Abstract
Objective
Fecal incontinence reduces the quality of life of many women but has no long-term cure. Research on mesenchymal stem cell (MSC)-based therapies has shown promising results. The primary aim of this study was to evaluate functional recovery after treatment with MSCs in two animal models of anal sphincter injury.
Methods
Seventy virgin female rats received a sphincterotomy (SP) to model episiotomy, a pudendal nerve crush (PNC) to model the nerve injuries of childbirth, a sham SP, or a sham PNC. Anal sphincter pressures and electromyography (EMG) were recorded after injury but before treatment and 10 days after injury. Twenty-four hours after injury, each animal received either 0.2 ml saline or 2 million MSCs labelled with green fluorescing protein (GFP) suspended in 0.2 ml saline, either intravenously (IV) into the tail vein or intramuscularly (IM) into the anal sphincter.
Results
MSCs delivered IV after SP resulted in a significant increase in resting anal sphincter pressure and peak pressure, as well as anal sphincter EMG amplitude and frequency 10 days after injury. MSCs delivered IM after SP resulted in a significant increase in resting anal sphincter pressure and anal sphincter EMG frequency but not amplitude. There was no improvement in anal sphincter pressure or EMG with in animals receiving MSCs after PNC. GFP-labelled cells were not found near the external anal sphincter in MSC-treated animals after SP.
Conclusion
MSC treatment resulted in significant improvement in anal pressures after SP but not after PNC, suggesting that MSCs could be utilized to facilitate recovery after anal sphincter injury.
Introduction
Psychological and social ostracism are common issues that patients debilitated by fecal incontinence (FI) encounter (Lazarescu et al., 2009). Although the cause of anal sphincter incontinence is multi-factorial (Kouraklis and Andromanakos, 2004; Safioleas et al., 2008), the prevalence is known to be higher in women due to childbirth injuries (Pretlove et al., 2006). However, the clinical manifestations of FI may not occur at the time of injury but most often manifest years later (Halverson and Hull, 2002).
Surgical repair is one of the treatments for a damaged anal sphincter; however, sphincter function deteriorates over time and long-term outcome remains unsatisfactory (Gutirrez et al., 2003; Halverson and Hull, 2002; Karoui et al., 2000; Malouf et al., 2000; Zutshi et al., 2009b). Newer treatment options include neuromodulation (Hosker et al., 2007), the Secca procedure (Takahashi-Monroy et al., 2008), bulking agents (Chan and Tjandra, 2006; Kenefick et al., 2007) and an artificial bowel sphincter (Altomare et al., 2009). The multiple treatment options and unsatisfactory long-term outcomes point to the need for innovative treatments for FI that have long-term durability.
Several studies have investigated the role of mesenchymal stem cells (MSCs) in improving anal sphincter function after direct injection of stem cells to the anal sphincter muscles (Kajbafzadeh et al., 2010; Kang et al., 2008; Lorenzi et al., 2008; Pathi et al., 2012). The results of these studies are promising; however, only ex vivo outcomes were utilized and the in vivo effects on anal pressures were not assessed. Pathi et al. (2012) investigated the effect of IV and direct injection on neurophysiology studies and studied mRNA levels of anti-inflammatory genes, genes highly expressed after an acute and genes involved in matrix synthesis as a function of time. In addition, investigations in animal models of heart failure demonstrate a therapeutic effect of MSCs infused intravenously (IV), which may provide a less invasive delivery route for MSCs than those previously tested for treatment of FI (Shabbir et al., 2009a, 2009b).
We have developed rat models of anal sphincter dysfunction induced via sphincterotomy (SP), or pudendal nerve injury to model the nerve injuries of childbirth, and have demonstrated changes in anal sphincter pressures in vivo lasting up to 4 weeks after the injury (Salcedo et al., 2010). We have also demonstrated upregulation of MCP-3 and SDF-1 in the anal sphincter complex after injury (Salcedo et al., 2011). The goal of this project was to investigate the changes in anal sphincter pressure after IV or intramuscular (IM) injection of MSCs in our previously established animal models, with the long-term goal of developing improved therapy for patients with FI.
Material and methods
This study was approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Mesenchymal stem cell harvesting and cell culturing
Virgin female Sprague–Dawley rats were euthanized and bone marrow was harvested from the tibia and femurs by gently flushing the bone with 1 ml Dulbecco's modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA, USA). To separate adherent cells, bone marrow clumps were passed through 18 and 20 gauge needles. The cells were centrifuged at 2500 rpm for 5 min with three changes of PBS. The washed cells were placed in a vented cell culture T75 flask (3151 Costar, Corning Incorporated, Corning, NY) with 25 ml DMEM (Gibco, Invitrogen Corp., USA) containing 10% FBS and 1% antibiotic and anti-mycotic solution (Gibco 15240, Invitrogen Corp., USA) and were incubated at 37 °C. At this stage, the cells were identified as P0. The media was changed 3 days later to remove non-adherent cells. Succeeding media changes were made every 3–4 days according to cellular confluence. At 70–80% confluence, the adherent cells were detached after incubation with 0.05% trypsin and 2 mM EDTA for 5–10 min.
At passage P4, cultures were negatively selected for MSC. Cell sorting for MSC was performed with an EasySep pycoerythrin (PE) selection kit according to the manufacturer's instructions (Stem Cell Technologies, Vancouver, B.C., Canada). The cultures were simultaneously depleted of CD45+ and CD34+ cells using 10 μl of each of the primary PE-conjugated antibodies: mouse anti-rat CD45+ (BD Biosciences, San Diego, CA, USA) and mouse anti-CD34+ (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for every 106 cells.
Green fluorescent protein (GFP) labelling
After sorting and MSC selection, when the cells reached 80–90% confluence, MSCs were transfected with a lentivirus vector pCCLsin.ppt.hPGK.GFP.pre (a generous gift from the Cossu Lab), which uses a human PGK promoter to constitutively express green fluorescent protein (GFP), and were processed overnight by incubation in a mixture of normal medium (6 ml), polybrene (6 μl) and 10× MOI (10 million viruses for each million cells). Transduction proceeded overnight and the medium was changed after 6–8 h incubation. MSC were checked for GFP-labelled cells under immunoflouroscopy and expanded until P12–20 when they were utilized for the study. Cultures were then trypsinized and spun at 2500 rpm for 5 min. Cells were resuspended in PBS (0.2 ml for 2 million cells) for animal experiments.
Animal models
Seventy age-matched female Sprague–Dawley rats weighing 240–260 g were randomly allocated into the following groups: sphincterotomy (SP; n=20), pudendal nerve crush (PNC; n= 20), sham SP (n=10) and sham PNC (n=20). SP was performed under ketamine (100 mg/kg) and xylazine (10 mg/kg) i.p. anesthesia by incising the external and internal anal sphincters 2–3 mm deep, as we have done previously (Zutshi et al., 2009a). Anal sphincter transection was confirmed with a dissecting microscope. Since the anal sphincter in the rat is small and superficial, even a minute incision could incorporate a part of the anal sphincter. Therefore, sham SP was created by pressing a Q-tip on the anal sphincter for 5 s.
PNC was performed under the same anesthesia via a posterior incision in the sacro-coccygeal area. The pudendal nerves were isolated bilaterally in the ischiorectal fossa and crushed twice for 30 s each with a Castroviejo needle holder, as we have done previously (Salcedo et al., 2010; Zutshi et al., 2009a). Sham PNC was created by making a similar incision in the sacro-coccygeal area and gently opening the ischiorectal fossa bilaterally without crushing the pudendal nerve.
MSC treatment
Twenty-four hours after injury, animals in each injury group received either 2 million MSC in 0.2 ml PBS (Sphincterotomy n=10 (IM AND IV) and pudendal nerve crush n=10 (IM and IV) sham SP n=10 (IM AND IV) and sham PNC n=10 (IM and IV) total n=40) or PBS without MSCs (Sphincterotomy n=10 (IM and IV), PNC n=10 (IM and IV) and sham PNC n=10 (IM and IV) total n=30) either IM in the anal sphincter via 4 injections, ¼ dose in each circumferential quadrant (n=35), or intravenously (IV) via the tail vein (n=35). This time point was selected since we have previously found that the stem cell homing cytokines SDF-1 and MCP-3 are maximally elevated 24 h after injury (Salcedo et al., 2011).
Functional testing
Anal pressure (basal pressure) and anal sphincter electromyography (EMG) were performed after injury but before treatment, as well as 10 days after injury (9 days after treatment) in all animals, as we have done previously (Zutshi et al., 2009a). Under ketamine and xylazine anesthesia, resting anal pressure was measured using a saline filled balloon (Kent Scientific, Torrington, CT; size 4) inserted superficially in the anal sphincter and, via tubing (PE-90), connected to a pressure transducer (Grass Astromed, PT300, Warwick, RI), a digital amplifier (Astromed Inc, Model P122), and digital data recording system (Dash 8×, Astromed Inc.) as in our previous studies (Zutshi et al., 2009a). Anal sphincter electromyography (EMG) data were recorded simultaneously with anal sphincter pressures by placing a 30 G concentric needle electrode (Viasys Healthcare, Hawthorne, NY) in the external anal sphincter at the left posterolateral position. Ten days after injury, functional testing of both resting anal pressures and EMG was repeated under anesthesia, after which the animals were euthanized with an overdose of pentobarbital (200 mg/kg) i.p.
Immunofluorescence
The anal sphincter complex was dissected, immersion fixed in formalin, paraffin embedded, sectioned (5 μm) and prepared for immunofluorescence studies to localize MSC via GFP. Antigen retrieval was performed by incubating the sections in 10 mM sodium citrate buffer (pH 6.0) at 95 °C for 1 h, then cooled for 20 min. The slides were then washed in PBS for 5 min three times at room temperature. Slides were then incubated with 1% universal blocking buffer for 3 h at 37 °C to reduce any non-specific binding of IgG. Slides were then incubated overnight with rabbit anti-GFP (SC 8334, Santa Cruz Biotechnology; 1:200) and mouse anti-smooth muscle α-actin (SC 1306, Santa Cruz Biotechnology; 1:30) antibodies overnight at 4 °C. Slides were then washed with PBS and incubated for 2 h with Alexa Fluor 488 (A21206, Invitrogen Corp.; 1:800). The slides were again washed with PBS then incubated for 2 h with goat-anti-mouse IgG Texas red (SC 2781, Santa Cruz Biotechnology; 1:100). After extensive washing with PBS, the cover slips were mounted with aqueous mounting medium (Vectashield Mounting Medium) with DAPI (4′,6-diamidino-2-phenylindole) as a nuclear counterstain (H-1200; Vector Laboratories, Burlingame, CA, USA).
Tissues were analyzed using an upright spectral laser scanning confocal microscope (Model TCS-SP Leica Microsystems, Heidelberg Germany). GFP-positive cells were counted per 20 × field and scanned for 10 fields.
Data analysis
Anal pressure data was analyzed to determine resting pressure (RP), the baseline anal pressure at the start of each recording and peak contraction pressure (PC), and the maximum anal pressure during anal contraction. Three typical pressure waves were analyzed before treatment and 10 days after treatment. A mean was taken of each variable in each animal and used in further group analysis (Salcedo et al., 2010).
EMG activity was quantified using mean amplitude and frequency as we have done previously (Salcedo et al., 2010; Zutshi et al., 2009a). Three 5 s intervals were segmented from the EMG at each time point after injury. The mean of the rectified signal for each 5 s interval was calculated to obtain amplitude (Myosotic™ SignaPoint 2007, Myosotic LLC, Woodenville, WA). To find the frequency of the signal, a threshold was calculated in the uninjured state of sham PNC animals that received PBS treatment in the absence of pressure contraction, and was set above the noise amplitude but below the amplitude of motor unit action potentials. Subsequently, we counted the number of threshold crossings. Half the number of threshold crossing was taken as an estimate of firing rate and their frequency was calculated. The mean of each variable in each animal was calculated and used in further group analysis.
Statistically significant differences in EMG amplitude and frequency as well as in anal pressures between groups at each of the two time points and between time points were determined using a one way ANOVA with a Tukey–Kramer post-hoc test for pairwise comparisons. P<0.05 indicated a significant difference between experimental groups. Values are presented as mean±standard error of the mean (SEM). Immunofluorescence data was analyzed quantitatively in a blinded fashion.
Results
No mortality was reported in any of the treatment arms.
Anal sphincter pressures
Neither resting pressure nor peak pressure of the anal sphincter complex was significantly different between groups after injury but before treatment. However, resting pressure was significantly greater 10 days after SP in animals treated with MSCs compared to those treated with PBS, via either IM (p=0.04) (Fig. 1 top) or IV (p=0.01) (Fig. 1, bottom) routes of administration. Resting pressures of the sham SP animals were not significantly different from that of animals that received SP and cells or PBS.
Figure 1.
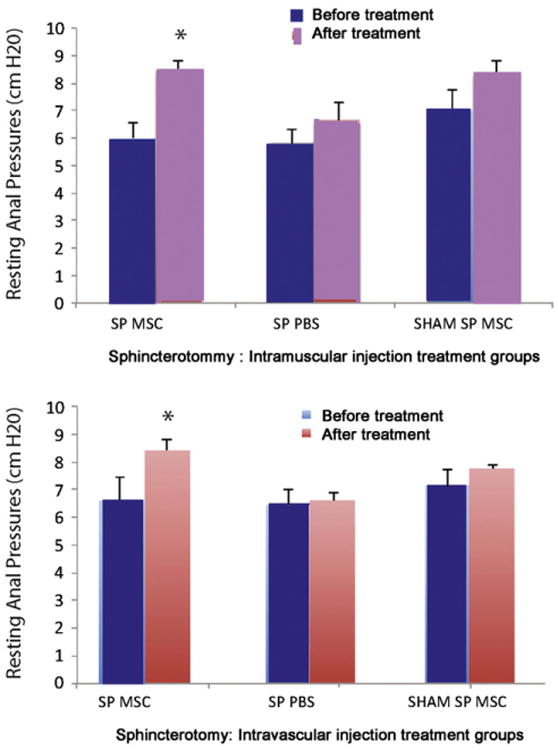
Anal sphincter pressures in animals 10 days after sphincterotomy (SP, n=10) or sham SP (n=10). Resting anal sphincter pressure in animals treated with intravascular (IV, n=5 (top) or intramuscular (IM, n=5 bottom) administration of mesenchymal stem cells (MSC) or saline (PBS, n=5). * indicates a significant difference compared to comparable saline treated group.
Although peak pressure of the anal sphincter increased in the SP group treated with MSCs delivered IM from 7.6±0.3 cm H2O after injury but before treatment to 13.9±1.5 cm H2O 9 days after treatment, peak pressure in MSC-treated animals with SP was not significantly greater than peak pressure in PBS-treated animals with SP (p=0.09). Peak pressure was significantly greater 9 days after treatment with MSC delivered IV after SP than 9 days after IV PBS treatment of SP (p= 0.04).
Resting pressure of the anal sphincter was significantly decreased 10 days after PNC compared to 10 days after sham PNC, when both were treated with PBS (p=0.01). Both PNC and sham PNC groups treated with MSCs delivered IV had resting pressure between that of PNC and sham PNC treated with PBS and were not significantly different from either (Fig. 2, bottom). There were no significant differences in either resting or peak pressure of the anal sphincter after PNC treated with MSC or PBS, IM or IV.
Figure 2.
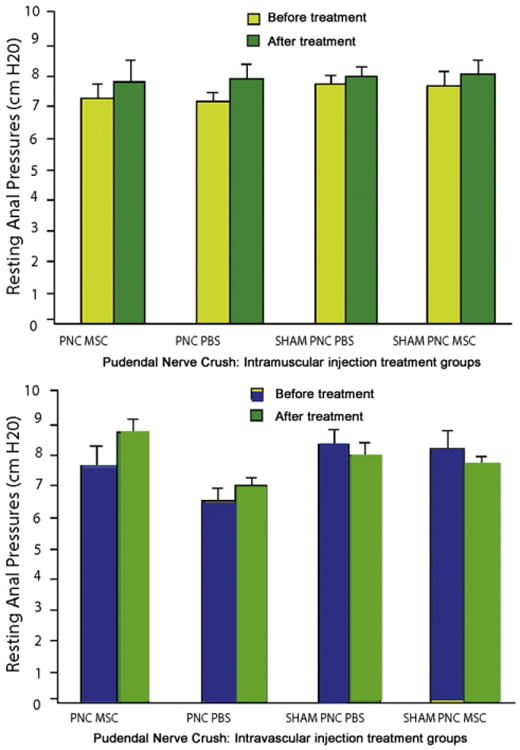
Anal sphincter pressures in animals 10 days after pudendal nerve crush (PNC, n=10) or sham PNC (n=10). Resting anal sphincter pressure in animals treated with intravascular (IV, n=5) (top) or intramuscular (IM, n=5) (bottom) administration of mesenchymal stem cells (MSC) or saline (n=5). * indicates a significant difference compared to comparable saline (PBS) treated group.
Anal sphincter EMG
Neither amplitude nor frequency of anal sphincter EMG was significantly different between groups after injury but before treatment.
Animals undergoing a sphincterotomy: Rats with SP treated with PBS had decreased external anal sphincter EMG amplitude 9 days after treatment compared to either rats with SP or rats with sham SP treated with MSCs. (Fig. 3) However, this difference was only significantly different between sham SP rats treated with MSCs delivered IM and SP rats treated with PBS given IM (p<0.001) and between rats with SP treated IV with PBS and rats with SP treated IV with MSCs (p=0.02). Frequency of external anal sphincter EMG, in contrast, was significantly decreased 9 days after PBS treatment compared to 9 days after MSC treatment via either IM (p=0.04) or IV (p= 0.003) routes of administration (Fig. 4).
Figure 3.
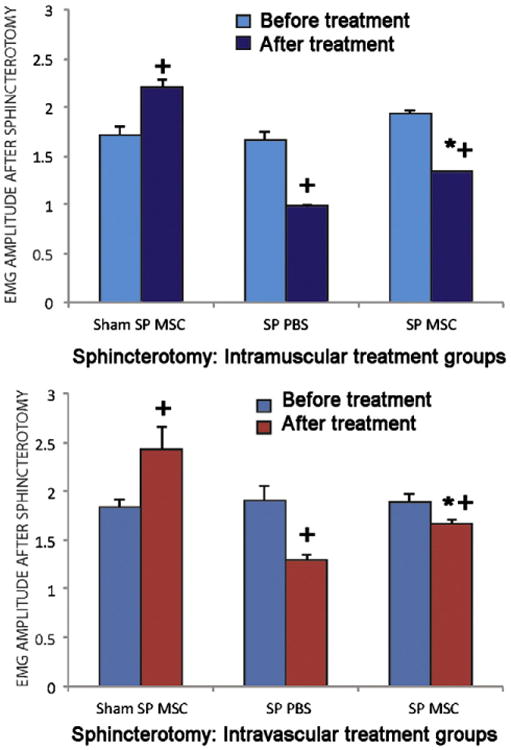
EMG amplitude in animals treated with intramuscular (IM, n=15) (top) and intravascular (IV, n=15) (bottom) injection of saline (control) or MSC 10 days after anal sphincterotomy (SP, n=20) and sham injury (Sham SP, n=10). MSC, mesenchymal stem cells; PBS, saline. * indicates a significant difference compared to comparable saline (PBS) treated group. + indicates a significant difference compared to before treatment.
Figure 4.
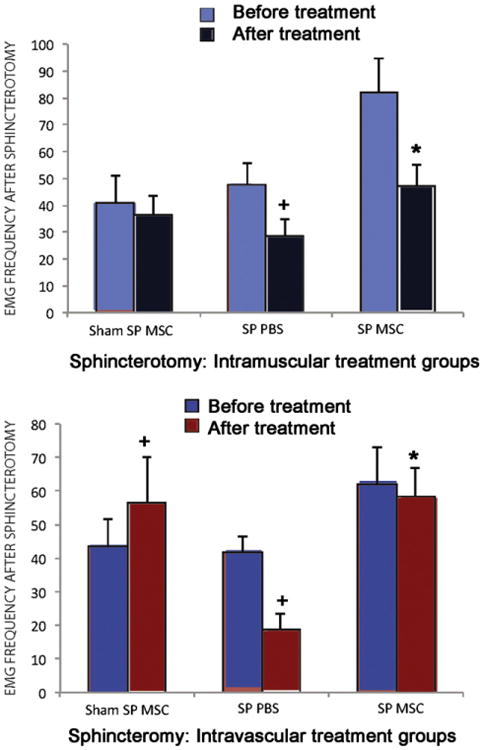
EMG frequency in animals treated with intramuscular (IM, n=15) (top) and intravascular (IV, n=15) (bottom) injection of saline (control) or MSC 10 days after anal sphincterotomy (SP, n=20) and sham injury (Sham SP, n=10). MSC, mesenchymal stem cells; PBS, saline. * indicates a significant difference compared to comparable saline (PBS) treated group. + indicates a significant difference compared to before treatment.
Animals undergoing a pudendal nerve crush: Although rats with PNC treated with MSCs had consistently greater external anal sphincter EMG amplitude (Fig. 5) and frequency (Fig. 6) than rats with PNC treated with PBS, these differences were not significantly different.
Figure 5.
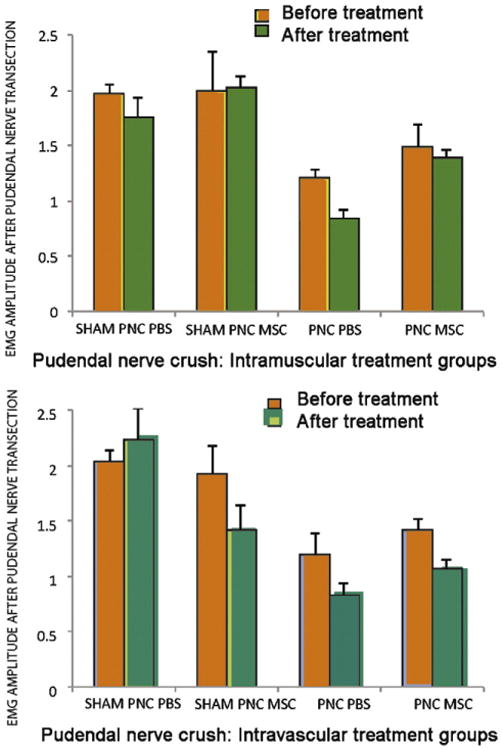
EMG amplitude in animals treated with intramuscular [IM, n=10 (top)] and intravenous (n=10) (bottom) injection of saline (n=10) (control) or MSC 10 days after pudendal nerve crush (PNC, n=20) and sham injury (Sham PNC, n=10). MSC, mesenchymal stem cells; PBS, saline.
Figure 6.
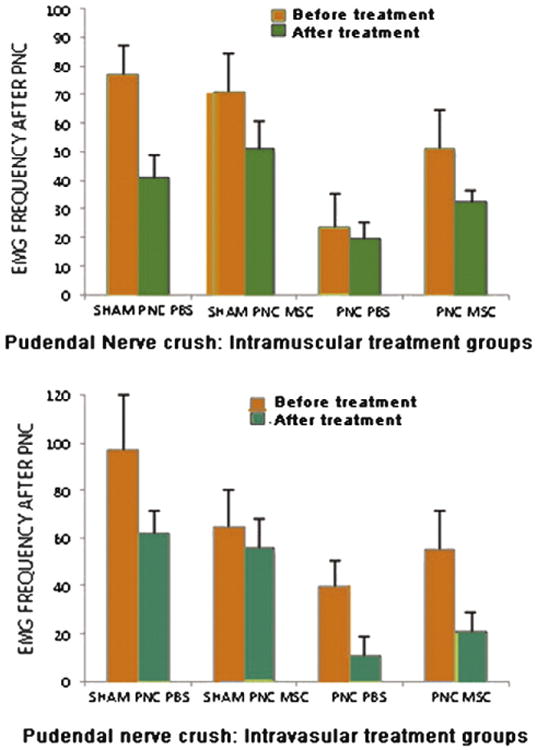
EMG frequency in animals treated with intramuscular (IM, n=10) (top) and intravascular (IV, n=10) (bottom) injection of saline (n=10) (control) or MSC 10 days after pudendal nerve crush (PNC) and sham injury (Sham PNC). MSC, mesenchymal stem cells; PBS, saline.
Immunofluorescence
Qualitative analysis under confocal microscopy did not show GFP-positive cells in the anal sphincter after either IM or IV administration of MSCs in animals that received either SP or PNC 9 days after injury.
Discussion
Lack of encouraging long-term outcomes for FI has prompted the search for innovative procedures (Altomare et al., 2009; Caplan and Dennis, 2006b; Hosker et al., 2007) and cell-based therapies have shown promising results in animal studies (Lorenzi et al., 2008; White et al., 2010). Stem cell therapy in gastrointestinal disease has been explored in familial adenomatous polyposis (Leedham et al., 2005) and in inflammatory bowel disease (Armaka et al., 2008). Several cell-based therapies are presently in clinical trials for treatment of pathologies of other organ systems, such as cardiac (Karussis et al., 2010; Perin et al., 2011), urinary (Furuta et al., 2007; Stangel-Wojcikiewicz et al., 2010), limb ischemia (Walter et al., 2011), amyotrophic lateral sclerosis (Mazzini et al., 2011) and multiple sclerosis (Karussis et al., 2010). This approach has motivated several researchers to create animal models of FI for pre-clinical testing of cell-based therapies (Kang et al., 2008; Lorenzi et al., 2008).
Healy et al. (2008) have developed a neurogenic model of FI, while White et al. (2010) tested the utility of myogenic stem cells for repair after anal sphincter transection and found that injection of myogenic stem cells at the time of repair resulted in enhanced contractility 90 days later compared with a repair alone. The results of this research can provide a better understanding of regenerative cell-based therapy; however, there is a need to further investigate the mechanisms that promote repair to optimize the results when translated.
Stem cell homing is an active process of migration of the stem cells through the vascular endothelium to a site and target organ (Lapidot et al., 2005). SDF-1 and MCP-3 have been identified as potential homing factors that are upregulated after myocardial infarction (Schenk et al., 2007). Studies have shown improvement in cardiac function with successful engraftment to sites where cells engineered to express SDF-1 and MCP-3 have been implanted in infarcted myocardial tissue (Penn, 2007). The improvement in function with MSC treatment has been attributed to improvement in function of the cells in the area surrounding the infarct (M. Zhang et al., 2007). Schenk et al. (2007) demonstrated engraftment of MSCs 1 month after injury, when homing was re-established by overexpressing SDF-1 at the site of the previous myocardial infarction.
The goals of the current study were to investigate the changes in anal sphincter pressure after IV or IM injection of MSCs in our previously established animal models. Our models (Salcedo et al., 2010; Zutshi et al., 2009a) allow measurement of anal sphincter pressure and EMG in vivo in a repeated, survival fashion, a useful attribute since our ultimate aim is to reinforce the anal sphincter complex with cell therapy. The results suggest that some of the deterioration of resting anal sphincter pressures after SP was preventable by treatment with MSCs. IV infusion appears to provide a more effective route than IM administration, which may have caused MSC retention in the lymphatic circulation (H. Zhang et al., 2007). This is in contrast to the findings of Pathi et al. (2012) who found increased levels of matrix synthesis genes TGF-β1 and lysyl oxidase only in animals receiving direct injection.
MSCs delivered either IV or IM did not appear to facilitate recovery from PNC. It is possible that the trophic effect mediated by the MSCs at the time points we studied could have been too subtle to be observed by our physiologic recordings. This could be secondary to the fact that nerve injuries do not cause significant muscular physiologic changes until several weeks later (Campbell, 2008). Based on our previous animal model investigations, a denervation injury causes significant changes to anal sphincter pressure and EMG due to anal sphincter atrophy (Salcedo et al., 2010; Zutshi et al., 2009a). Future research should be aimed at investigating pudendal nerve recovery over a longer time course, particularly considering that clinical trials using cells to treat stress urinary incontinence show a delay to functional improvement after treatment (Stangel-Wojcikiewicz et al., 2010).
In animals receiving a sphincterotomy followed by IV MSC therapy, green fluorescing MSCs were not observed in the external and internal anal sphincters. This is similar to the findings of Pathi et al. (2012). The increase in the anal pressure compared to the controls thus suggests that MSCs may have had a short term engraftment and had a local effect via secretion of paracrine factors (Caplan and Dennis, 2006a; Shabbir et al., 2009b). This finding is also similar to that observed by Schenk et al. (2007) and M. Zhang et al. (2007) who demonstrated in a cardiac model that cardiac function improved without new tissue formation. They attributed the functional improvement to trophic factors released by MSCs that home to the site of injury. Studies by Shabbir et al. (2009b) and others (Caplan and Dennis, 2006b) have similarly shown improvement in cardiac myocyte regeneration and demonstrated that stem cells produce trophic cellular mediators like HGF, IGF-II and VEGF responsible for physiologic changes. Shabbir et al. have also shown that MSC administration increases bone marrow progenitor cells along with increased myocardial progenitor cells. Therefore, we postulate that as of yet unknown chemotactic secretions by the MSCs could be the source of the improvements observed in our study. We have demonstrated the upregulation of MCP-3 and SDF-1 (Salcedo et al., 2011) after anal sphincter injury and have documented in vitro-migration of MSCs towards SDF-1 and MCP-3 (unpublished data). More research is needed to evaluate the molecular mechanisms leading to functional improvement with MSCs treatment.
Other studies that evaluated MSCs for improving anal continence used in vitro muscle contractility testing, and concluded that MSCs provide a beneficial effect when used as a cellular therapy (Kang et al., 2008; Lorenzi et al., 2008; Pathi et al., 2012; White et al., 2010). Although the mechanisms of this effect were not elucidated, the main premise is that MSCs provide a useful therapeutic adjunct for improving anal sphincter function (Kajbafzadeh et al., 2010). A study by White et al. (2010) confirms that injection of myogenic stem cells at the time of external anal sphincter repair results in improvement of function, while the same group (Pathi et al., 2012) demonstrated evidence of increased collagen bundles in animals injected directly with MSCs with increase in both TGF-β 1 and lysyl oxidase mRNA. They, however, did not find GFP-labelled cells in the area beyond 7 days in these animals.
A limitation of the study is the potential bias of the IV approach of administration. The proximity of the tail vein to the anal sphincter may suggest that MSCs could be trapped during their systemic course. Ours was an initial study and a longer time course of recovery is underway with different doses and timing of MSC administration. In addition, since FI often develops years or decades after the initial injury, treatment with MSCs at a time remote from injury needs to be investigated, along with methods to upregulate homing cytokines at this later time (Abbott et al., 2004; Chavakis and Dimmeler, 2011; Chavakis et al., 2008; Ghadge et al., 2011; Kuliszewski et al., 2011; Ma et al., 2005). The mechanism of action of MSCs ought to be investigated in these studies.
Another limitation is the short time period that the study involved. As this was a pilot study to test our hypothesis that MSCs do home to the anal sphincter and because we have demonstrated the process of repair to be demonstrated by 2 weeks we chose 10 days as the time to test the hypothesis. A future study involves an extended time period to study longer time changes after MSC mediated repair and to look for engraftment vs. new tissue formation due to paracrine effects.
We also did not use fibroblasts as a negative control as done in cardiac studies. This field is not investigated enough in the anal sphincter for a true population of cells to be defined. Other researchers have used other cell types such as muscle derived stem cells (Kang et al., 2008) with some good results. Further investigation into the mechanism of action is warranted.
Conclusion
MSC treatment either IM or IV resulted in improved anal sphincter pressures after a direct injury to the anal sphincter complex. The possible secretion of unknown chemotactic factors by MSCs that were present at the site of injury could have contributed to this improvement in resting anal pressures after IV MSC treatment. Only after IV MSC treatment of sphincterotomy was the improvement in EMG significant. MSC administration after pudendal nerve crush did not result in functional improvement and requires further investigation into changes in the timing of MSC therapy as well as the mechanism of regeneration after a nerve injury.
Acknowledgments
This work funded in part by the Department of Colorectal Surgery and the Cleveland Clinic Innovations of the Cleveland Clinic Cleveland, Ohio and supported by Rehabilitation Research and Development Service of the Department of Veterans Affairs. The authors would like to thank Jonathan Glaab, Farjad Forudi, Feng Dong, Linda Vargo, Matthew Kiedrowski and Nikolai Sopko for assistance and expertise.
Footnotes
Moderated Poster Presentation: 39th Annual Scientific Meeting of International Continence Society, September 29–October 3, 2009, San Francisco, CA.
References
- Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- Altomare DF, Ratto C, Ganio E, Lolli P, Masin A, Villani RD. Long-term outcome of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2009;52(1):11–17. doi: 10.1007/DCR.0b013e3181974444. [DOI] [PubMed] [Google Scholar]
- Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205(2):331–337. doi: 10.1084/jem.20070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119(9):1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006a;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006b;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Chan MK, Tjandra JJ. Injectable silicone biomaterial (PTQ) to treat fecal incontinence after hemorrhoidectomy. Dis Colon Rectum. 2006;49(4):433–439. doi: 10.1007/s10350-005-0307-2. [DOI] [PubMed] [Google Scholar]
- Chavakis E, Dimmeler S. Homing of progenitor cells to ischemic tissues. Antioxid Redox Signal. 2011;15(4):967–980. doi: 10.1089/ars.2010.3582. [DOI] [PubMed] [Google Scholar]
- Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45(4):514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Furuta A, Jankowski RJ, Pruchnic R, Yoshimura N, Chancellor MB. The potential of muscle-derived stem cells for stress urinary incontinence. Expert Opin Biol Ther. 2007;7(10):1483–1486. doi: 10.1517/14712598.7.10.1483. [DOI] [PubMed] [Google Scholar]
- Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129(1):97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Gutirrez A, Madoff R, Lowery A, Parker S, Buie W, Baxter N. Long term results of anterior sphincteroplasty. Dis Colon Rectum. 2003;47:727–731. doi: 10.1007/s10350-003-0114-6. [DOI] [PubMed] [Google Scholar]
- Halverson AL, Hull TL. Long-term outcome of overlapping anal sphincter repair. Dis Colon Rectum. 2002;45(3):345–348. doi: 10.1007/s10350-004-6180-6. [DOI] [PubMed] [Google Scholar]
- Healy CF, O'Herlihy C, O'Brien C, O'Connell PR, Jones JF. Experimental models of neuropathic fecal incontinence: an animal model of childbirth injury to the pudendal nerve and external anal sphincter. Dis Colon Rectum. 2008;51(11):1619–1626. doi: 10.1007/s10350-008-9283-7. discussion 1626. [DOI] [PubMed] [Google Scholar]
- Hosker G, Cody JD, Norton CC. Electrical stimulation for faecal incontinence in adults. Cochrane Database Syst Rev. 2007;3:CD001310. doi: 10.1002/14651858.CD001310.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajbafzadeh AM, Elmi A, Talab SS, Esfahani SA, Tourchi A. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon Rectum. 2010;53(10):1415–1421. doi: 10.1007/DCR.0b013e3181e53088. [DOI] [PubMed] [Google Scholar]
- Kang SB, Lee HN, Lee JY, Park JS, Lee HS, Lee JY. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum. 2008;51(9):1367–1373. doi: 10.1007/s10350-008-9360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoui S, Leroi A, Koning E, Menard J, Michot F, Denis P. Results of sphincteroplasty in 86 patients with anal incontinence. Dis Colon Rectum. 2000;45(3):345–348. doi: 10.1007/BF02238020. [DOI] [PubMed] [Google Scholar]
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenefick N, Vaizey C, Malouf A, Norton C, Marshall M, Kamm M. Injectable silicone biomaterial for faecal incontinence due to internal sphincter dysfunction. Gut. 2007;51(2):225–228. doi: 10.1136/gut.51.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kouraklis G, Andromanakos N. Anorectal incontinence: aetiology, pathophysiology and evaluation. Acta Chir Belg. 2004;104(1):81–91. doi: 10.1080/00015458.2003.11978401. [DOI] [PubMed] [Google Scholar]
- Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong-Poi H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol Ther. 2011;19(5):895–902. doi: 10.1038/mt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Lazarescu A, Turnbull GK, Vanner S. Investigating and treating fecal incontinence: when and how. Can J Gastroenterol. 2009;23(4):301–308. doi: 10.1155/2009/905359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leedham SJ, Thliveris AT, Halberg RB, Newton MA, Wright NA. Gastrointestinal stem cells and cancer: bridging the molecular gap. Stem Cell Rev. 2005;1(3):233–241. doi: 10.1385/SCR:1:3:233. [DOI] [PubMed] [Google Scholar]
- Lorenzi B, Pessina F, Lorenzoni P, Urbani S, Vernillo R, Sgaragli G, et al. Treatment of experimental injury of anal sphincters with primary surgical repair and injection of bone marrow-derived mesenchymal stem cells. Dis Colon Rectum. 2008;51(4):411–420. doi: 10.1007/s10350-007-9153-8. [DOI] [PubMed] [Google Scholar]
- Ma J, Ge J, Zhang S, Sun A, Shen J, Chen L, et al. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res Cardiol. 2005;100(3):217–223. doi: 10.1007/s00395-005-0521-z. [DOI] [PubMed] [Google Scholar]
- Malouf A, Norton C, Engel A, Nicholls J. Longterm results of overlapping anterior anal-sphincter repair for obstetric trauma. Lancet. 2000;355:260–265. doi: 10.1016/S0140-6736(99)05218-6. [DOI] [PubMed] [Google Scholar]
- Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, Servo S, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2011;1:56–60. doi: 10.3109/14653249.2011.613929. [DOI] [PubMed] [Google Scholar]
- Pathi SD, Acevedo JF, Keller PW, Kishore AH, Miller RT, Wai CY, et al. Recovery of the injured external anal sphincter after injection of local or intravenous mesenchymal stem cells. Obstet Gynecol. 2012;119(1):134–144. doi: 10.1097/AOG.0b013e3182397009. [DOI] [PubMed] [Google Scholar]
- Penn MS. Cell-based gene therapy for the prevention and treatment of cardiac dysfunction. Natl Clin Pract Cardiovasc Med. 2007;4(Suppl. 1):S83–S88. doi: 10.1038/ncpcardio0733. [DOI] [PubMed] [Google Scholar]
- Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF). [Randomized Controlled Trial] Am Heart J. 2011;161(6):1078–1087. doi: 10.1016/j.ahj.2011.01.028. e1073. [DOI] [PubMed] [Google Scholar]
- Pretlove SJ, Radley S, Toozs-Hobson PM, Thompson PJ, Coomarasamy A, Khan KS. Prevalence of anal incontinence according to age and gender: a systematic review and meta-regression analysis. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(4):407–417. doi: 10.1007/s00192-005-0014-5. [DOI] [PubMed] [Google Scholar]
- Safioleas M, Andromanakos N, Lygidakis N. Anorectal incontinence: therapeutic strategy of a complex surgical problem. Hepatogastroenterology. 2008;55(85):1320–1326. [PubMed] [Google Scholar]
- Salcedo L, Damaser M, Butler R, Jiang HH, Hull T, Zutshi M. Long-term effects on pressure and electromyography in a rat model of anal sphincter injury. Dis Colon Rectum. 2010;53(8):1209–1217. doi: 10.1007/DCR.0b013e3181de7fe0. [DOI] [PubMed] [Google Scholar]
- Salcedo L, Sopko N, Jiang HH, Damaser M, Penn M, Zutshi M. Chemokine upregulation in response to anal sphincter and pudendal nerve injury: potential signals for stem cell homing. Int J Color Dis. 2011:577–581. doi: 10.1007/s00384-011-1269-6. [DOI] [PubMed] [Google Scholar]
- Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25(1):245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy bynonautologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. 2009a;87(9):1275–1282. doi: 10.1097/TP.0b013e3181a1719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009b;296(6):H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangel-Wojcikiewicz K, Majka M, Basta A, Stec M, Pabian W, Piwowar M, et al. Adult stem cells therapy for urine incontinence in women. Ginekol Pol. 2010;81(5):378–381. [PubMed] [Google Scholar]
- Takahashi-Monroy T, Morales M, Garcia-Osogobio S, Valdovinos MA, Belmonte C, Barreto C, et al. SECCA procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359. doi: 10.1007/s10350-007-9169-0. [DOI] [PubMed] [Google Scholar]
- Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schluter M, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4(1):26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- White AB, Keller PW, Acevedo JF, Word RA, Wai CY. Effect of myogenic stem cells on contractile properties of the repaired and unrepaired transected external anal sphincter in an animal model. Obstet Gynecol. 2010;115(4):815–823. doi: 10.1097/AOG.0b013e3181d56cc5. [DOI] [PubMed] [Google Scholar]
- Zhang H, Song P, Tang Y, Zhang XL, Zhao SH, Wei YJ, et al. Injection of bone marrow mesenchymal stem cells in the borderline area of infarcted myocardium: heart status and cell distribution. J Thorac Cardiovasc Surg. 2007;134(5):1234–1240. doi: 10.1016/j.jtcvs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21(12):3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- Zutshi M, Salcedo LB, Zaszczurynski PJ, Hull TL, Butler RS, Damaser MS. Effects of sphincterotomy and pudendal nerve transection on the anal sphincter in a rat model. Dis Colon Rectum. 2009a;52(7):1321–1329. doi: 10.1007/DCR.0b013e31819f746d. [DOI] [PubMed] [Google Scholar]
- Zutshi M, Tracey TH, Bast J, Halverson A, Na J. Ten-year outcome after anal sphincter repair for fecal incontinence. Dis Colon Rectum. 2009b;52(6):1089–1094. doi: 10.1007/DCR.0b013e3181a0a79c. [DOI] [PubMed] [Google Scholar]


