Abstract
Introduction
Reachable workspace is a measure that provides clinically meaningful information regarding arm function. In this study, a Kinect sensor was used to determine the spectrum of 3D reachable workspace encountered in a cross-sectional cohort of individuals with ALS.
Method
Bilateral 3D reachable workspace was recorded from 10 subjects with ALS and 23 healthy controls. The data were normalized by each individual's arm length to obtain a reachable workspace relative surface area (RSA). Concurrent validity was assessed by correlation with ALSFRSr scores.
Results
The Kinect-measured reachable workspace RSA differed significantly between the ALS and control subjects (0.579±0.226 vs. 0.786±0.069; P<0.001). The RSA demonstrated correlation with ALSFRSr upper extremity items (Spearman correlation ρ=0.569; P=0.009). With worsening upper extremity function as categorized by the ALSFRSr, the reachable workspace also decreased progressively.
Conclusions
This study demonstrates the feasibility and potential of using a novel Kinect-based reachable workspace outcome measure in ALS.
Keywords: Reachable workspace, ALS, upper extremity, Kinect, outcome measure
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive, lethal motor neuron disease with prevalence of 3.9 per 100,000 in the US1 and 5.4 in Europe.2 In most cases the disease appears sporadically, with only about 15% of patients possessing a known pathologic genetic mutation.3 It is characterized by upper and lower motor neuron loss resulting in pervasive muscle atrophy and weakness, including upper extremity muscles. The upper extremities are the most common site of disease onset, producing weakness and functional impairment with reduced ability to perform self-care and activities of daily living (ADLs). Several recent and ongoing ALS therapeutic clinical trials have aimed at improving both cervical-innervated respiratory and arm functions.4 However, measuring and tracking clinical severity in ALS presents inherent challenges, as the disease is often asymmetric in onset and spread in a single individual. It is also heterogeneous with highly variable rates of progression from patient to patient. Outcome measures that are easily performed, cost effective, portable, sensitive, and reliable for tracking upper extremity function in ALS are severely lacking.
Currently, there are several measures used commonly to assess upper extremity involvement in ALS. Manual muscle testing (MMT) and hand-held dynamometry (HHD) are used often, but share several limitations in that they are evaluator dependent and are cumbersome. Both measures provide results that can track upper extremity weakness in ALS, but they capture only isolated strength over specific joints and do not measure overall arm function. Furthermore, they are time-consuming and quite effort intensive, thereby making them unpopular with study participants. The Accurate Test of Limb Isometric Strength (ATLIS) system, a newer limb strength outcome measure, promises to be an improvement but is equally time consuming, and the required equipment is not easily portable5, limiting its potential for remote assessments. Hand grip testing is another upper limb measure employed in ALS clinical trials. It is quick and simple to administer, but assesses only distal finger flexor muscles. These tests are used in many but not all trials, illustrating the lack of consensus regarding their utility.
The most commonly used outcome measure is the ALS Functional Rating Scale revised (ALSFRSr).6 It captures arm function in 3 items (items 4-6), with each item scored on a 5 point scale (0-4). Although ALSFRSr was designed specifically for ALS and has been used widely in clinical trials, it is an ordinal rating scale with test metrics that have prompted some investigators to propose that it does not meet modern outcome measurement standards.5,7 Practically, the ALSFRSr scores show linear decline in most studies, but the scale is multidimensional (bulbar, limb, and respiratory) and has limitations that corrupt its metric quality.7 Cognitive impairment in ALS, which often is characterized by poor insight, is another potential source of inaccuracy.8 Lastly, this scale was not designed to adequately capture the potential asymmetry of extremity impairments that can be observed in ALS. Not only does this limit test sensitivity to detect impairment, but it is a severe limitation for trials using a single side for intervention and the other as control.9
Recently, we demonstrated that 3D reachable workspace can be measured reliably using a simple, lightweight, single camera system (the depth-sensing Microsoft Kinect).10 The Kinect-acquired results were comparable to that obtained by a more complex and expensive multi-camera motion capture system.10 The Kinect-based system enables quick and reproducible upper extremity motion capture with accurate and reliable reconstruction of the 3D reachable workspace. One of the earliest uses of the Kinect system was in ergonomics, where it was used to evaluate and design pilot workspace in an airplane.11 Later the concept was developed as a functional measure of the upper extremity based on kinematic and goniometry information. Reachable workspace has been applied as a relevant upper extremity outcome measure in various neuromuscular diseases [facioscapulohumeral muscular dystrophy (FSHD), Becker muscular dystrophy (BMD), Duchenne muscular dystrophy (DMD)] 12-14 and shows promise as an upper extremity assessment tool and novel outcome measure in ALS.
In this study, our objective was to determine the spectrum of reachable workspace encountered in ALS subjects and compare it to healthy controls. In addition, we aimed to evaluate the concurrent validity of the novel Kinect-measured reachable workspace as an outcome measure in ALS by assessing correlation with the ALSFRSr upper extremity items. We also made a similar comparison to the Brooke Upper Extremity Functional Scale, a measure developed to assess upper limb function in Duchenne muscular dystrophy.15
MATERIALS AND METHODS
Participants
A total of 10 subjects with ALS (7 women, 3 men) and 17 healthy controls (10 women, 7 men) participated in the study. The ALS subjects were recruited from the UC Davis ALS clinic. Subjects were diagnosed by El Escorial-Awaji criteria (2 definite, 6 probable, 2 possible) and had ALSFRSr scores ranging from 18 to 43 (average 31). The protocol was approved by the UC Davis Institutional Review Board (IRB). The healthy controls were without any neuromuscular or musculoskeletal disorder and were recruited using IRB approved advertising. Exclusion criteria for the healthy controls consisted of any impairment in arm function that prevented abduction in a full circle until both arms touched above the head. Written informed consent was obtained before any study procedures were conducted on both ALS subjects and healthy controls. Clinical history, demographic and anthropometric information (age, gender, height, and weight) was obtained from each subject.
ALSFRSr
The upper extremity sub-score of the ALSFRSr6, i.e. items number 4-6 (score range: 0-12), was used to characterize the functional limitations of the limbs of each study subject. The ALSFRSr upper extremity items are: Item 4, “Has your handwriting changed?”; Item 5A, for patients who eat most of their food by mouth, “Cutting food and handling utensils”, or Item 5B, for those who primarily rely on a feeding tube, “Using a feeding tube (PEG)”; and Item 6, “Has your ability to dress and perform self-care activities (i.e. bathing, teeth brushing, shaving, combing your hair, other hygienic activities) changed?” Each item is scored from 0-4 on an ordinal scale, where 4 represents normal and 0 represents no remaining function. As another comparison for the study cohort, the Brooke15 upper extremity function scores were also assigned. Brooke scores range from 1-6: 1 (full abduction of arms above the head); 2 (can raise arms above head only by flexing the elbow and shortening the circumference of the movement); 3 (cannot raise hands above head, but able to raise an 8-oz glass of water to the mouth); 4 (can raise hands to mouth but cannot raise an 8 oz. glass/cup of water to mouth); 5 (cannot raise hands to the mouth, but can use hands to hold a pen or pick up pennies from the table); and 6 (cannot raise hands to the mouth and has no useful function of hands).
Upper Extremity Reachable Workspace Protocol
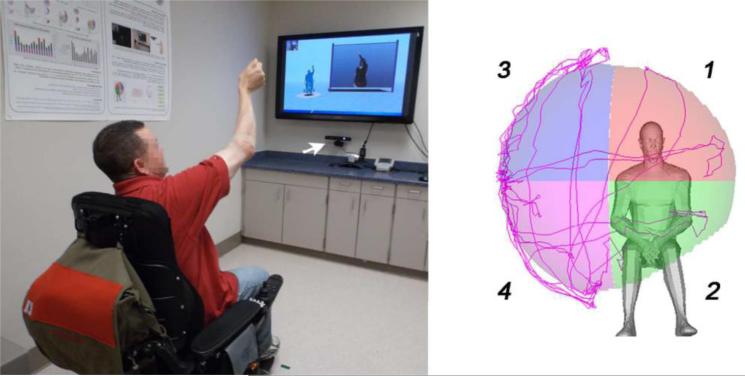
The upper extremity 3D reachable workspace reconstruction by the Kinect system, based on arm movement, followed the previously published protocol (Fig.1).10-13 Assessment of each limb took approximately 1 minute using the methods we published previously. Briefly, the arm movement protocol requires the subject to sit on a narrow chair without armrests and move each arm independently under the direction of a clinical evaluator through a set of standardized movements. We instructed the subjects to reach as far as they could with an extended elbow, without leaning forward or twisting their shoulders or body. When they were unable to reach further they were instructed to return to starting position with the arms at their side. We standardized the assessment by having the participants follow a pre-recorded video. This encouraged participants to maintain a similar execution speed to limit variability due to the velocity of movement. In addition to the instructional video, the study evaluator also demonstrated the movements for the subjects to limit compensatory trunk rotation or forward leaning. If such movements were observed, the recording was repeated.
Figure 1.
An image of a patient performing the reachable workspace protocol with a Kinect sensor (arrow points to the location of the mounted Kinect sensor) and a visualization of the reachable workspace output. This example shows the reachable workspace quadrants 1, 2, 3 and 4 in the right upper extremity perspective.
Reachable Workspace Surface Envelope Analysis
After recording the arm motion with a Kinect sensor, we transformed the trajectories and constructed a graphic output using methods described previously.10 To enable a better understanding of where reductions of RSA occur, we divided each arm's RSA hemisphere into 4 quadrants. We used the shoulder as the anchor point for the horizontal and vertical divisions. We then calculated the total absolute reachable workspace surface envelope area (m2) and also the reachable workspace in each quadrant. Next, we normalized the absolute surface envelope area by arm length to calculate a relative surface area (RSA). The RSA facilitates comparison between subjects with different heights or arm lengths. The RSA represents the portion of the frontal hemispherical space that can be reached by the individual's hand movements (Fig. 1). It is determined by dividing the absolute reachable workspace surface envelope area by the ½ of 4πr2, where r represents the radius of the sphere (i.e. the extended arm length between the shoulder joint and hand).
Statistical Analysis
In this study we tested a new outcome measure that could be used to describe the central tendency and variability of RSA in subjects with ALS compared to healthy controls. We determined whether subjects with impaired upper extremity function had reduced RSA. Differences were determined between groups using the Welsh 2-sample t-test, as the ALS subjects RSAs were not distributed normally. The concordance correlation was calculated using the Spearman method to compare the ordinal ALSFRSr with the continuous RSA variable. ANOVA was used to assess differences between groups, and a post-hoc Tukey test was used to determine sub-group differences. All statistical analysis was conducted using SAS 9.4 software. For all analyses, a P-value of 0.05 was accepted as the level of statistical significance.
RESULTS
Study Participants
A total of 10 subjects with ALS (average age 67.4±8.8 years) and 17 healthy controls (average age 66.2±7.4 years) participated in the study. No significant group differences were found for age, height, or weight. Table 1 lists the basic anthropometric information, height, weight, Brooke score, ALSFRSr, and ALSFRSr upper extremity score. All 10 ALS subjects were able to perform the testing, despite several individuals having marked impairment of arm function.
Table 1.
ALS study subject demographics and characteristics.
| ID | Gender | Age (yrs) | Height (cm) | Weight (kg) | Brooke | ALSFRSr Total | ALSFRSr Upper Extremity scores | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Handwriting | Cut Food | Dressing/Hygiene | Upper Combined | |||||||
| 1 | W | 54.9 | 165 | 73.9 | 1 | 41 | 4 | 4 | 4 | 12 |
| 2 | W | 67.1 | 155 | 58.7 | 1 | 37 | 4 | 3 | 3 | 10 |
| 3 | W | 68.5 | 170 | 94.8 | 1 | 32 | 4 | 4 | 1 | 9 |
| 4 | M | 69.7 | 178 | 69.8 | 1 | 43 | 3 | 3 | 3 | 9 |
| 5 | M | 76.1 | 178 | 81.8 | 2 | 34 | 3 | 3 | 2 | 8 |
| 6 | W | 76.5 | 156 | 64.9 | 3 | 37 | 3 | 2 | 3 | 8 |
| 7 | W | 73.0 | 157 | 53.1 | 1 | 18 | 2 | 3 | 2 | 7 |
| 8 | M | 59.2 | 186 | 104.8 | 3 | 25 | 2 | 3 | 1 | 6 |
| 9 | W | 76.6 | 165 | 49.8 | 2 | 29 | 3 | 1 | 0 | 4 |
| 10 | W | 52.1 | 155 | 59.1 | 4 | 26 | 3 | 0 | 1 | 4 |
Reachable Workspace Comparison between Healthy Controls and Subjects with ALS
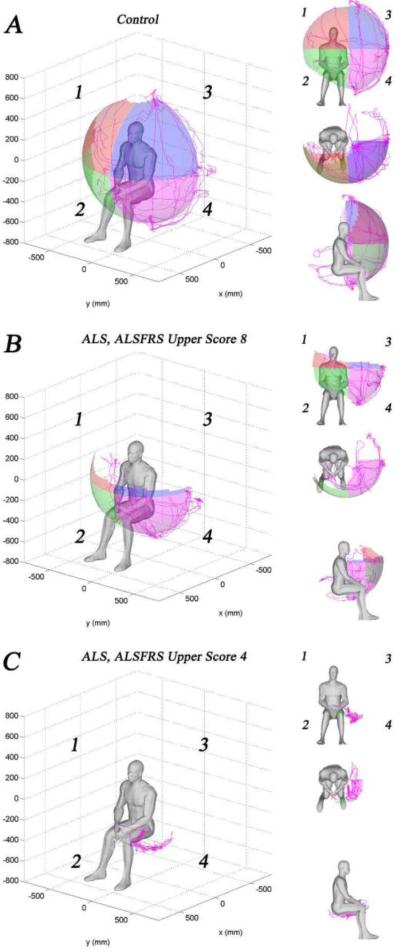
The reachable workspace of the ALS subjects was significantly reduced compared to controls (0.579±0.226 vs.0.786±0.069; P<0.0001). Figure 2 shows the graphical outputs of a normal control, a moderately affected ALS subject, and a severely affected ALS subject (ALSFRSr combined upper extremity scores of 8 and 4, respectively). The RSA quadrants that were most affected by ALS were the upper quadrants 1 and 3, but the difference compared to controls was statistically significant in all quadrants as shown in Table 2. The total and quadrant RSAs of ALS subjects and controls are represented graphically in Figure 3.
Figure 2.
Example of graphical outputs of Kinect-based reachable workspace RSA (left upper extremity perspective shown). (A) Normal control showing a nearly hemispherical surface area. (B) A moderately impaired subject with ALS (ALSFRSr upper extremity score = 8) demonstrating reduced RSA primarily in the upper quadrants. (C) A severely impaired subject with ALS (ALSFRSr upper extremity score = 4) demonstrating markedly reduced RSA in all quadrants.
Table 2.
Relative surface area (RSA) of the reachable workspace surface envelope from controls and ALS subjects by quadrant and total area.
| Quad 1 | Quad 2 | Quad 3 | Quad 4 | TOTAL | |
|---|---|---|---|---|---|
| Contralateral Quadrant | Ipsilateral Quadrant | ||||
| Cohort (n) | Upper | Lower | Upper | Lower | |
| ALS (20) | 0.125±0.068 | 0.097±0.054 | 0.163±0.084 | 0.195±0.062 | 0.579±0.226 |
| Control (34) | 0.196±0.041 | 0.135±0.044 | 0.227±0.014 | 0.228±0.013 | 0.785±0.070 |
| ALS vs. Control (P-value) | <0.0001 | 0.0124 | <0.0001 | 0.0036 | <0.0001 |
Means ± SD and sample size (n) are presented as the average for dominant and non-dominant sides. ALS vs. Control by a 2-sample t-test.
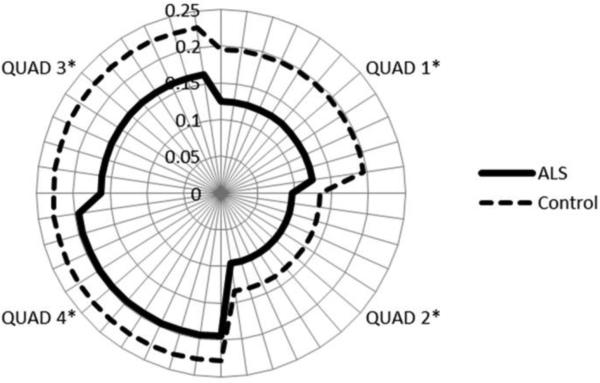
Figure 3.
A reachable workspace plot demonstrating graphical comparison of total and quadrant RSAs of individuals with ALS and healthy controls (right upper extremity perspective shown). The lower quadrant RSAs are relatively preserved compared to the upper quadrants in the ALS subjects.
Reachable Workspace and Hand dominance
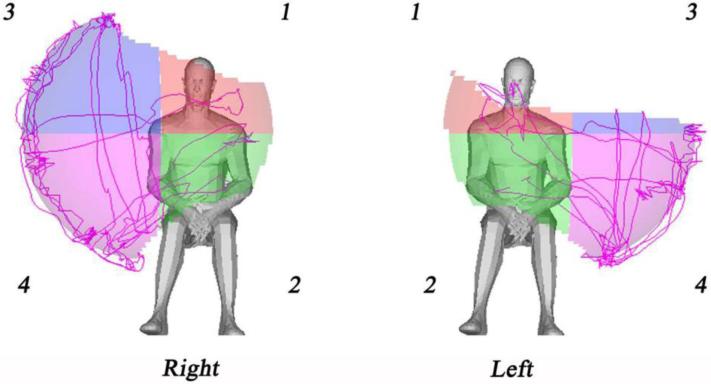
Association between reachable workspace and hand dominance in the ALS study cohort was examined. We observed no significant differences in reachable workspace between dominant and non-dominant sides in this cohort of ALS subjects (data not shown; P=0.824). Although no side-to-side difference in RSA between dominant and non-dominant upper limbs was noted in the ALS cohort, the Kinect-based system demonstrates the capability to detect asymmetry in reachable workspace that can be seen in patients with ALS. Figure 4 illustrates such an arm asymmetry in reachable workspace found in an individual with ALS.
Figure 4.
Asymmetry of upper extremity reachable workspace in a single study subject with ALS demonstrated by the Kinect-based RSA.
Reachable Workspace by ALSFRSr arm score and Brooke
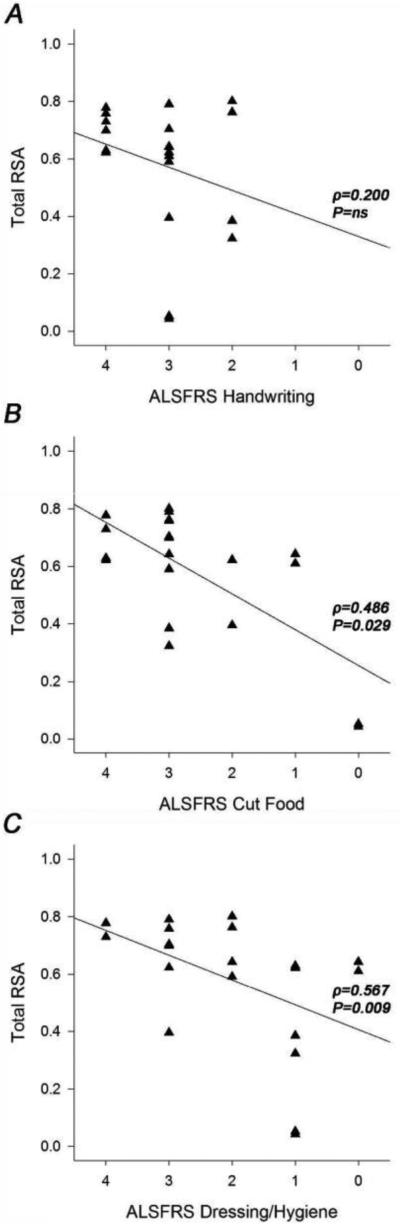
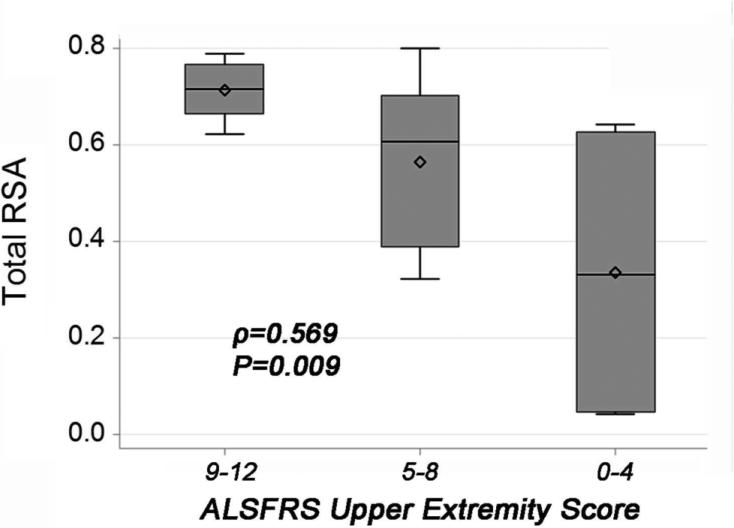
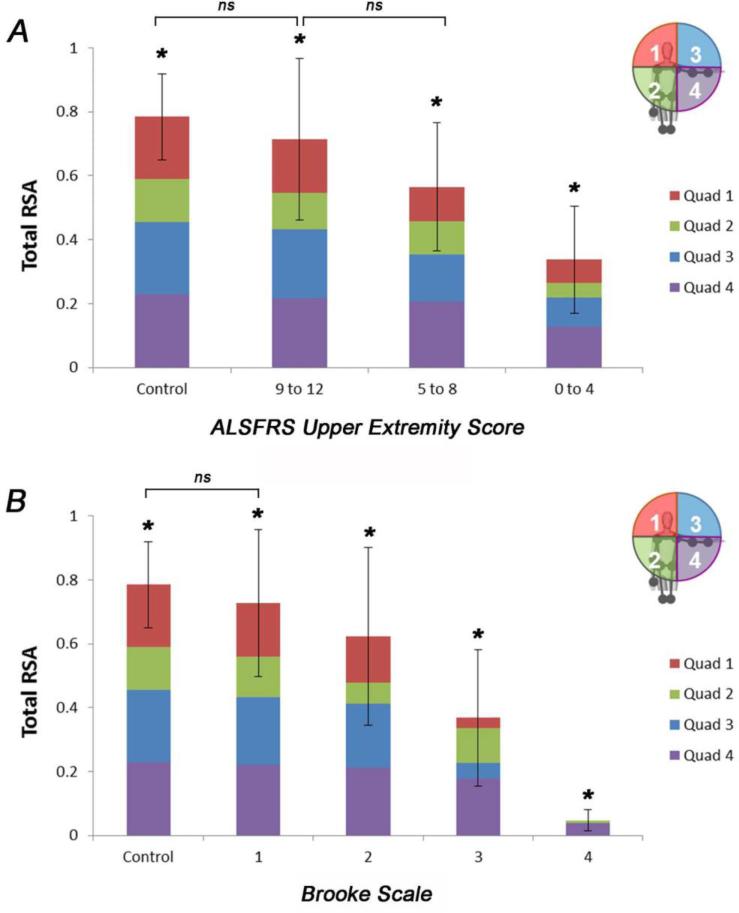
The ALSFRSr scores for item 4 were all between 2 and 4, and there was no statistically significant relationship between ALSFRSr item 4 and the RSA (Figure 5A). Looking at the other 2 ALSFRSr upper limb items individually compared to the RSA, there is a statistically significant relationship for both items 5 and 6 with the reachable workspace RSA; this is illustrated in Figure 5B and C. An overall lower score on the ALSFRSr arm items (combined) corresponded to a contracted reachable workspace RSA, as would be predicted, since they are both measures of upper extremity function. This is illustrated by stratifying the ALSFRSr upper extremity scores to low (0-4), medium (5-8), and high (9-12) scores in relationship to the RSA as shown in Figure 6. The total RSA as measured by Kinect correlates with the ALSFRSr arm score (ρ=0.569; P=0.009). Comparing each quadrant of the RSA to the low, medium, and high arm function by the ALSFRSr shows a relatively larger loss in the upper quadrants (Figure 7A). The Brooke score shows a very strong correlation with the RSA (ρ=0.865, P<0.0001), and its relationship to total and quadrant RSA is shown in Figure 7B.
Figure 5.
Scatterplots of ALS subjects’ total RSA by ALSFRSr upper extremity item score for A) item 4: “Handwriting”, B) item 5: “Cutting food”, and C) item 6: “Dressing/Hygiene”.
Figure 6.
Box-and-whiskers plot showing the total RSA of the ALS cohort categorized by the combined ALSFRSr upper extremity item scores in tertiles (mild impairment: 9-12; moderate impairment: 5-8; and severe impairment: 0-4).
Figure 7.
Bar graphs showing the gradual reduction in the total and quadrant RSAs of the ALS cohort compared to controls by (A) combined ALSFRSr upper extremity item scores (divided in tertiles), and (B) Brooke grades 1-4. Note the decrease in RSA that occurs with impairment in arm movement as measured by these 2 scales (*denotes significant difference between groups, P<0.05; ns = no significant difference).
DISCUSSION
The relentless and progressive nature of ALS results in eventual and nearly ubiquitous arm dysfunction in patients with ALS. The rate of disability and relative symmetry of dysfunction are highly variable among patients, however, the upper extremities are the most commonly affected region of the body. The importance of upper extremity impairment in ALS is supported by the inclusion of measures of upper extremity strength and function in the vast majority of ALS clinical trials. Several outcome measures of arm function are available, but the ALSFRSr is the most widely-used measure in ALS clinical trials due to the relative ease with which it can be administered, its brevity and lack of costly equipment, and its ability to be adapted for remote telephone administration; however, it is not a continuous variable and lacks granularity to identify small but perhaps significant changes in function.
In our previously published papers, we have demonstrated the feasibility of using the Kinect sensor system to acquire a reachable workspace outcome measure, which is capable of discerning changes in upper extremity function for clinical trials. We have shown that reachable workspace RSA is both reliable and has discriminant validity to identify significant differences between healthy controls and patients with other neuromuscular diseases.12,14 In this paper, we follow up on previously published work by extending its application to a cohort of ALS patients with varying upper extremity involvement. The findings demonstrate the capacity of the instrument to discriminate between groups of individuals with and without disease (P<0.0001). In addition, we compared the RSA to the ALSFRSr upper extremity measure and found a moderate correlation between the two measures as well as a strikingly parallel correlation using the Brooke score. We believe the moderate correlation between the RSA and ALSFRSr can be explained by important differences in the measures; the RSA reflects multi-segment arm strength, and the ALSFRSr indicates mostly hand function.
We show that the reachable workspace RSA is measured quickly and easily even in severely affected individuals with ALS, illustrating its ability to capture and discern data across all disease severities (range of ALSFRSr upper extremity measure from 4 to 12, and Brooke grade from 1 to 4). Although the sample size was small, the data suggest that the system and our methodology is not only capable of detecting differences in reachable workspace RSA, but may be sensitive enough to differentiate observed changes within each quadrant between individuals with normal function and those with mild, moderate, and severe impairment as categorized by both the Brooke scale and the commonly used ALSFRSr upper extremity items. Unlike the Brooke scale and ALSFRSr, reachable workspace RSA is a continuous variable, which may allow for increased statistical sensitivity to detect change compared to these ordinal measures. Verification of these results is needed with data from a larger sample. In addition, we have shown that reachable workspace RSA has the capacity to capture asymmetric upper limb dysfunction within a single patient (Figure 4).
Additional attributes of the Kinect sensor system include its portability and potential as a remote assessment tool. The Kinect system was developed as an in-home gaming system. It is capable of transmitting data electronically and can be programed with instructional video to reduce reliance on clinical evaluators. ALS is a rapidly debilitating disease, and many trial subjects become unable to travel to a study site before their death. The potential to conveniently capture functional data from a patients’ home using this methodology is particularly promising.16
There were several limitations of this study; as a pilot feasibility study it is limited by small sample size and recruitment of a convenience sample. Reachable work space RSA, while being a multi-joint upper limb measure, is largely influenced by movements of the shoulder joint, and to a lesser degree the elbow and wrist joints; whether this affects its ability to capture change in patients with varying patterns of upper extremity weakness remains to be determined. In addition, most outcome measures currently used in ALS trials are more sensitive to lower motor neuron dysfunction as opposed to upper motor neuron dysfunction. The published experience with reachable workspace has been centered on neuromuscular conditions. There are no published data on central nervous system disorders, which may be an area for further studies.
Finally, reachable workspace RSA is not a measure of global ALS disease severity. As this was a cross sectional study, we were not able to compare disease progression slopes to those of global measures, such as the ALSFRSr. However, we believe the value of reachable workspace RSA lies in its ability to precisely capture small incremental change in upper limb function as a continuous variable, and, as a companion outcome measure, to expand the granularity of global measures to detect small but significant change. We believe that the data collected in this study confirm the utility of the novel reachable workspace RSA as an outcome measure in ALS and substantiate its potential to be a superior upper limb outcome measure. Additional prospective longitudinal studies are needed and are currently ongoing to validate this preliminary experience, to further characterize common patterns of upper limb functional loss in patients with ALS and to compare the performance of reachable RSA to other measures used to track ALS progression.
CONCLUSION
As an outcome measure, the Kinect-measured reachable workspace holds promise as a quick, affordable, scalable, and unobtrusive tool to monitor disease progression in ALS. It may be used to evaluate efficacy of novel therapeutics in clinical trials. The measure could be of particular use in trials specifically targeting the upper extremity/cervical spinal cord. We plan to conduct a longitudinal study with a larger sample to further examine its sensitivity to change, validity as compared to other outcome measures, and reliability in ALS.
Acknowledgements
The research was supported in part by grant funds from the National Institutes of Health (NIH), NIAMS: U01 AR065113-01; the U.S. Department of Education (NIDRR): #H133B090001; and the National Science Foundation (NSF) #1111965. We would like to thank the study participants for their time and effort; Craig McDonald and Michelle Cregan for project support; and research assistants Chia-Yuan Michael Lee, Daniel Alekyan, Nida Ahmed, Sarah Macabales, Carly Davis, and Jaijeet Toor, for their help in preparing data for this manuscript. B.O. was supported by NIH UL1 TR000002 and KL2 TR000134. N.C.J. was supported by NIH 5K12HD001097-15.
ABBREVIATIONS
- 3D
Three-dimensional
- ADLs
activities of daily living
- ALS
Amyotrophic lateral sclerosis
- ALSFRSr
ALS Functional Rating Scale revised
- ATLIS
Accurate Test of Limb Isometric Strength
- BMD
Becker muscular dystrophy
- DMD
Duchenne muscular dystrophy
- FSHD
Facioscapulohumeral muscular dystrophy
- HHD
hand-held dynamometry
- MMT
Manual muscle testing
- RSA
Relative surface area
REFERENCES
- 1.Mehta P, Antao V, Kaye W, Sanchez M, Williamson D, Bryan L, et al. Prevalence of amyotrophic lateral sclerosis - United States, 2010-2011. Morbidity and mortality weekly report Surveillance summaries. 2014;63(Suppl 7):1–14. [Google Scholar]
- 2.Chio A, Logroscino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118–130. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shefner JM, Watson ML, Meng L, Wolff AA. Neals/Cytokinetics ST. A study to evaluate safety and tolerability of repeated doses of tirasemtiv in patients with amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2013;14(7-8):574–581. doi: 10.3109/21678421.2013.822517. [DOI] [PubMed] [Google Scholar]
- 5.Andres PL, Skerry LM, Munsat TL, Thornell BJ, Szymonifka J, Schoenfeld DA, et al. Validation of a new strength measurement device for amyotrophic lateral sclerosis clinical trials. Muscle Nerve. 2012;45(1):81–85. doi: 10.1002/mus.22253. [DOI] [PubMed] [Google Scholar]
- 6.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS- R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169(1-2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 7.Franchignoni F, Mora G, Giordano A, Volanti P, Chio A. Evidence of multidimensionality in the ALSFRS-R Scale: a critical appraisal on its measurement properties using Rasch analysis. J Neurol Neurosurg Psychiatry. 2013;84(12):1340–1345. doi: 10.1136/jnnp-2012-304701. [DOI] [PubMed] [Google Scholar]
- 8.York C, Olm C, Boller A, McCluskey L, Elman L, Haley J, et al. Action verb comprehension in amyotrophic lateral sclerosis and Parkinson's disease. Journal of neurology. 2014;261(6):1073–1079. doi: 10.1007/s00415-014-7314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75(3):363–373. doi: 10.1002/ana.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurillo G, Chen A, Bajcsy R, Han JJ. Evaluation of upper extremity reachable workspace using Kinect camera. Technology and health care : official journal of the European Society for Engineering and Medicine. 2013;21(6):641–656. doi: 10.3233/THC-130764. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy KW. Reach capability of men and women: A three-dimensional analysis: DTIC Document. 1978 [Google Scholar]
- 12.Han JJ, Kurillo G, Abresch RT, de Bie E, Nicorici A, Bajcsy R. Reachable workspace in facioscapulohumeral muscular dystrophy (FSHD) by kinect. Muscle Nerve. 2015;51(2):168–175. doi: 10.1002/mus.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurillo G, Han JJ, Abresch RT, Nicorici A, Yan P, Bajcsy R. Development and application of stereo camera-based upper extremity workspace evaluation in patients with neuromuscular diseases. PloS one. 2012;7(9):e45341. doi: 10.1371/journal.pone.0045341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han JJ, Kurillo G, Abresch RT, de Bie E, Nicorici Lewis A, Bajcsy R. Upper extremity 3D reachable workspace analysis in dystrophinopathy using Kinect. Muscle Nerve. 2015 doi: 10.1002/mus.24567. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981;4(3):186–197. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- 16.Kurillo G, Han JJ, Nicorici A, Bajcsy R. Tele-MFAsT: Kinect-Based Tele-Medicine Tool for Remote Motion and Function Assessment. Studies in health technology and informatics. 2014;196:215–221. [PubMed] [Google Scholar]