Abstract
In collaboration with Marshall Nirenberg, we performed in vivo RNA interference (RNAi) genome-wide screening in Drosophila embryos. Pebble has been shown to be involved in Drosophila neuronal development. We have also reported that depletion of Ect2, a mammalian ortholog of Pebble, induces differentiation in NG108-15 neuronal cells. However, the precise role of Ect2 in neuronal development has yet to be studied. Here, we confirmed in PC12 pheochromocytoma cells that inhibition of Ect2 expression by RNAi stimulated neurite outgrowth, and in the mouse embryonic cortex that Ect2 was accumulated throughout the ventricular and subventricular zones with neuronal progenitor cells. Next, the effects of Ect2 depletion were studied in primary cultures of mouse embryonic cortical neurons: Loss of Ect2 did not affect the differentiation stages of neuritogenesis, the number of neurites, or axon length, while the numbers of growth cones and growth cone-like structures were increased. Taken together, our results suggest that Ect2 contributes to neuronal morphological differentiation through regulation of growth cone dynamics.
Keywords: GEF, Neuronal development, Cerebral cortex, Growth cones
1. Introduction
RNA interference (RNAi) has been used successfully to screen large numbers of genes in Caenorhabditis elegans (Gonczy et al., 2000), Drosophila embryos (Kim et al., 2004), and Drosophila cell cultures (Boutros et al., 2004; Kiger et al., 2003) to identify genes that are involved in biological phenomena. In collaboration with Marshall Nirenberg, we previously performed in vivo RNAi to screen for Drosophila genes required in the development of the embryonic nervous system (Ivanov et al., 2004; Koizumi et al., 2007). Two genetic screening studies, including our RNAi screens, indicated that pebble is a gene affecting development of the Drosophila nervous system (Kraut et al., 2001; Prokopenko et al., 2000; Ivanov et al., 2004; Koizumi et al., 2007). However, the functional role of Ect2, a mammalian ortholog of Pebble, has not been examined in the development of the mammalian nervous system, except in pituitary development (Islam et al., 2010).
Ect2 and Pebble are guanine nucleotide exchange factors (GEF) that function during cytokinesis. They both activate RhoA (Schmidt and Hall, 2002), which regulates contractile ring formation through actomyosin contraction (Kishi et al., 1993; Mabuchi et al., 1993). Prokopenko et al. reported that Pebble mutants failed to form the contractile ring and showed defects in cytokinesis (Prokopenko et al., 1999). Tatsumoto et al. reported that downregulation of Ect2 function, either by dominant-negative Ect2 expression or by microinjection of anti-Ect2 antibody, blocked cell division and generated multinucleate cells (Tatsumoto et al., 1999).
We previously showed the induction of binucleate cells by Ect2 RNAi in mouse neuroblastoma × rat glioma hybrid NG108-15 cells, a useful neuronal model produced in Nirenberg’s laboratory (Nirenberg et al., 1983; Puro and Nirenberg, 1976; McGee et al., 1978; Tsuji et al., 2011). Although there have been many analyses of Ect2 in cytokinesis, a recent study revealed possible functions of Ect2 in cellular differentiation and neuronal regulation; Smallhorn et al. reported that Pebble was involved in epithelial-mesenchymal transition and mesoderm migration in Drosophila (Smallhorn et al., 2004). Pebble was identified as a candidate substrate of UBE3A ligase, a gene responsible for Angelman syndrome that causes severe developmental delay and mental retardation, associated with autism in a certain ratio (Steffenburg et al., 1996), by two-dimensional gel and MALDI-TOF analyses (Reiter et al., 2006). Moreover, the expression level and pattern of Ect2 were remarkably altered in the hippocampus and cerebellum of UBE3A null mice (Reiter et al., 2006). However, no previous reports have revealed the precise roles of Ect2 in mammalian neuronal development.
In the present study, we showed that inhibition of Ect2 by RNAi also stimulated neurite outgrowth in PC12 cells, a nerve growth factor (NGF)-regulated adrenergic clone derived from pheochromocytoma cells (Greene and Tischler, 1976). Next, we examined Ect2 expression in the mouse embryonic cortex and found its accumulation throughout the ventricular and subventricular zone (VZ, SVZ). Furthermore, to assess the role of Ect2, RNAi was performed in primary cultures of mouse embryonic cortical neurons. We demonstrated that Ect2 depletion did not affect the defined stages of neuritogenesis (de Lima et al., 1997) of cultured cortical neurons. While neither the number of neurites nor axon length showed differences related to the loss of Ect2, the numbers of growth cones and growth cone-like structures were increased by Ect2 depletion.
2. Materials and methods
2.1. RNA interference in PC12 cells
PC12 cells were maintained in DMEM supplemented with 5% bovine serum and 10% horse serum (Torocsik et al., 2002). For Ect2 knockdown, siRNA Ect2 #1 (Ect2-RSS360274; Invitrogen, Carlsbad CA, USA) and #2 (Ect2-RSS360275; Invitrogen) were used. Stealth RNAi Negative Control Duplex (Invitrogen) was used for control RNAi. The siRNA (0.6 μl of 20 μM siRNA duplex) and 2 μl of Lipofectamine RNAiMAX (Invitrogen) were mixed in 200 μl DMEM and added to 1 ml of culture medium according to the manufacturer’s protocol. After 48 h, NGF (50 ng/ml) was applied to the replaced culture medium (DMEM supplemented with 0.05% bovine serum and 0.1% horse serum). As the medium was replaced, the cells were again transfected with each siRNA. For morphological analyses, cells were seeded onto glass coverslips coated with poly-D-lysine (0.1 mg/ml; Sigma, St. Louis, MO, USA). Cells were fixed with 3.7% paraformaldehyde (PFA) and stained with DAPI. To determine the knockdown efficiency, the whole cell lysates of PC12 cells were subjected to SDS–PAGE, followed by immunoblotting as described (Islam et al., 2010).
2.2. Immunohistochemistry
All animal experiments were performed in Slc:ICR mice purchased from Japan SLC, Inc. (Hamamatsu, Japan). The experiments were carried out in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology of Japan. For immunohistochemistry, embryonic day (E) 14 mice were immersion fixed with 4% PFA in PBS for 16 h. Procedures for immunohistochemistry using cryosections were conducted essentially as reported (Islam et al., 2010).
2.3. Primary cultures of embryonic cortical neurons
Cultures of primary cortical neurons were prepared from E14 mice essentially as described with minor modifications (de Lima et al., 1997). Briefly, dissected cortical tissue was minced and dissociated with a solution of trypsin–EDTA in PBS for 5 min at 37 °C. Dissociation was completed by repeatedly passing the suspension through a Pasteur pipette. Dissociated cells (1 × 106 cells) were resuspended in 8 μl of Resuspension buffer (Invitrogen) with 60 pmol of siRNA Ect2 (Ect2-MSS203768; Invitrogen) or Stealth RNAi Negative Control Duplex. Neurons were transfected by electroporation (MicroPorator MP-100; NanoEnTek, Seoul, Korea) according to the optimized protocol for primary mouse neurons. Neurons were plated onto glass coverslips coated with poly-D-lysine (0.1 mg/ml; Sigma) and cultured in Neurobasal culture medium supplemented with 2% B27, 0.5 mM L-glutamine, and 1% FBS. After 24 h, medium was replaced with serum-free culture medium. Neurons were fixed 48 h after plating with 3.7% PFA and stained with Texas Red-X phalloidin (Invitrogen). The efficiency of knockdown was examined by immunoblotting and RT-PCR analysis as described previously (Islam et al., 2010; Tsuji et al., 2011).
2.4. Analysis of cell morphology
Images were taken using an Olympus IX71 inverted microscope equipped with a CoolSNAP HQ2 camera (Molecular Devices, Sunnyvale, CA, USA) to determine the morphology of PC12 cells and cortical primary neurons. Using the MetaMorph software (Molecular Devices, Sunnyvale, CA, USA), images were analyzed manually by the experimenter blind to the experimental groups. PC12 cells possessing at least one neurite with a length longer than one cell diameter (≥22 μm) were defined as neurite-bearing cells. In the cortical primary neurons, growth cones or growth cone-like structures were scored if the tip of neurites or branches longer than 10 μm exhibited either a lamellipodium or ≥3 filopodia. Axon lengths were measured from the cell body to the distal extent of the central region of the growth cone. An axon was defined as a process that was at least double the length of the next longest minor process. Neurites were scored if they were longer than 10 μm from the cell body by two independent observers. Samples were blinded to them to minimize unconscious bias and to ensure objectivity and reproducibility. The statistical significance of differences between each group was analyzed by the two-tailed Student’s t test.
3. Results
3.1. Ect2 negatively regulates the differentiation of PC12 cells
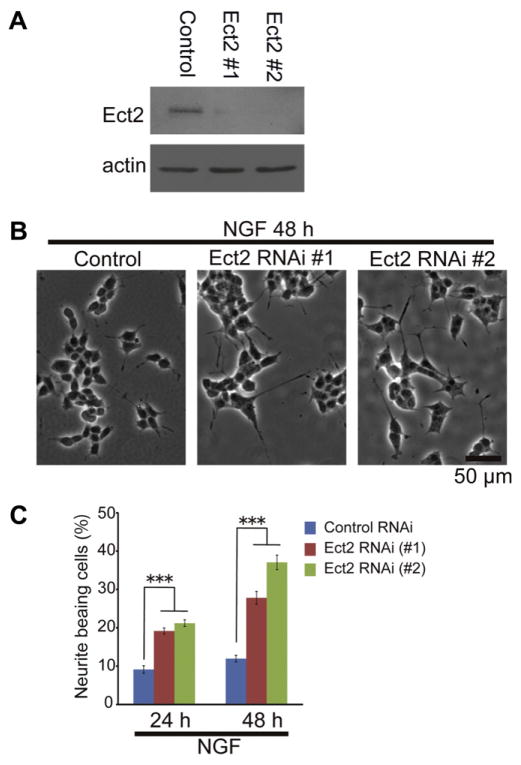
We previously performed Ect2 RNAi in the NG108-15 cell line (Tsuji et al., 2011). Depletion of Ect2 stimulated neurite outgrowth and concomitantly increased acetylcholine esterase mRNA levels. To confirm negative regulation of Ect2 in neuronal differentiation in another cell line, we performed loss-of-function experiments using the RNAi method for Ect2 in PC12 cells. Immunoblotting showed that the amount of Ect2 was markedly reduced by Ect2 knockdown (Fig. 1A). Previous studies have indicated that the downregulation of Ect2 function generates multinucleated cells (Tatsumoto et al., 1999). Consistent with these findings, Ect2-RNAi in growth medium for 72 h increased the proportion of multinucleated cells compared to control-RNAi (mean ± SD, 14.7 ± 1.4% (control), 44.3 ± 3.4% (#1), 37.9 ± 2.2% (#2), n = 3 independent experiments, in each of which >150 cells were examined). Furthermore, the number of cells showing neurite outgrowth after NGF application for 48 h was increased by depletion of Ect2 (Fig. 1B and C). These results suggested that Ect2 negatively regulates neurite outgrowth in NGF-stimulated PC12 cells.
Fig. 1.
Inhibition of Ect2 expression induces neurite formation by NGF-stimulated PC12 cells. (A) Depletion of Ect2 protein by siRNA treatment. PC12 cells were transfected with control siRNA or two Ect2 siRNAs, #1 and #2. After 48 h, the cells were harvested and subjected to immunoblotting with anti-Ect2 or anti-actin antibody. (B) Representative images of PC12 cells stimulated with NGF for 48 h. siRNAs were transfected 48 h prior to the application of NGF. (C) Frequencies of neurite-bearing PC12 cells (means ± SEM; n = 4 independent experiments, in each of which >368 cells were examined). ***P < 0.001 compared with controls.
3.2. Role of Ect2 in the early differentiation of cortical neurons
We previously reported Ect2 expression in the mouse embryonic brain by immunoblotting and RT-PCR analysis (Tsuji et al., 2011). To extend these observations and determine the spatial distribution of Ect2, we performed immunohistochemistry on sections from the E14 brain cortex. Ect2 was detected throughout both VZ and SVZ, i.e., the two layers rich in progenitor cells (Fig. 2).
Fig. 2.
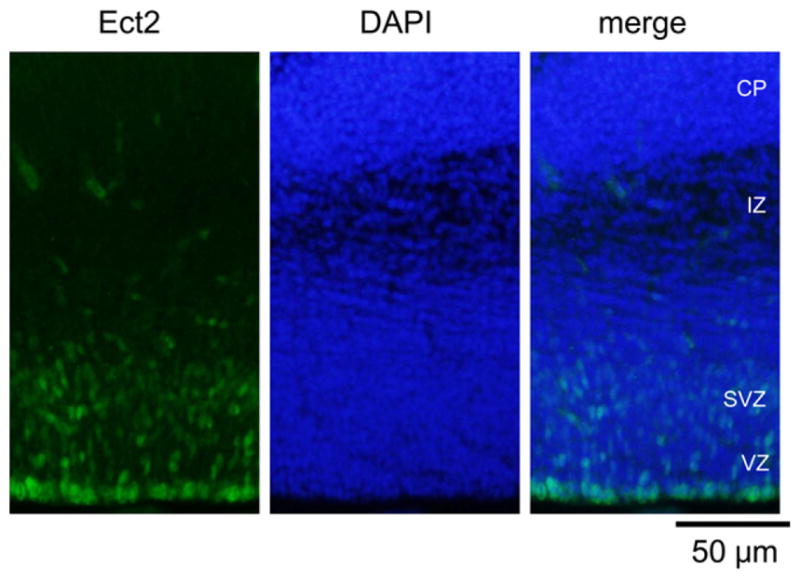
Expression of Ect2 in the developing mouse cerebral cortex. Coronal sections from E14 were stained with anti-Ect2 (green, left) and DAPI (blue, middle). Ect2 staining was concentrated in the VZ and SVZ. CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone.
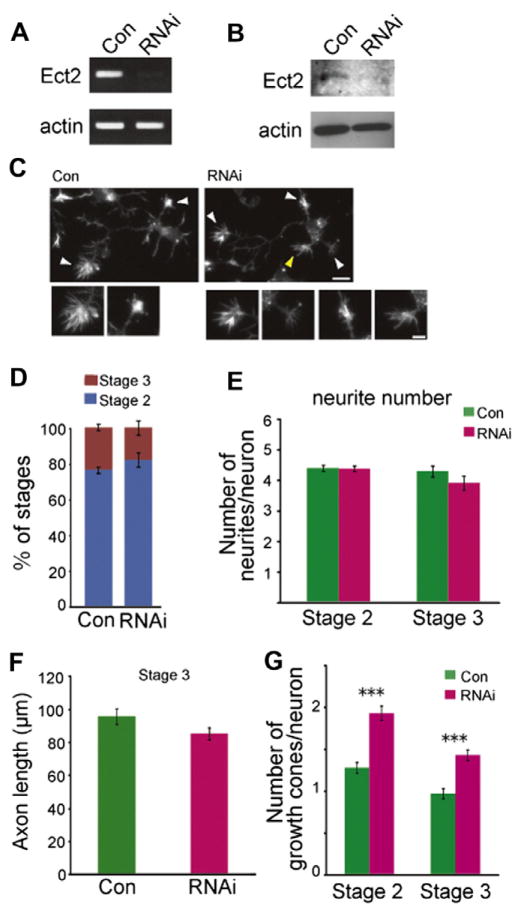
Next, the role of Ect2 in early neuronal differentiation was examined by loss-of-function experiments in cultured embryonic cortical neurons. We transfected cells with siRNA by electroporation at the time of plating on dishes and analyzed morphological changes 48 h later. The knockdown of Ect2 was determined by RT-PCR and immunoblotting (Fig. 3A and B). de Lima et al. (1997) defined neuritogenesis in dissociated cortical neurons: during the early differentiation step of stage 1, cortical neurons are round and devoid of neurites; stage 2 neurons possess primordial neurites, but not an established axon; stage 3 neurons extend a single axon that is often ramified. According to this definition, after 48 h in the control RNAi culture, 76.2% of embryonic cortical neurons remained in stage 2 and 23.8% reached stage 3 in our experiments. Almost no neurons were detected in stage 1. No obvious differences in the ratio of neurons in each differentiation stage were observed with Ect2 depletion compared to controls (Fig. 3C and D). Ect2 depletion had no effect on either the axon length in stage 3 neurons or the number of neurites (Fig. 3C, E, F). However, we found significant increases in numbers of growth cones and growth cone-like structures in Ect2-depleted neurons (Fig. 3C and G). These results indicated Ect2 expression in the neural/progenitor cells and suggested that Ect2 plays a role in neuronal morphological differentiation through regulating growth cone dynamics.
Fig. 3.
Effects of Ect2 depletion in cultured mouse cortical neurons. (A) Depletion of Ect2 mRNA by siRNA treatment. Primary cultured neurons were transfected with control siRNA or Ect2 siRNA. After 48 h of culture, the cells were harvested and subjected to RT-PCR analysis. (B) Depletion of Ect2 protein by siRNA treatment. The cells were harvested after 48 h and subjected to immunoblotting. (C) F-actin structures visualized with fluorescent phalloidin. Note that Ect2-depleted neurons showed increased numbers of growth cones and growth cone-like structures. White arrowheads indicate the growth cones or growth cone-like structures. A lamellipodium or ≥3 filopodia at the tip of neurite shorter than 10 μm is not scored as a growth cone, shown by a yellow arrowhead. The structures shown by arrowheads are enlarged. Bars 10 μm. (D) Scoring for developmental stages after 48 h in culture showed that depletion of Ect2 did not affect the differentiation stages. There were almost no neurons in stage 1. Data represent mean percentages ± SEM (n = 3 independent experiments). In each of which >627 neurons and >192 neurons were examined for stages 2 and stage 3, respectively. (E) The numbers of neurites per neuron in the two stages were unaffected by Ect2 depletion (n = 3 independent experiments; in each of which >339 neurons were counted for control and Ect2 RNAi). (F) The axon length was unaffected by Ect2 depletion (n = 3 independent experiments; 80 neurons and 81 neurons were counted for control and Ect2 RNAi, respectively). (G) The numbers of growth cones and growth cone-like structures per neuron were increased by loss of Ect2 (Stage 2: 1.28 ± 0.07, 1.93 ± 0.09, control and RNAi, respectively; stage 3, 0.97 ± 0.06, 1.43 ± 0.06, control and RNAi, respectively; means ± SEM) (n = 3 independent experiments; in each of which >130 and >140 neurons were examined in stage 2 and stage 3, respectively). ***P < 0.001 compared to control.
4. Discussion
In the present study, we demonstrated that Ect2 depletion induces neurite outgrowth stimulated by NGF in PC12 cells. Although Ect2 depletion alone had no such effect, the Ect2 suppressive signal and simultaneous NGF-induced intracellular signal induced morphological differentiation of PC12 cells. In contrast, we reported previously that Ect2 inhibition alone can elicit morphological differentiation in NG108-15 cells characterized by the outgrowth of neurites and by a concomitant increase in acetylcholine esterase mRNA level (Tsuji et al., 2011). These findings suggest that Ect2 tonically inhibits cellular differentiation and reduction of this inhibitory signal leads toward neuronal differentiation. The requirement for the additional signal may be dependent on cell type.
Several reports have suggested that regulation of cell cycle, particularly the transition of G1 to S phase, is important for the differentiation of neuroepithelial cells. During embryonic development, through cell cycle lengthening, neuroepithelial cells progress from proliferative to neurogenic division and then transit to radial glial cells (Gotz and Huttner, 2005; Takahashi et al., 1995). Inactivation of Rho causes accumulation of p21Cip/WAF and p27Kip, CDK inhibitors, resulting in G1/S arrest (Hirai et al., 1997; Olson et al., 1998). Increased expression of CDK inhibitor induces neuronal differentiation (Durand et al., 1997; Molofsky et al., 2004; Ohnuma et al., 1999). Scoumanne and Chen reported that Ect2 was negatively regulated by p53 and required for the transition from G1 to S phase in H1299 cells derived from lung carcinoma. They also showed that knockdown of Ect2 triggered cell cycle arrest in G1 (Scoumanne and Chen, 2006). Taken together with our data on neuronal cell lines, these observations suggest that Ect2 may be involved in neuronal differentiation through cell cycle regulation. Therefore, we expected the induction by Ect2 depletion to advance neuritic differentiation according to the classification of de Lima et al. (1997) in primary cortical embryonic neurons. However, the ratio of neurons in each differentiation stage did not differ between Ect2 RNAi and control cells (Fig. 3D). This discrepancy may be related to differences in length of cell cycle or in the characteristics of cell proliferation: regulated cell proliferation and cell cycle in the embryonic neurons and disordered regulation in the cell proliferation and cell cycle in the tumor cells.
In this study, we found that Ect2 depletion increases the number of growth cones and growth cone-like structures. This result indicates that in embryonic neurons, probably immature neurons, Ect2 may not be involved in neuritic differentiation (de Lima et al., 1997) through the cell cycle, but directly participating in reorganization of the actin cytoskeleton at the tips of neurites (growth cones). To our knowledge, this is the first report indicating that Ect2 may be directly involved in actin reorganization other than during cytokinesis.
The increases in numbers of growth cones and growth cone-like structures by Ect2 depletion observed in the present study may have been due to inhibition of the Rho-ROCK pathway and sequential activation of the Rac pathway when Ect2 was depleted in neurons. Pebble and Ect2 have been identified as GEFs that mediate Rho activation during cytokinesis (Lehner, 1992; Tatsumoto et al., 1999). Although Ect2 catalyzes guanine nucleotide exchange on three representative Rho GTPases, i.e., Rho, Rac1, and Cdc42, in vitro, a very distinct mechanism of regulation toward Rho alone by localization would be exerting, as shown for Ect2 in HeLa cells (Tatsumoto et al., 1999). In neurons, it has been reported that the Rho-ROCK pathway is associated with repulsive cues and growth cone collapse. Rac/Cdc42 is with attractive cues and forward protrusion (Hall and Lalli, 2010). The Rho-ROCK pathway often appears to function antagonistically to the Rac/Cdc42 pathway (Tsuji et al., 2002). Further studies are necessary to elucidate the molecular mechanism underlying the regulation of growth cone dynamics by Ect2.
As mentioned above, Ect2 is also degraded by UBE3A, one of the candidate genes responsible for Angelman syndrome with autistic symptoms (Reiter et al., 2006). Pediatric mental disorders, including autism spectrum disorders (ASDs), are postulated to involve aberrations in neuronal circuits (Ramocki and Zoghbi, 2008). Considering our data indicating the developmental expression pattern of Ect2 in the brain and its involvement in the morphological regulation of growth cones, Ect2 may be one of the key regulators in remodeling the neuronal circuit. Therefore, further studies of the spatiotemporal regulation of Ect2 by UBE3A in vivo will contribute to the elucidation of ASD and the discovery of therapeutic targets for ASD in the future.
References
- Boutros M, Kiger AA, Armknecht S, Kerr K, Hild M, Koch B, Haas SA, Paro R, Perrimon N. Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science. 2004;303:832–835. doi: 10.1126/science.1091266. [DOI] [PubMed] [Google Scholar]
- de Lima AD, Merten MD, Voigt T. Neuritic differentiation and synaptogenesis in serum-free neuronal cultures of the rat cerebral cortex. J Comp Neurol. 1997;382:230–246. doi: 10.1002/(sici)1096-9861(19970602)382:2<230::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Durand B, Gao FB, Raff M. Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J. 1997;16:306–317. doi: 10.1093/emboj/16.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P, Echeverri C, Oegema K, Coulson A, Jones SJ, Copley RR, Duperon J, Oegema J, Brehm M, Cassin E, et al. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331– 336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Lalli G Rho. Ras GTPases in axon growth, guidance, and branching. Cold Spring Harbor Perspect Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai A, Nakamura S, Noguchi Y, Yasuda T, Kitagawa M, Tatsuno I, Oeda T, Tahara K, Terano T, Narumiya S, et al. Geranylgeranylated rho small GTPase(s) are essential for the degradation of p27Kip1 and facilitate the progression from G1 to S phase in growth-stimulated rat FRTL-5 cells. J Biol Chem. 1997;272:13–16. [PubMed] [Google Scholar]
- Islam MS, Tsuji T, Higashida C, Takahashi M, Higashida H, Koizumi K. Expression of a Rho guanine nucleotide exchange factor, Ect2, in the developing mouse pituitary. J Neuroendocrinol. 2010;22:477–482. doi: 10.1111/j.1365-2826.2010.01962.x. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Rovescalli AC, Pozzi P, Yoo S, Mozer B, Li HP, Yu SH, Higashida H, Guo V, Spencer M, Nirenberg M. Genes required for Drosophila nervous system development identified by RNA interference. Proc Natl Acad Sci USA. 2004;101:16216–16221. doi: 10.1073/pnas.0407188101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger AA, Baum B, Jones S, Jones MR, Coulson A, Echeverri C, Perrimon N. A functional genomic analysis of cell morphology using RNA interference. J Biol. 2003;2:27. doi: 10.1186/1475-4924-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YO, Park SJ, Balaban RS, Nirenberg M, Kim Y. A functional genomic screen for cardiogenic genes using RNA interference in developing Drosophila embryos. Proc Natl Acad Sci USA. 2004;101:159–164. doi: 10.1073/pnas.0307205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Higashida H, Yoo S, Islam MS, Ivanov AI, Guo V, Pozzi P, Yu SH, Rovescalli AC, Tang D, Nirenberg M. RNA interference screen to identify genes required for Drosophila embryonic nervous system development. Proc Natl Acad Sci USA. 2007;104:5626–5631. doi: 10.1073/pnas.0611687104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut R, Menon K, Zinn K. A gain-of-function screen for genes controlling motor axon guidance and synaptogenesis in Drosophila. Curr Biol. 2001;11:417–430. doi: 10.1016/s0960-9822(01)00124-5. [DOI] [PubMed] [Google Scholar]
- Lehner CF. The pebble gene is required for cytokinesis in Drosophila. J Cell Sci. 1992;103(Pt 4):1021–1030. doi: 10.1242/jcs.103.4.1021. [DOI] [PubMed] [Google Scholar]
- Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organisation of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- McGee R, Simpson P, Christian C, Mata M, Nelson P, Nirenberg M. Regulation of acetylcholine release from neuroblastoma × glioma hybrid cells. Proc Natl Acad Sci USA. 1978;75:1314–1318. doi: 10.1073/pnas.75.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Nirenberg M, Wilson S, Higashida H, Rotter A, Krueger K, Busis N, Ray R, Kenimer JG, Adler M. Modulation of synapse formation by cyclic adenosine monophosphate. Science. 1983;222:794–799. doi: 10.1126/science.6314503. [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Philpott A, Wang K, Holt CE, Harris WA. P27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell. 1999;99:499–510. doi: 10.1016/s0092-8674(00)81538-x. [DOI] [PubMed] [Google Scholar]
- Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- Prokopenko SN, Brumby A, O’Keefe L, Prior L, He Y, Saint R, Bellen HJ. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko SN, He Y, Lu Y, Bellen HJ. Mutations affecting the development of the peripheral nervous system in Drosophila: a molecular screen for novel proteins. Genetics. 2000;156:1691–1715. doi: 10.1093/genetics/156.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro DG, Nirenberg M. On the specificity of synapse formation. Proc Natl Acad Sci USA. 1976;73:3544–3548. doi: 10.1073/pnas.73.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter LT, Seagroves TN, Bowers M, Bier E. Expression of the Rho-GEF Pbl/ECT2 is regulated by the UBE3A E3 ubiquitin ligase. Hum Mol Genet. 2006;15:2825–2835. doi: 10.1093/hmg/ddl225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Scoumanne A, Chen X. The epithelial cell transforming sequence 2, a guanine nucleotide exchange factor for Rho GTPases, is repressed by p53 via protein methyltransferases and is required for G1–S transition. Cancer Res. 2006;66:6271–6279. doi: 10.1158/0008-5472.CAN-06-0121. [DOI] [PubMed] [Google Scholar]
- Smallhorn M, Murray MJ, Saint R. The epithelial–mesenchymal transition of the Drosophila mesoderm requires the Rho GTP exchange factor Pebble. Development. 2004;131:2641–2651. doi: 10.1242/dev.01150. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg CL, Steffenburg U, Kyllerman M. Autism in Angelman syndrome: a population-based study. Pediatr Neurol. 1996;14:131–136. doi: 10.1016/0887-8994(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol. 1999;147:921–928. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torocsik B, Angelastro JM, Greene LA. The basic region and leucine zipper transcription factor MafK is a new nerve growth factor-responsive immediate early gene that regulates neurite outgrowth. J Neurosci. 2002;22:8971–8980. doi: 10.1523/JNEUROSCI.22-20-08971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Higashida C, Yoshida Y, Islam MS, Dohmoto M, Koizumi K, Higashida H. Ect2, an ortholog of Drosophila’s pebble, negatively regulates neurite outgrowth in neuroblastoma × glioma hybrid NG108-15 cells. Cell Mol Neurobiol. 2011;31:663–668. doi: 10.1007/s10571-011-9668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Ishizaki T, Okamoto M, Higashida C, Kimura K, Furuyashiki T, Arakawa Y, Birge RB, Nakamoto T, Hirai H, Narumiya S. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J Cell Biol. 2002;157:819–830. doi: 10.1083/jcb.200112107. [DOI] [PMC free article] [PubMed] [Google Scholar]