-Way ANOVA with Bonferroni's post-test or Student's t Test where appropriate. *p<0.05, **p<0.01, ***p<0.001. See also supplemental figure S6.
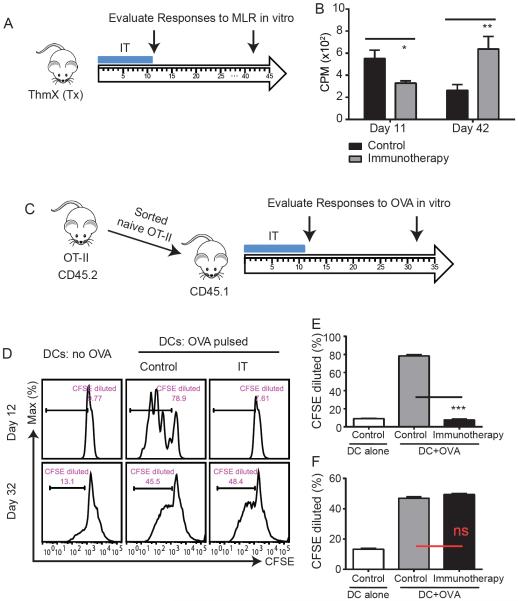
Given that the impaired naïve CD4+ T cell responsiveness was not permanent, we sought to determine whether the effect on naïve CD4+ T cells was truly transient or whether new thymic output accounted for the gain back of function. We treated thymectomized (ThmX) mice with immunotherapy and evaluated MLR responses ex vivo during immunotherapy or at day 42, a time point in which kinetic studies revealed that MLR hyporesponsiveness is resolved (Figure 4a). During treatment with immunotherapy, ThmX mice were also hyporesponsive to MLR stimulation (Figure 4b) which resolved by day 42, illustrating restoration of naïve CD4+ T cell function in the absence of thymic output and demonstrated that the naïve CD4+ T cells exposed to therapy were capable of recovering function.
Figure 4. Naïve CD4 paralysis following strong immune stimulation is transient.
(A–B) Thymectomized mice were treated with immunotherapy and splenocytes were harvested and subjected to functional assays. (A) Experimental schema. (B) At day 11 or 42 post initiation of treatment, splenocytes were harvested and subjected to MLRs. (C–E) Naïve ova-specific CD4+ T cells were flow cytometry-sorted from OT-II TCR transgenic mice (CD45.2) and adoptively transferred into congenic Ly5.2 (CD45.1) mice. Congenic mice were treated with immunotherapy and adoptively transferred naïve OT-II cells were subjected to downstream functional assays. (C) Experimental schema. (D–E) CD4+ T cells were purified from congenic recipients at indicated time points, stained with CFSE, and cultured in the presence of OVA-pulsed BMDCs for 72hrs. After 72h, dilution of CFSE in adoptively transferred (CD45.2) OT-II cells was assessed. All murine bar graphs indicate mean±SEM. Data are representative of at least 2 independent experiments. Statistical analysis was performed using Two-Way ANOVA with Bonferroni's post-test or Student's t Test where appropriate. *p<0.05, **p<0.01, ***p<0.001.
To definitively assess the duration of the naïve CD4+ T cell hyporesponsiveness, we performed an adoptive transfer of sorted, naïve CD4+ T cells from TCR transgenic mice (OTII: OVA-specific, CD45.2+) into congenic CD45.1 mice, treated with immunotherapy, and evaluated responses to OVA in the transferred congenic cells utilizing CFSE dye dilution as a measure of proliferation (Figure 4c). Adoptively transferred OT-II CD4+ T cells from immunotherapy-treated mice were profoundly impaired in response to OVA challenge compared to control mice (p<0.001) whereas the two groups were indistinguishable at day 32 confirming the transient nature of the antigen-specific functional deficiency (Figure 4d–f). Thus, in contrast to permanent anergy or tolerance, which can occur from lack of Signal 2 during T cell priming, out of sequence Signal 3 induces a period of short-term hyporesponsiveness, or paralysis, of naïve CD4+ T cells that reverts once the inflammatory stimulus recedes if cognate antigen is not encountered.
Naïve CD4+ T cell paralysis is hallmarked by impaired JAK-STAT signaling and SOCS3 upregulation
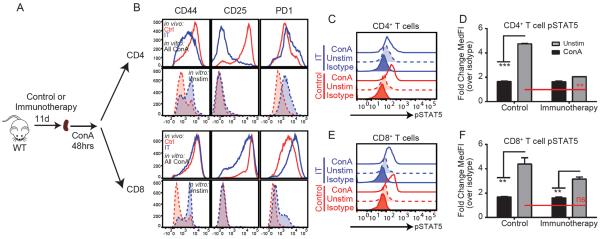
We next sought to better understand the molecular mechanisms underlying the naive CD4+ T cell paralysis. Because sorting experiments revealed that immunotherapy-treated CD8+ T cell populations were behaving similarly to controls upon subsequent stimulation through TCR, we compared the expression of activation markers in CD4+ and CD8+ T cells following in vitro stimulation after immunotherapy. Typically, in response to TCR ligation, murine lymphocytes will upregulate CD44, CD25 (a component of the high affinity IL-2 receptor), and the inhibitory receptor PD-1, among other acute activation markers. Following ex vivo stimulation with ConA (Figure 5a), both CD4+ and CD8+ T cells from control mice exhibited robust upregulation of CD44, CD25, and PD-1. Conversely, while CD8+ T cells from immunotherapy treated mice comparably upregulated all three activation markers, CD4+ T cells expressed far less CD25 than control counterparts (Figure 5b).
Figure 5. Paralyzed CD4+ T cells have impaired JAK-STAT signaling upon restimulation through TCR engagement.
Splenocytes from control (red) or immunotherapy (blue) treated mice were restimulated ex vivo with ConA to mimic TCR engagement. (A) Experimental schema. (B) Activation marker upregulation in CD4 and CD8+ T cells following restimulation with anti-CD3/28. (C) Representative histograms and (D–E) fold change of pSTAT5b on CD4 and CD8+ T cells following restimulation with ConA. All bar graphs indicate mean±SEM. Data are representative of 3–4 independent experiments with 2-3 mice per group. Statistical analysis was performed using One or Two-Way ANOVA with Bonferroni's post-test where appropriate. *p<0.05, **p<0.01, ***p<0.001.
CD25 is a component of the high affinity IL-2 receptor conferring heightened sensitivity to low IL-2 concentrations (Boyman et al., 2006). Following TCR engagement, an extensive activation program ensues in which several genes, including CD25 (Willerford et al., 1995), are induced. Autocrine IL-2 signaling resulting in STAT5b phosphorylation post TCR engagement is required to stabilize the expression of CD25 allowing for complete generation of the T cell activation program (Verdeil et al., 2006). Similar to the paralysis phenotype observed herein, T cells from STAT5b−/− mice display reduced proliferative responses to TCR engagement as well (Moriggl et al., 1999). We hypothesized that this pathway may be affected during immunotherapy-induced paralysis. To investigate this, we evaluated STAT5b phosphorylation in control and treated CD4+ T cells following ex vivo stimulation with ConA. Following stimulation, CD4+ T cells from control mice exhibited significant (p<0.001) phosphorylation of STAT5b and upregulation of CD25 while STAT5b phosphorylation and CD25 expression did not significantly differ between unstimulated and stimulated CD4+ T cells from immunotherapy treated mice. Conversely, CD8+ T cells from both control and immunotherapy-treated mice exhibited rapid, comparable phosphorylation of STAT5b following ex vivo stimulation with ConA (Figure 5c–f).
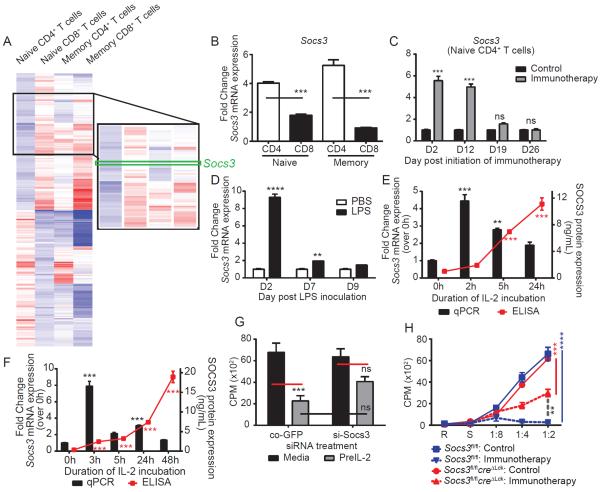
Genetic alterations observed in the different T cell populations using microarray analysis on the sorted T cell subpopulations revealed an assortment of genes differentially expressed between naïve CD4+ and CD8+ T cells following immunotherapy (Figure 6a and Figure S6a–b). Overall, genes associated with negative regulation of immune responses, negative regulation of signaling, and cell death and/or apoptosis were upregulated in CD4+ T cells while genes involved with immune activation and proliferation were more highly expressed in naïve CD8+ T cells. Socs3 was upregulated in both naïve and memory CD4+ yet not naïve or memory CD8+ T cells after immunotherapy. JAK-STAT-dependent cytokine signaling pathways are regulated by the suppressors of cytokine signaling (SOCS) (Yoshimura et al., 2007) family. SOCS1, SOCS3, and CIS have been implicated in modulation of T cell immunity and have been shown to be induced directly by IL-2 (Palmer and Restifo, 2009), among other cytokines. We therefore validated the array data with qPCR by evaluatuating the various T cell populations for expression of Cish, Socs1, and Socs3. Cish and Socs1 expression did not vary among subsets (Figure S6c), but Socs3 was highly upregulated in both naïve and memory CD4+ T cells but not in naïve or memory CD8+ T cells (Figure 6b), mirroring the functionality of the cells. Elevated Socs3 expression was detected in naïve CD4+ T cells whenever functional paralysis occurred: following high dose IL-2 alone (Figure S6d), in the absence of IFN-γ (Figure S6e), following adrenalectomy (Figure S6f) and following systemic LPS (Figure 6d). The kinetics of Socs3 expression strongly coincided with the onset of paralysis as it was detectible by day 2 and reverted to control levels within one week of treatment cessation (Figure 6c). A similar expression pattern of Socs3 in naïve CD4+ T cells was also noted following LPS exposure (Figure 6d, presumably through exposure to IL-6 induced by LPS). Furthermore, Socs3 expression was significantly increased in both human (Figure 6e) and murine (Figure 6f) naïve CD4+ T cells following paralysis-inducing IL-2 exposure and IL-6 (Figure S7) exposure in vitro. Interference of the Socs3 gene, either through ex vivo silencing of Socs3 in purified naïve CD4+ T cells (Figure 6g) or in vivo using a transgenic mouse model (Socs3fl/flcreΔlck) partially restored proliferative responses to MLR (Figure 6h) indicating the importance of this suppressive pathway on down-regulating naïve CD4+ T cell responses following out of sequence Signal 3.
Figure 6. SOCS3 is elevated and contributes to paralyzed phenotype of CD4+ T cells during strong immune stimulation.
(A) Microarray showing clustering of genes differentially expressed in naïve CD4 and CD8+ T cells in all T cell populations. (B) Naïve and memory CD4 and CD8+ T cell populations were sorted from splenocytes of control or immunotherapy treated mice and fold change in SOCS3 gene expression was quantified by qPCR. Expression of SOCS3 in flow cytometry-sorted naïve CD4+ T cells from (C) immunotherapy or (D) LPS treated mice at indicated time points. Naïve T cells were purified from (E) human PBMCs or (F) murine splenocytes using magnetic bead separation and incubated with high dose rhIL-2. At indicated time points, Socs3 expression was quantified by qPCR (bars) and ELISA (red lines). (G) Magnet purified murine, naïve CD4+ T cells were transfected with scrambled GFP tagged siRNA or Socs3 siRNA, incubated in high dose IL-2, and subjected to MLR (1:1). (H) Purified naïve CD4+ T cells from control or immunotherapy treated SOCS3fl/fl or Socs3fl/flcreΔLck mice were subjected to MLR. All graphs indicate mean±SEM. Data are representative of 2–4 independent experiments with 2–3 mice per group for murine studies or 5 human samples for human studies. Statistical analysis was performed using One or Two-Way ANOVA with Bonferroni's post-test where appropriate. *p<0.05, **p<0.01, ***p<0.001. See also supplemental figures S6–7.
CD4 paralysis results in impaired memory generation, viral clearance and protects from induction of autoimmunity in vivo
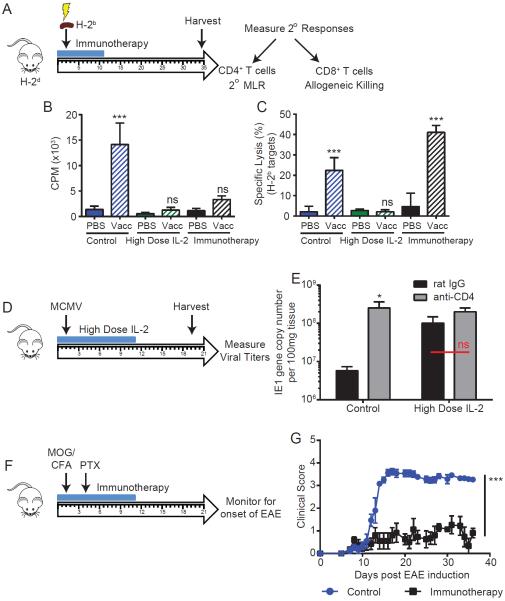
We next evaluated the extent and immunological consequences of primary CD4+ T cell paralysis due to out of sequence Signal 3 stimulation in vivo. Mice were immunized with irradiated allogeneic splenocytes while undergoing immunotherapy and evaluated for secondary responses long after immunotherapy had ended (Figure 7a). Since the immunotherapy regimen utilizes agonistic anti-CD40 which has been demonstrated to bypass the need for CD4 help in the activation of CD8+ T cells (Schoenberger et al., 1998), we included an IL-2 alone cohort as we have observed naïve CD4+ T cell paralysis and Socs3 upregulation occur during this regimen as well (Figure S2c and S6d). Strong secondary CD4+ and CD8+ T cell responses were observed in control mice (p<0.001). Following immunotherapy, secondary MLR responses were totally absent while CD8+ T cell recall responses to allogeneic targets were comparable (p<0.001), if not greater than, control counterparts consistent with the role of anti-CD40 in bypassing the need for CD4+ T cell help. In contrast, vaccination with allogeneic targets during high dose IL-2 administration resulted in both absent CD4+ and CD8+ T cell secondary allogeneic responses highlighting the profound impact of this paralysis on T cell responses overall(Figure 7b–c).
Figure 7. Engagement of cognate antigen during paralysis inducing conditions results in impaired CD4 and CD8 priming and memory formation in vivo.
(A–C) Balb/c mice were treated as controls or with immunotherapy or high dose (high dose) IL-2. On day 2 of treatment, mice were inoculated with 10×106 irradiated C57BL/6 splenocytes ip. On day 35 following treatment, splenocytes were harvested and evaluated for functional assays. (A) Schema of experiment. (B) Splenocytes were harvested and subjected to secondary MLR. (C) CD8+ T cells were enriched from splenocytes by magnetic bead purification and restimulated with irradiated allogeneic splenocytes for 4 days. On day 4, lysis of H-2b specific targets (C1498 line) was measured by standard 4-hr chromium-51 release assay. (D–E) C57BL/6 mice were treated with high dose IL-2 and infected with murine cytomegalovirus (MCMV) on day 2 of treatment (D). (E) Salivary gland viral titers as assessed by viral IE1 gene copy number were measured on day 19 following treatment. (F–G) C57BL/6 mice were treated with immunotherapy and induction of EAE was initiated on day 2. Mice were then monitored for onset of neurological symptoms using a five-point scale (limp tail or waddling gait = 1; limp tail and waddling gait = 2; single limb paresis and ataxia 2.5; double limb paresis = 3; single limb paralysis and paresis of second limb = 3.5; full paralysis of 2 limbs = 4; moribund = 4.5; and death = 5). All graphs indicate mean±SEM. Data are representative of 1–2 independent experiments with 4–6 mice per group. Statistical analysis was performed using One or Two-Way ANOVA with Bonferroni's post-test where appropriate. *p<0.05, **p<0.01, ***p<0.001.
We next sought a more physiologic inflammatory model to assess the impact of the T cell paralysis. Many acute viral models do not require CD4+ T cells to facilitate resolution of infection (Johnson et al., 1999; Matloubian et al., 1994; Stevenson et al., 1998). Murine cytomegalovirus (MCMV) infection has been described to occur similarly in CD4 sufficient and depleted hosts with the exception of an elevated reservoir of virus in the salivary glands of depleted hosts after 14 days of infection(Jonjic et al., 1989). For this model, we initiated high dose high dose IL-2 therapy, infected the mice with MCMV on day 2 of treatment, and evaluated viral loads in the salivary glands at day 17p.i. (Figure 7d). NK cells were depleted in vivo prior to treatment to eliminate any compensatory effect activated NK cells may have had on viral loads. Mice receiving high dose IL-2 showed reduced viral clearance in the salivary gland (Figure 7e), comparable to that of CD4+ T cell depleted control and CD4 depleted high dose IL-2-treated mice indicating viral resistance impairment due to the T cell paralysis.
One potential purpose for CD4+ T cell paralysis during systemic immune activation is in the protection against the induction of autoimmunity to self-antigens. To assess this possibility we utilized the experimental autoimmune encephalitis (EAE) model of multiple sclerosis which is highly CD4+ T cell-dependent in susceptible strains. Induction of EAE was initiated on day 2 of immunotherapy (Figure 7f) and mice were then evaluated for the onset of EAE. It is worth noting that early symptoms of EAE closely mimicked the symptoms of immunotherapy-related toxicities that occur during treatment (ie., slow walk or waddle, poor tail posture, hunching, etc). Mice receiving immunotherapy did not develop EAE as evidenced by clinical scores that were sustained around 1 (consistent with treatment related toxicities) whereas control mice had clinical scores averaging 3.5–4.0 (consistent with onset of EAE) (Figure 7g) providing further evidence of impaired primary CD4+ T cell-mediated immunity during immunotherapy. Taken together, these results demonstrate that out of sequence Signal 3 induced naïve CD4+ T cell paralysis not only occurs ex vivo, but can result in impaired T cell-mediated activation, secondary responses, and viral clearance in vivo.
Discussion
It is critical that T cell activation and expansion be tightly controlled, particularly with CD4+ T cells in instances of strong inflammatory responses. This is evidenced by the “three signal” model of naïve T cell activation. Lack of co-stimulation, or Signal 2, following TCR engagement can result in anergy of naïve T cells. We now demonstrate that both human and mouse naïve CD4 but not CD8+ T cells were further regulated by exposure to cytokine (Signal 3) in the absence of TCR engagement resulting in a transient form of anergy that we have termed paralysis. During paralysis, cytokine exposure induces expression of suppressor of cytokine signaling-3 (SOCS3) which stifles primary CD4 activation culminating in an inability to mount CD4-dependent adaptive responses both in vitro and in vivo (graphical abstract). SOCS3 represents a critical, although likely not sole, pathway in inducing the CD4+ T cell paralysis or anergy. This paralysis is transient and contingent upon continued cytokine exposure of the CD4+ T cells.
Paralysis occurred rapidly within two days of the initiation of treatment and was accompanied by marked acute thymic involution (data not shown). It is widely accepted that corticosterone plays an important role in acute thymic involution and previous studies have shown that corticosterone can induce SOCS3 expression as well (Madiehe et al., 2001). However, it appears corticosterone did not play a major role in paralysis as 1) corticosterone elevation did not mimic the pattern of paralysis in vivo, 2) adrenalectomy did not improve responsiveness of naïve CD4+ T cells despite normalization of corticosterone responses, and 3) more importantly, SOCS3 remained elevated during adrenalectomy following immunotherapy. Nevertheless, while it may not play a direct role in the development of naïve CD4+ T cell paralysis, thymic involution as a result of corticosterone elevation may potentially impact naïve T cell responses through delay in the output of new naïve T cells which likely further contributes to the delay in the total recovery of naïve CD4+ T cell responses post stimulation. This would be of particular concern in aged recipients where thymic output is already diminished.
There appear to be fundamental differences between CD4 and CD8+ T cells with regard to this suppression by out of sequence Signal 3, likely due to the pivotal nature of CD4+ T cells in orchestrating and amplifying immune responses in general. This hints toward alternative activation and regulatory pathways between naïve CD4+ and CD8+ T cells. Indeed, microarray data presented herein showed global differences in gene expression patterns. Evidence suggesting differential phenotype, function, and viability between activated CD4+ and CD8+ T cells following various immunostimulatory regimens exists(Berner et al., 2007). While differences in CD44high CD4+ and CD8+ T cells appears to be due to IFNγ-mediated activation induced cell death (AICD), in naïve T cells this appeared to not be the case as IFNγ-receptor(R)−/− mice still exhibit paralysis following therapy. CD4+ T cells represent the ideal target as conventional dogma holds that CD4+ T cell activation is essential to productive and sustained adaptive responses (including humoral responses). While CD8 responses can be generated in the absence of CD4+ T cells, it has been shown that these “helpless” CD8+ T cells often go on to form impaired memory (Sun and Bevan, 2003).
The induction of the CD4+ T cells paralysis paradoxically correlated with the expansion of “bystander” phenotype CD8+ T cells and non-specific lytic capacity in immunotherapy associated with successful initial anti-tumor effects. We and others have previously shown that these “bystander” activated CD8+ T cells perform their function in a more “NK-like” fashion using alternative receptors such as NKG2D for targeting transformed and virally infected cells rather than TCR mediated recognition (Chu et al., 2013; Sckisel et al., 2014; Tietze et al., 2011). Thus, the induction of bystander memory T activation, which is driven by cytokines and not antigen, necessitates the emergence of another control pathway which suppresses naïve CD4+ T cell expansion even with TCR triggering resulting in paralysis of primary T cell responses. In our model we have observed that both naïve and memory CD4+ T cells undergo this paralysis. As the cytokine milieu declines and bystander activation wanes so does the SOCS3-mediated suppression of the naive CD4+ T cells and restoration of primary T responses ensue.
The implications and practical application of these findings are considerable. The immunotherapy regimens employed result in successful anti-tumor effects but these data suggest that long-term maintenance or responses to epitope-spreading will likely be impaired. Indeed, few correlations have been uncovered linking tumor-specific T cell generation and objective tumor responses with many immunotherapies (Attia et al., 2005; Comin-Anduix et al., 2008; Maker et al., 2005; Tietze et al., 2011). Our vaccination model suggests that while pre-existing CD8 immunity may remain intact, the development of de novo responses to novel tumor antigens during immunotherapy may be hampered without a secondary stimulant which may bypass the need for CD4 help, agonistic anti-CD40 in our case. The impact on pre-existing memory CD8 responses to the tumor is less clear. While our data suggest that cognate antigen recognition in memory CD8+ T cells remains intact during high dose cytokine therapy, it has been shown that persistent CD8 activation in the absence of CD4 responses (as occurs in AIDS and other chronic infections) leads to accelerated exhaustion and premature decline in immune efficacy (Appay and Rowland-Jones, 2002; Khaitan and Unutmaz, 2011; Sachdeva et al., 2010; Sauce et al., 2013). In addition to tumor-specific CD8+ T cells, the bystander activation of CD8+ T cells is another important consideration that is less well understood. A recent study by our lab suggests that bystander activation and function occurs similarly, if not better, in CD4 depleted mice suggesting that CD4+ T cells are not necessary for acquisition of function (Monjazeb et al., 2014). However, this study does not address the long-term consequences of prolonged bystander activation in the absence of CD4+ T cells to understand whether CD4+ T cells would be necessary for their maintenance as has been shown with antigen-specific CD8+ T cells. Thus the long-term impact of CD4 paralysis on CD8 function (both antigen specific and bystander) is an important avenue for future studies to address.
We have presented data herein describing the profound immune paralysis, particularly in the CD4+ T cell subset, that occurs following infection or treatment exposures that lead to supraphysiological cytokine production. However, the implications for this process during physiological inflammation, such as acute or chronic viral infections, cancer, or even aging, remain to be elucidated. Finally, the successful prevention of autoimmunity in our model suggests that inducing CD4+ T cell paralysis by pre-administering Signal 3 activation alone presents a potential viable target for inducing tolerance in transplantation.
Experimental Procedures
Mice
Female 8–12 week old female C57BL/6 or BALB/c mice were purchased from the animal production area at the National Cancer Institute (NCI-APA, Frederick, MD) or The Jackson Laboratory (Bar Harbor, ME). Congenic B6.sJL/N were purchased from the NCI-APA. Foxp3-DTR mice were purchased from the Mutant Mouse Regional Resource Center at the Jackson Laboratory. C57BL/6-IFNg-receptor (IFNgR)-KO and OT-II Tg(TcraTcrb)425Cbn (The Jackson Laboratory) mice were bred in house at the University of California, Davis (UC Davis). SOCS3fl/fl (C57BL/6 background), a kind gift from Dr Warren Alexander, were crossed to creΔLck mice (Taconic Farms) to generate Socs3fl/flcreΔLck mice. All mice were housed in the animal facilities at the University of California, Davis under specific pathogen-free conditions and studies were approved by the UC Davis Institutional Animal Care and Use committee.
Human High Dose IL-2 Trial
Blood samples were obtained from patients with metastatic melanoma enrolled in a randomized Phase II trial and receiving high-dose IL-2 alone as previously described (Seung et al., 2012). Patients began treatment on a Monday (day 1) and high-dose IL-2 was administered at 600,000 IU/kg by i.v. bolus infusion given every 8 hours for 14 planned doses depending on how well the IL-2 was tolerated. The median number of doses in this cycle of the study was 11. Blood samples were obtained at baseline, day 2, and day 8. For the data shown at day 2, all patients were still undergoing therapy. The day 8 data will vary from patient to patient depending on how many doses that they tolerated. Peripheral blood mononuclear cells (PBMCs) were cryopreserved from Ficoll-separated blood and stored at −170C. Signed informed consent was obtained before enrollment. The study was approved by the Providence Health System Regional Institutional Review Board, Oregon.
In vivo antibodies and reagents
The agonistic anti-mouse CD40 antibody (FGK115.B3) was generated as previously described(Murphy et al., 2003). Recombinant human interleukin-2 (rhIL2; Teceleukin, Roche, Germany) was provided by the National Cancer Institute (NCI, Frederick, MD). Rat IgG (Jackson ImmunoResearch Laboratories Inc, West Grove, PA) was used as a control for anti-CD40. In some experiments, NK cells were depleted using 300 ug anti-NK1.1 (PK136) grown by National Cell Culture Center (Minneapolis, MN). CD4+ T cells were depleted with anti-CD4 at 500 ug (clone GK1.5, a kind gift from Garry B. Huffnagle, University of Michigan). LPS was purchased from Sigma (St. Louis, MO). Diphtheria toxin (Sigma) was administered at 1ug/0.2ml ip every other day beginning at day -2.
Immunotherapeutic Regimen
Mice were administered agonistic anti-CD40 and rhIL-2 as previously described(Berner et al., 2007). Briefly, agonistic anti-CD40 or rat IgG (Jackson Immunoresearch) was administered for 5 days (days 0–4). rhIL-2 or PBS alone was administered twice per week for 2 weeks. For studies where the regimen was doubled, a second round was administered identically to the first round starting on day 14.
Microarray data
Microarray data have been deposited into the NCBI GEO public database, tracking number: 17380730.
Data Analysis and Statistics
Statistical analysis was performed using Prism software (GraphPad Software Inc.). Data were expressed as mean ± SEM. For analysis of three or more groups, the non-parametric ANOVA test was performed with the Bonferroni post-test. Analysis of differences between two normally distributed test groups was performed using the Student's t-test. Welch's correction was applied to Student's t-test data sets with significant differences in variance. * P<0.05, ** P<0.01, *** P<0.001.
Supplementary Material
Highlights.
Pre-exposure to elevated cytokines impairs TCR-mediated activation in CD4 T cells
Cytokines directly induce Socs3, suppressing IL-2 signaling and proliferation
Contact with antigen during paralysis inhibits CD4 T cell help and memory responses
Paralysis can protect from potential autoimmunity during systemic inflammation
Acknowledgements
We would like to thank Monja Metcalf and Weihong Ma for excellent technical assistance with these studies. We would also like to thank Dr. Jonathan Weiss for helpful discussions with the manuscript. We would also like to thank Dr. Fu-Tong Liu for donating his OT-II Tg colony which was further propagated for studies in this manuscript. This work was funded by grants from the National Institutes of Health (NIH) R01 CA 095572 and R01 CA 072669. Samples from patients undergoing systemic high dose IL-2 therapy were part of a larger study evaluating combination of radiotherapy with systemic IL-2. This trial is supported in part by Prometheus Laboratories, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: G.D.S. designed and carried out experiments, analyzed data, and prepared manuscript for submission. M.C. and B.D.C. collected and provided samples for human IL-2 trial data. W.A. provided SOCS3fl/fl mice. M.N.B., D.E.C.W, K.A.A., C.M.S., E.A., A.M., and A.R. performed experiments and analyzed data. A.S. helped design and carry out studies involving EAE model. A.M.M, B.R.B, D.L.L., and R.H.W. helped in experimental design and preparation of manuscript. W.J.M. designed experiments, assisted in data interpretation, and helped prepare manuscript.
References
- Appay V, Rowland-Jones SL. Premature ageing of the immune system: the cause of AIDS? Trends in immunology. 2002;23:580–585. doi: 10.1016/s1471-4906(02)02338-4. [DOI] [PubMed] [Google Scholar]
- Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, et al. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nature medicine. 2007;13:354–360. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell reports. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, Bozon VA, Bulanhagui CA, Seja E, Villanueva A, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. Journal of translational medicine. 2008;6:22. doi: 10.1186/1479-5876-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. The Journal of experimental medicine. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. The Journal of experimental medicine. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Njenga MK, Hansen MJ, Kuhns ST, Chen L, Rodriguez M, Pease LR. Prevalent class I-restricted T-cell response to the Theiler's virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. Journal of virology. 1999;73:3702–3708. doi: 10.1128/jvi.73.5.3702-3708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjic S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. The Journal of experimental medicine. 1989;169:1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaitan A, Unutmaz D. Revisiting immune exhaustion during HIV infection. Current HIV/AIDS reports. 2011;8:4–11. doi: 10.1007/s11904-010-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura MY, Pobezinsky LA, Guinter TI, Thomas J, Adams A, Park JH, Tai X, Singer A. IL-7 signaling must be intermittent, not continuous, during CD8(+) T cell homeostasis to promote cell survival instead of cell death. Nature immunology. 2013;14:143–151. doi: 10.1038/ni.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiehe AM, Lin L, White C, Braymer HD, Bray GA, York DA. Constitutive activation of STAT-3 and downregulation of SOCS-3 expression induced by adrenalectomy. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;281:R2048–2058. doi: 10.1152/ajpregu.2001.281.6.R2048. [DOI] [PubMed] [Google Scholar]
- Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Annals of surgical oncology. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. Journal of virology. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjazeb AM, Tietze JK, Grossenbacher SK, Hsiao HH, Zamora AE, Mirsoian A, Koehn B, Blazar BR, Weiss JM, Wiltrout RH, et al. Bystander activation and anti-tumor effects of CD8+ T cells following Interleukin-2 based immunotherapy is independent of CD4+ T cell help. PloS one. 2014;9:e102709. doi: 10.1371/journal.pone.0102709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, van Deursen J, Sangster MY, Bunting KD, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Welniak L, Back T, Hixon J, Subleski J, Seki N, Wigginton JM, Wilson SE, Blazar BR, Malyguine AM, et al. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. Journal of immunology. 2003;170:2727–2733. doi: 10.4049/jimmunol.170.5.2727. [DOI] [PubMed] [Google Scholar]
- Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends in immunology. 2009;30:592–602. doi: 10.1016/j.it.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona-Wright G, Mohrs K, Mohrs M. Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nature immunology. 2010;11:520–526. doi: 10.1038/ni.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. Journal of acquired immune deficiency syndromes. 2010;54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauce D, Elbim C, Appay V. Monitoring cellular immune markers in HIV infection: from activation to exhaustion. Current opinion in HIV and AIDS. 2013;8:125–131. doi: 10.1097/COH.0b013e32835d08a9. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. T cell anergy. Annual review of immunology. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Sckisel GD, Tietze JK, Zamora AE, Hsiao HH, Priest SO, Wilkins DE, Lanier LL, Blazar BR, Baumgarth N, Murphy WJ. Influenza infection results in local expansion of memory CD8(+) T cells with antigen non-specific phenotype and function. Clinical and experimental immunology. 2014;175:79–91. doi: 10.1111/cei.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, Miller W, Payne R, Glenn L, Bageac A, Urba WJ. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Science translational medicine. 2012;4:137–174. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Belz GT, Altman JD, Doherty PC. Virus-specific CD8(+) T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15565–15570. doi: 10.1073/pnas.95.26.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze JK, Sckisel GD, Hsiao HH, Murphy WJ. Antigen-specific versus antigen-nonspecific immunotherapeutic approaches for human melanoma: the need for integration for optimal efficacy? International reviews of immunology. 2011;30:238–293. doi: 10.3109/08830185.2011.598977. [DOI] [PubMed] [Google Scholar]
- Tietze JK, Sckisel GD, Zamora AE, Hsiao HH, Priest SO, Wilkins DE, Lanier LL, Blazar BR, Baumgarth N, Murphy WJ. Influenza infection results in local expansion of memory CD8 T cells with antigen-nonspecific phenotype and function. Clinical and experimental immunology. 2013 doi: 10.1111/cei.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze JK, Wilkins DE, Sckisel GD, Bouchlaka MN, Alderson KL, Weiss JM, Ames E, Bruhn KW, Craft N, Wiltrout RH, et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough DF, Sun S, Zhang X, Sprent J. Stimulation of naive and memory T cells by cytokines. Immunological reviews. 1999;170:39–47. doi: 10.1111/j.1600-065x.1999.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Verdeil G, Puthier D, Nguyen C, Schmitt-Verhulst AM, Auphan-Anezin N. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. Journal of immunology. 2006;176:4834–4842. doi: 10.4049/jimmunol.176.8.4834. [DOI] [PubMed] [Google Scholar]
- Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nature reviews. Immunology. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang T, He H, Xie Y. Interleukin-2 inhibits polarization to T helper type 1 cells and prevents mouse acute graft-versus-host disease through up-regulating suppressors of cytokine signalling-3 expression of naive CD4+ T cells. Clinical and experimental immunology. 2010;160:479–488. doi: 10.1111/j.1365-2249.2010.04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.