Abstract
Background
Glucarpidase rapidly reduces methotrexate plasma concentrations in patients experiencing methotrexate-induced renal dysfunction. Debate exists regarding the role of glucarpidase in therapy given its high cost. The use of reduced-dose glucarpidase has been reported, and may allow more institutions to supply this drug to their patients. This report explores the relationship between glucarpidase dosage and patient outcomes in pediatric oncology patients.
Methods
The authors evaluated data from 26 patients who received glucarpidase after high-dose methotrexate. Decrease in plasma methotrexate concentrations and time to renal recovery were evaluated for an association with glucarpidase dosage, which ranged from 13 to 90 units/kg.
Results
No significant relationship was found between glucarpidase dosage (units/kg) and percent decrease in methotrexate plasma concentrations measured by TDx (P >0.1) or HPLC (P >0.5). Patients who received glucarpidase dosages <50 units/kg had a median percent reduction in methotrexate plasma concentration of 99.4% (range, 98–100) measured by HPLC compared to a median percent reduction of 99.4% (range, 77.2–100) in patients who received ≥50 units/kg. Time to SCr recovery was not related to glucarpidase dosage (P >0.8).
Conclusions
The efficacy of glucarpidase in the treatment of HDMTX-induced kidney injury was not dosage-dependent in this retrospective analysis of pediatric oncology patients. Pediatr Blood Cancer 2015;62:1518–1522.
Keywords: glucarpidase, carboxypeptidase, methotrexate, pediatric, nephrotoxicity
INTRODUCTION
High-dose methotrexate, defined as dosages ≥1 gm/m2, remains a mainstay component of conventional chemotherapy for multiple malignancies, both hematologic and non-hematologic. Despite supportive care measures such as, aggressive alkalinized hydration, patients receiving high-dose methotrexate are at risk for methotrexate-induced renal dysfunction [1–9]. The development of impaired renal function and subsequent delayed methotrexate excretion also puts patients at greater risk for other severe or life-threatening toxicities, such as, mucositis, myelosuppression, and liver dysfunction [1,10,11].
Glucarpidase (Voraxaze®), a recombinant carboxypeptidase G2 enzyme that hydrolyzes antifolates such as, methotrexate to inactive metabolites, was approved by the US FDA in January 2012 for the treatment of methotrexate concentrations >1 mmol/l in patients with delayed methotrexate clearance due to impaired renal function [12]. Glucarpidase is currently supplied from the manufacturer as single-use vials containing 1,000 units per vial as lyophilized powder. The manufacturer’s dosing recommendations state that glucarpidase should be given as a single intravenous injection of 50 units/kg [13].
Although glucarpidase has been shown to be effective in rapidly reducing methotrexate plasma concentrations [2–9,12,14–27], the pharmacoeconomic impact of its use is substantial. The current average wholesale price (AWP) of glucarpidase is $27,000 per 1,000 unit vial. Therefore, one dose of glucarpidase in a 70 kg patient (i.e., four vials needed to supply 3,500 units) may cost >$100,000 if dosed per the FDA approved dosage. While this cost must be weighed against the cost of other interventions such as, dialysis and/or prolonged hospitalization, justification for such drug expenditures may be challenging. Current Centers for Medicare and Medicaid Services guidelines allow for reimbursement of two glucarpidase vials per administration [28]. The successful use of reduced-dose glucarpidase has been reported [22,29,30]. This has led some clinicians to question whether choosing a lowerdose of glucarpidase or capping the dose at a full vial size would be a more cost-effective approach to treating patients with methotrexate induced renal dysfunction. This report explores the relationship between glucarpidase dosage and patient outcomes in pediatric oncology patients, and provides perspective on the potential cost-savings of a capped-dose (i.e., maximum of 2,000 units) approach.
METHODS
A retrospective chart review was performed examining all patients who received glucarpidase after high-dose methotrexate at St. Jude Children’s Research Hospital prior to August 2012. One patient received glucarpidase after its FDA approval in January 2012. Otherwise, glucarpidase was provided through investigational drug protocols and per-patient emergency use, and written informed consent was obtained from the parent, legal guardian, and/or patient as appropriate. Criteria used for glucarpidase administration varied by protocol. All patients who received glucarpidase had an increased serum creatinine of ≥1.5 times their baseline value. The methotrexate plasma concentration was >50 μM at 24 hr from the start of the methotrexate infusion for all patients with osteosarcoma with one exception. This patient had a 24 hr methotrexate plasma concentration of 45.1 μM and a corresponding SCr that was 3.75 times baseline. The methotrexate plasma concentration was >10 μM at 42 hr from the start of the methotrexate infusion for all other patients with the exception of one patient who received glucarpidase prior to 42 hr. All patients received standard supportive care including, aggressive alkalinized hydration and leucovorin rescue before and after glucarpidase administration; this supportive care was individualized based on standards of care at our institution. Leucovorin administration was held for 2 hr before and after glucarpidase administration per protocol. This study was approved by the Institutional Review Board.
Methotrexate dosages and infusion times varied by diagnosis, therapeutic protocol, and/or patient specific factors. Patients with osteosarcoma received 4- or 6-hr infusions at methotrexate dosages of 12 g/m2. Patients with acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma received either 4- or 24-hr infusions with methotrexate dosages ranging from 2.5 to 8 g/m2. All methotrexate plasma concentrations were analyzed with a commercially available fluorescence polarization immunoassay using an Abbott TDxFLx analyzer (Abbott Laboratories, Abbott Park, IL). A subset of patients had methotrexate plasma concentrations analyzed via high performance liquid chromatography (HPLC) as previously reported [16]. HPLC analysis was performed due to the non-specificity of commercial immunoassays for 4-[[2,4-diamino-6-(pteridinyl) methyl]-methylamino]-benzoic acid (DAMPA), the byproduct of the enzymatic reaction between glucarpidase and methotrexate. This metabolite is known to cause overestimation of methotrexate concentrations measured by immunoassays [13].
DATA ANALYSIS
Data collection included demographic information, methotrexate, and glucarpidase regimens, methotrexate pharmacokinetic data including, methotrexate plasma concentrations measured by TDx and HPLC, when available, and serum creatinine (SCr). All patients’ SCr was measured at least once daily. Percent change in plasma methotrexate concentration after glucarpidase administration measured by TDx and HPLC and time to recovery of SCr in relation to glucarpidase dosage (units/kg) were compared using the Spearman correlation coefficient test or the Exact Wilcoxon Two-Sample test when tested as categorical variables. A multivariable analysis was used to examine the relationship between change in plasma methotrexate concentration after glucarpidase administration and other variables. Time to recovery of SCr was defined as the time for SCr to return to ≤1.5x the pre-methotrexate baseline from the time of glucarpidase administration.
RESULTS
Retrospective data from 26 pediatric patients who received glucarpidase after high-dose methotrexate were analyzed. The median age of this population was 11.7 years (range, 4.0–20.4 years) (Table I). Twenty-one of 26 doses of glucarpidase were started within 48 hr of the start of the high-dose methotrexate infusion, and five doses were given more than 48 hr after the start of methotrexate (at 48.25, 49, 51.5, 52.5, and 95 hr). Glucarpidase dosages administered ranged from 13–90 units/kg. All glucarpidase dosages <50 units/kg were administered as a result of capping a dose at a full vial size. Likewise, in all cases, dosages >50 units/kg were administered due to rounding up to the next full vial.
TABLE I.
Patient Characteristics of 26 Patients at the Time They Received Glucarpidase for Delayed Methotrexate Clearance
| Characteristic | All patients (n =26) | OS (n =10) | ALL (n =12) | Other (n =4) |
|---|---|---|---|---|
| Age, years Median Range | 11.7 4.0–20.4 | 11.7 9.8–20.4 | 6.2 4.6–18.7 | 19.2 4.0–20.2 |
| Sex, no. of pts Male Female | 18 8 | 5 5 | 10 2 | 3 1 |
| Methotrexate dosage, g/m2 Median Range | 7.4 2.5–12.4 | 12.0 11.5–12.4 | 4.6 2.5–8 | 6.1 3.3–8 |
| Methotrexate infusion time, hr Median Range | 11 4–24.1 | 4 4–6 | 24 4–24.1 | 11 4–20 |
| Glucarpidase dosage, units/kg Median Range | 50.8 13.0–90.1 | 51.6 15.0–65.6 | 50.8 13.0–90.1 | 39.3 21.0–65.0 |
| Pre-glucarpidase MTX concentration (TDx), μmol Median Range | 38.6 1.31–590.6 | 154.3 32.2–590.6 | 15.7 1.31–222.1 | 54.6 16.5–239.8 |
| Post-glucarpidase MTX concentration (TDx), μmol Median Range | 5.6 0.35–82.8 | 8.8 0.7–82.8 | 4 0.35–36.9 | 6.8 1–26.8 |
| Post-glucarpidase MTX concentration (HPLC), μmol Median Range | 0.75 <0.15–5 | 1.4 0.3–5 | 0.15 <0.15–3.6 | |
| Time to glucarpidase dose from start of MTX, hr Median Range | 44.5 25.8–95 | 29 25.8–46.5 | 47 26.5–95 | 42.3 28.8–48 |
| Time to post-glucarpidase MTX concentration from glucarpidase administration, hr Median Range | 3.5 0.5–22 | 3.0 1–20.25 | 5.5 1–20.5 | 1.75 0.5–11.5 |
| Decrease in MTX concentration (TDx), % Median Range | 81.8 61.9–98.9 | 84.5 71.1–98.9 | 78.5 61.9–94.6 | 86.0 70.5–95.9 |
| Decrease in MTX concentration (HPLC), % Median Range | 99 77.2–100 | 99.3 98.6–100 | 99.8 77.2–100 | |
| SCr at baseline (pre-MTX), mg/dL Median Range | 0.45 0.2–0.9 | 0.45 0.3–0.9 | 0.35 0.2–0.8 | 0.7 0.3–0.9 |
| SCr at time of glucaridase administration, mg/dl Median Range | 1.75 0.7–4.8 | 1.85 1–3.4 | 1.65 0.7–2.8 | 2.55 1.3–4.8 |
| Time to recovery of renal function, days Median Range | 18 2–54 | 17 2–28 | 23 6–54 | 16 4–40 |
Pre- and post-glucarpidase plasma methotrexate concentrations were analyzed via TDx for all 26 patients, and 14 patients had post-glucarpidase plasma methotrexate concentrations analyzed via HPLC. All 26 patients experienced a reduction in plasma methotrexate concentration from the pre-glucarpidase measurement to the first post-glucarpidase measurement, at a median of 3.5 hr (range, 0.5–22 hr) post-glucarpidase (Table I).
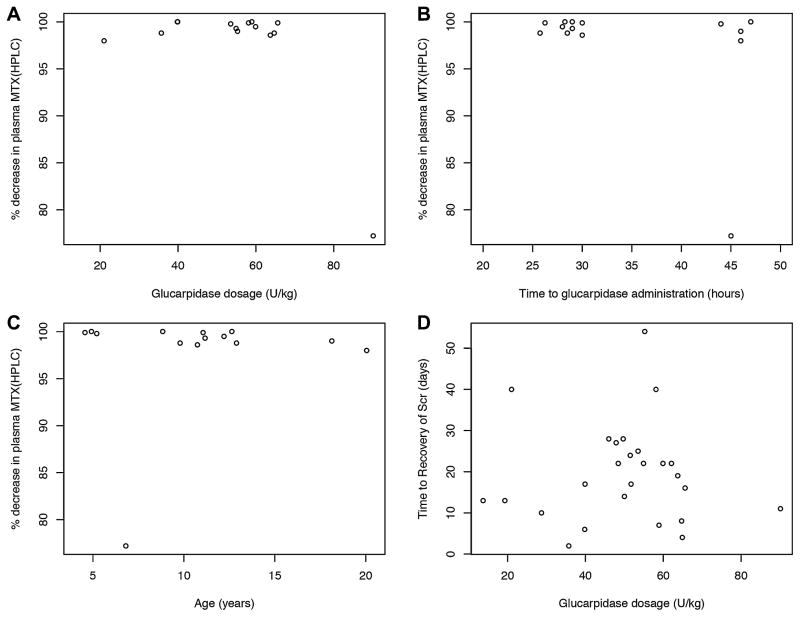
A multivariable analysis revealed no statistically significant relationship between glucarpidase dosage (units/kg) and percent decrease in plasma methotrexate concentration measured by TDx (P >0.1) or HPLC (P >0.5) (Table I, Fig. 1). The only statistically significant correlation noted was between patient disease type and the percentage decrease in plasma methotrexate concentration by TDx. The association between age and percent decrease in methotrexate plasma concentration approached statistical significance (P =0.06). A majority of doses of glucarpidase (81%) were started over the relatively narrow time period of 25 to 48 hours from the start of the methotrexate infusion, and the percent decrease in plasma methotrexate concentration after glucarpidase was similar in these patients. No significant association was observed between the time from the start of the methotrexate infusion to glucarpidase administration and the percent decrease in plasma methotrexate concentration by TDx when data from all patients were included (r2 =0.13, P =0.08). Similarly, no statistically significant association was found between glucarpidase dosage (units/kg) and time to recovery of SCr (P >0.8) (Table I, Figure 1).
Fig. 1.
Dot plots showing (A) the percent decrease in plasma methotrexate concentration measured by HPLC following glucarpidase administration vs glucarpidase dosage in all patients with a measurement; (B) percent decrease in plasma methotrexate concentration measured by HPLC following glucarpidase administration vs time from start of methotrexate infusion to glucarpidase administration in all patients with a measurement; (C) percent decrease in plasma methotrexate concentration measured by HPLC following glucarpidase administration versus patient age in years in all patients with a measurement; and (D) time to recovery of serum creatinine versus glucarpidase dosage following a single glucarpidase dose in all patients studied.
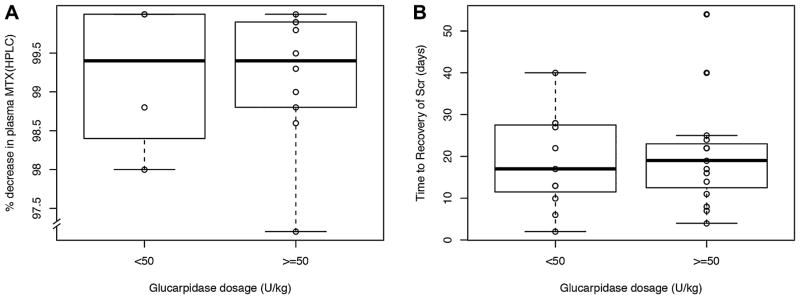
Forty-two percent of patients received glucarpidase dosages <50 units/kg. Patients who received glucarpidase dosages <50 units/kg had a median percent reduction in methotrexate plasma concentration of 99.4% (range, 98–100) measured by HPLC and 78.2% (range, 69.8–98.9) measured by TDx compared to a median percent reduction of 99.4% (range, 77.2–100) measured by HPLC and 82.9% (range, 61.9–99.4) measured by TDx in patients who received ≥50 units/kg (P >0.4)(Fig. 2). Five patients, ranging in body weight from 50.1 to 145.7 kg, received a glucarpidase dose capped at 2,000 units, which resulted in a dosage of less than 50 U/ kg (range, 13.7–39.9 U/kg). Of these five patients, two had post-glucarpidase methotrexate plasma concentrations measured by HPLC. A reduction in methotrexate plasma concentration of 98% and >99%, respectively was achieved.
Fig. 2.
(A) Percent decrease in plasma methotrexate concentration following a dose of glucarpidase in patients receiving high-dose methotrexate (>2.5 g/m2) displayed by glucarpidase dosage group; the percent decrease in plasma methotrexate concentration was not different in patients who received <50 units/kg of glucarpidase (n =4) versus those who received >50 units/kg of glucarpidase (n =10; P >0.4; Wilcoxon rank sum test). Each data point indicates the percent decrease in plasma methotrexate (pre-glucarpidase–post-glucarpidase) for an individual patient measured by HPLC. (B) Time to renal recovery following a dose of glucarpidase in patients receiving high-dose methotrexate (>2.5 g/m2) displayed by glucarpidase dosage group; the time to renal recovery was not different in patients who received <50 units/kg of glucarpidase (n =11) vs those who received >50 units/kg of glucarpidase (n =15; P >0.8; Wilcoxon rank sum test). Each data point indicates the time in days for an individual patient’s serum creatinine to return to <1.5x the pre-methotrexate baseline from the time of glucarpidase administration. Boxes include data between the 25th and 75th percentiles; bars indicate the median value, and whiskers indicate the minimal and maximal values (excluding outliers).
Patients who received glucarpidase dosages <50 units/kg had similar time to recovery of SCr as compared to patients who received ≥50 units/kg (P >0.9). One patient with lymphoma received hemodialysis for electrolyte abnormalities. This patient received 48.4 units/kg of glucarpidase. Methotrexate plasma concentration data measured via HPLC were not available for this patient. SCr did recover to ≤1.5x baseline in all patients included in this analysis.
DISCUSSION
High-dose methotrexate-induced acute kidney injury is an oncologic emergency that can potentially result in serious toxicity [31]. The close monitoring of patients and early intervention to decrease elevated plasma methotrexate concentrations are necessary elements of patient management [11]. Glucarpidase provides an important and effective treatment to rapidly decrease methotrexate concentrations in patients with delayed methotrexate elimination [27].
In this analysis we observed that glucarpidase dosages varying from 13 to 90.1 units/kg were effective in rapidly decreasing plasma methotrexate concentrations. These data demonstrate that glucarpidase dosages lower than 50 units/kg were effective in reducing methotrexate plasma concentrations in pediatric cancer patients. Furthermore, higher dosages of glucarpidase were not associated with more rapid recovery of renal function (measured by SCr) in this population. A statistically significant association between patient disease type and percent decrease in methotrexate plasma concentration and a trend towards significance between patient age and percent decrease in methotrexate plasma concentration were observed. These associations may be attributed to differing MTX dosages used for different diagnoses. The ALL patients included in the analysis had a lower median age and received lower MTX dosages over 24 hr as compared to patients with a diagnosis of osteosarcoma and non-lymphoblastic lymphoma, who received higher dosages of MTX over 4 hr, and had higher median pre-glucarpidase MTX plasma concentrations.
It should be noted that in our small sample size there were few courses of glucarpidase that were started more than 48 hr from the start of methotrexate, and thus we observed a high percent decrease in methotrexate plasma concentrations in all patients. Other studies have found that delays in the administration of glucarpidase can greatly interfere with the effectiveness of the drug [7]. This lack of effectiveness has been attributed to delayed administration of glucarpidase at which time most of the MTX is not circulating, but is retained intracellularly; therefore, although we would not expect a lower dose of glucarpidase to hydrolyze MTX less efficiently if administered at a later time point, we cannot address whether glucarpidase dosage may be associated with efficacy in cases where glucarpidase administration is delayed.
Given the cost of glucarpidase, the pharmacoeconomic impact of glucarpidase dose selection is noteworthy. There is debate regarding glucarpidase’s place in therapy for patients experiencing methotrexate-induced renal insufficiency. Much of this debate surrounds the fact that this drug is very expensive, and arguably unnecessary in some situations [32]. However, the cost of glucarpidase administration may be offset by the high cost of alternative interventions such as dialysis and/or prolonged hospitalization.
These data indicate that rounding glucarpidase doses down to, for example, the nearest vial size when patients’ doses require opening additional vials to administer a full 50 units/kg, or capping doses may be a more cost-effective approach to effectively lower methotrexate plasma concentrations. At least one study has suggested that capping glucarpidase doses is feasible [29]. Regarding the potential pharmacoeconomic impact of dose rounding in this report, had all glucarpidase doses in this report been capped at 2,000 units, the institution’s total use of glucarpidase would have decreased by 20 vials, resulting in a cost savings to the institution of >$500,000 based on glucarpidase AWP. Our institution has since adopted the practice of capping individual glucarpidase doses at a maximum of 2,000 units. Given that the current Centers for Medicare and Medicaid Services guidelines allow for reimbursement of two glucarpidase vials per administration [28], the utilization of a capped dose of, for example, 2,000 units might allow more institutions to provide glucarpidase therapy to their patients.
The information presented in this report regarding the potential for dose selection and decreased cost of glucarpidase utilization is vital to consider during discussions surrounding an institution’s use of glucarpidase, potentially allowing for a more efficient use of this important agent. Further studies are needed to validate this dose capping/ vial-size rounding approach; however, these data indicate that the utilization of glucarpidase dosages less than 50 units/kg in pediatric oncology patients are effective in decreasing methotrexate plasma concentrations.
Acknowledgments
Grant sponsor: NIH CA; Grant number: 21765 ; Grant sponsor: GM; Grant number: 92666; Grant sponsor: American Lebanese Syrian Associated Charities (ALSAC)
We thank our patients and their families. We also thank Charles Rose for outstanding technical assistance.
Abbreviations
- ALL
acute lymphoblastic leukemia
- OS
osteosarcoma
- MTX
methotrexate
- HPLC
high performance liquid chromatography
- TDx
fluorescence polymerization immunoassay
- SCr
serum creatinine
Footnotes
Conflict of interest: Nothing to declare.
References
- 1.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 2.Snyder RL. Resumption of high-dose methotrexate after methotrexate-induced nephrotoxicity and carboxypeptidase G2 use. Am J Health Syst Pharm. 2007;1:1163–1169. doi: 10.2146/ajhp060187. [DOI] [PubMed] [Google Scholar]
- 3.Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, Bacci G, Craft AW, Adamson PC. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;15:2222–2232. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis LM, Tong WP, Lin S, Fleisher M, Bertino JR. Carboxypeptidase G2 rescue after high-dose methotrexate. J Clin Oncol. 1996;14:2145–2149. doi: 10.1200/JCO.1996.14.7.2145. [DOI] [PubMed] [Google Scholar]
- 5.Krause AS, Weihrauch MR, Bode U, Fleischhack G, Elter T, Heuer T, Engert A, Diehl V, Josting A. Carboxypeptidase-G2 rescue in cancer patients with delayed methotrexate elimination after high-dose methotrexate therapy. Leukemia lymphoma. 2002;43:2139–2143. doi: 10.1080/1042819021000032953. [DOI] [PubMed] [Google Scholar]
- 6.Mohty M, Peyriere H, Guinet C, Hillaire-Buys D, Blayac JP, Rossi JF. Carboxypeptidase G2 rescue in delayed methotrexate elimination in renal failure. Leukemia lymphoma. 2000;37:441–443. doi: 10.3109/10428190009089446. [DOI] [PubMed] [Google Scholar]
- 7.Widemann BC, Balis FM, Kim A, Boron M, Jayaprakash N, Shalabi A, O’Brien M, Eby M, Cole DE, Murphy RF, Fox E, Ivy P, Adamson PC. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;1:3979–3986. doi: 10.1200/JCO.2009.25.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widemann BC, Balis FM, Murphy RF, Sorensen JM, Montello MJ, O’Brien M, Adamson PC. Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol. 1997;15:2125–2134. doi: 10.1200/JCO.1997.15.5.2125. [DOI] [PubMed] [Google Scholar]
- 9.Widemann BC, Hetherington ML, Murphy RF, Balis FM, Adamson PC. Carboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicity. Cancer. 1995;1:521–526. doi: 10.1002/1097-0142(19950801)76:3<521::aid-cncr2820760325>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Abelson HT, Fosburg MT, Beardsley GP, Goorin AM, Gorka C, Link M, Link D. Methotrexate-induced renal impairment: Clinical studies and rescue from systemic toxicity with high-dose leucovorin and thymidine. J Clin Oncol. 1983;1:208–216. doi: 10.1200/JCO.1983.1.3.208. [DOI] [PubMed] [Google Scholar]
- 11.Relling MV, Fairclough D, Ayers D, Crom WR, Rodman JH, Pui CH, Evans WE. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12:1667–1672. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 12.Green JM. Glucarpidase to combat toxic levels of methotrexate in patients. Ther Clin Risk Manag. 2012;8:403–413. doi: 10.2147/TCRM.S30135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voraxaze;1; [package insert] BTG International Inc; Brentwood, TN: 2012. [Google Scholar]
- 14.Patterson DM, Lee SM. Glucarpidase following high-dose methotrexate: Update on development. Expert Opin Biol Ther. 2010;10:105–111. doi: 10.1517/14712590903468677. [DOI] [PubMed] [Google Scholar]
- 15.Buchen S, Ngampolo D, Melton RG, Hasan C, Zoubek A, Henze G, Bode U, Fleischhack G. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Brit J Cancer. 2005;14:480–487. doi: 10.1038/sj.bjc.6602337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen AM, Pauley JL, Molinelli AR, Panetta JC, Ward DA, Stewart CF, Hoffman JM, Howard SC, Pui CH, Pappo AS, Relling MV, Crews KR. Resumption of high-dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patients. Cancer. 2012;1:4321–4330. doi: 10.1002/cncr.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteve MA, Devictor-Pierre B, Galy G, Andre N, Coze C, Lacarelle B, Bernard JL, Monjanel-Mouterde S. Severe acute toxicity associated with high-dose methotrexate (MTX) therapy: Use of therapeutic drug monitoring and test-dose to guide carboxypeptidase G2 rescue and MTX continuation. Eur J Clin Pharmacol. 2007;63:39–42. doi: 10.1007/s00228-006-0212-1. [DOI] [PubMed] [Google Scholar]
- 18.Hum M, Kamen BA. Successful carboxypeptidase G2 rescue in delayed MTX-elimination due to renal failure. Pediatr Hemat Oncol. 1995;12:521–524. doi: 10.3109/08880019509030765. [DOI] [PubMed] [Google Scholar]
- 19.Nowicki TS, Bjornard K, Kudlowitz D, Sandoval C, Jayabose S. Early recognition of renal toxicity of high-dose methotrexate therapy: A case report. Pediatr Hemat Oncol. 2008;30:950–952. doi: 10.1097/MPH.0b013e318182e73e. [DOI] [PubMed] [Google Scholar]
- 20.Park ES, Han KH, Choi HS, Shin HY, Ahn HS. Carboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicity. Cancer Res Treat. 2005;37:133–135. doi: 10.4143/crt.2005.37.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peyriere H, Cociglio M, Margueritte G, Vallat C, Blayac JP, Hillaire-Buys D. Optimal management of methotrexate intoxication in a child with osteosarcoma. Ann Pharmacother. 2004;38:422–427. doi: 10.1345/aph.1D237. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz S, Borner K, Muller K, Fischer L, Korfel A, Auton T, Thiel E. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. Oncologist. 2007;12:1299–1308. doi: 10.1634/theoncologist.12-11-1299. [DOI] [PubMed] [Google Scholar]
- 23.Sieniawski M, Rimpler M, Herrmann R, Josting A. Successful carboxypeptidase G2 rescue of a high-risk elderly Hodgkin lymphoma patient with methotrexate intoxication and renal failure. Leukemia lymphoma. 2007;48:1641–1643. doi: 10.1080/10428190701447338. [DOI] [PubMed] [Google Scholar]
- 24.Tuffaha HW, Al Omar Glucarpidase rescue in a patient with high-dose methotrexate-induced nephrotoxicity. J Oncol Pharma Pract. 2011;17:136–140. doi: 10.1177/1078155209348720. [DOI] [PubMed] [Google Scholar]
- 25.Vilay AM, Mueller BA, Haines H, Alten JA, Askenazi DJ. Treatment of methotrexate intoxication with various modalities of continuous extracorporeal therapy and glucarpidase. Pharmacotherapy. 2010;30:111. doi: 10.1592/phco.30.1.111. [DOI] [PubMed] [Google Scholar]
- 26.Zoubek A, Zaunschirm HA, Lion T, Fischmeister G, Vollnhofer G, Gadner H, Pillwein K, Schalhorn A, Bode U. Successful carboxypeptidase G2 rescue in delayed methotrexate elimination due to renal failure. Pediatr Hemat Oncol. 1995;12:471–477. doi: 10.3109/08880019509009477. [DOI] [PubMed] [Google Scholar]
- 27.Widemann BC, Schwartz S, Jayaprakash N, Christensen R, Pui CH, Chauhan N, Daugherty C, King TR, Rush JE, Howard SC. Efficacy of glucarpidase (carboxypeptidase g2) in patients with acute kidney injury after high-dose methotrexate therapy. Pharmacotherapy. 2014;34:427–439. doi: 10.1002/phar.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson CA. CMS to compensate hospitals for inpatient use of fidaxomicin, glucarpidase. Am J Health Syst Pharm. 2012;1:1618–1619. doi: 10.2146/news120070. [DOI] [PubMed] [Google Scholar]
- 29.Trifilio S. Reduced-dose carboxypeptidase-g2 successfully lowers elevated methotrexate levels in an adult with acute methotrexate-induced renal failure. Clin Adv Hematol Oncol. 2013;11:322–325. [PubMed] [Google Scholar]
- 30.Widemann BC. Using a lower dose of glucarpidase to reduce plasma levels of methotrexate. Clin Adv Hematol Oncol. 2013;11:324–325. [PubMed] [Google Scholar]
- 31.Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996;42(8 Pt 2):1322–1329. [PubMed] [Google Scholar]
- 32.Meyers PA, Flombaum C. High-dose methotrexate-induced renal dysfunction: Is glucarpidase necessary for rescue? J Clin Oncol. 2011;1:E180–E180. doi: 10.1200/JCO.2010.32.8245. [DOI] [PubMed] [Google Scholar]