Mediterranean high-mountain ecosystems are increasingly threatened by climate change, causing biodiversity loss, habitat degradation and landscape modifications. In this work, we used phytosociological relevés to conduct a re-visitation study in order to analyze changes in floristic composition over the last 42 years in the central Apennines (Majella National Park). We observed changes in floristic composition, along with a significant increase in thermophilic and nutrient-demanding species. Such changes are likely attributable to the combined effect of higher temperatures and the increase in soil nutrients triggered by global change.
Keywords: Global change, Landolt indicators, life forms, Mediterranean mountains, phytosociological relevés, re-visitation study
Abstract
High-mountain ecosystems are increasingly threatened by climate change, causing biodiversity loss, habitat degradation and landscape modifications. However, very few detailed studies have focussed on plant biodiversity in the high mountains of the Mediterranean. In this study, we investigated the long-term changes that have occurred in the composition, structure and ecology of high-mountain vegetation in the central Apennines (Majella) over the last 42 years. We performed a re-visitation study, using historical and newly collected vegetation data to explore which ecological and structural features have been the most successful in coping with climatic changes. Vegetation changes were analysed by comparing geo-referenced phytosociological relevés collected in high-mountain habitats (dolines, gentle slopes and ridges) on the Majella massif in 1972 and in 2014. Composition analysis was performed by detrended correspondence analysis, followed by an analysis of similarities for statistical significance assessment and by similarity percentage procedure (SIMPER) for identifying which species indicate temporal changes. Changes in ecological and structural indicators were analysed by a permutational multivariate analysis of variance, followed by a post hoc comparison. Over the last 42 years, clear floristic changes and significant ecological and structural variations occurred. We observed a significant increase in the thermophilic and mesonitrophilic plant species and an increment in the frequencies of hemicryptophytes. This re-visitation study in the Apennines agrees with observations in other alpine ecosystems, providing new insights for a better understanding of the effects of global change on Mediterranean high-mountain biodiversity. The observed changes in floristic composition, the thermophilization process and the shift towards a more nutrient-demanding vegetation are likely attributable to the combined effect of higher temperatures and the increase in soil nutrients triggered by global change. The re-visitation approach adopted herein represents a powerful tool for studying climate-related changes in sensitive high-mountain habitats.
Introduction
High-mountain ecosystems are increasingly threatened by climate change, causing biodiversity loss, habitat degradation and landscape modifications (e.g. Körner 2003; Bruun et al. 2006). Mountain habitats in Europe contain ∼20 % of the native flora (Väre et al. 2003) and are authentic hotspots of plant diversity, hosting highly specialized vascular plants (Barthlott et al. 1996; Myers et al. 2000) and many endemics (Pauli et al. 2007). These ecosystems are sensitive to climatic factors and respond differently according to the rate of climatic change, the species pool and the biogeographical region (Beniston 2003; Pauli et al. 2012). Among these mountain habitats, the Mediterranean mountains in Europe deserve particular attention. They are represented by a few isolated peaks, which constituted the major refugia of plant species during the ice age of the Pleistocene, and at present, they host a high number of endemic and rare plants (Pauli et al. 2003; Kazakis et al. 2007; Stanisci et al. 2011; van Gils et al. 2012; Gutiérrez Girón and Gavilán 2013). Moreover, the orographic discontinuity of the Mediterranean mountains makes them particularly vulnerable to biodiversity loss (Nogués Bravo et al. 2008).
The last Intergovernmental Panel on Climate Change (IPCC) clearly noted the anomalous rates of change occurring in many environmental parameters such as temperature, atmospheric moisture content and atmospheric nitrogen (N) deposition (IPCC 2014). For example, the period between 1983 and 2012 was the warmest 30-year period of the millennium in the northern hemisphere. In Italy, during the period 1955–2005, the mean temperatures increased by 1.6 °C in spring and of 1.9 °C in summer (Brunetti et al. 2006; Toreti and Desiato 2008). Besides the increase in average temperatures, the IPCC (2013) also reported an increase in the atmospheric moisture content, which was related to recent changes in precipitation patterns and with the increment in evapotranspiration rates. Yet, atmospheric N deposition increased in Europe in recent decades, reaching rates 20 times higher than before the Industrial Revolution (e.g. Vitousek et al. 1997; Bobbink et al. 2010).
Global change processes attracted the interest of many ecologists, and a rich scientific literature addresses their effects on biodiversity at different spatial and temporal scales (see Parmesan 2006; Bellard et al. 2012). For instance, the increase of soluble N deposition, recently recorded in high-mountain habitats (Hättenschwiler and Körner 1997; Tørseth and Semb 1997), has been reported to limit plant growth and diversity in terrestrial ecosystems (Vitousek and Howarth 1991; Gong et al. 2015) and may restrict plant growth in alpine species (Hiltbrunner et al. 2005). Other studies indicated that early melting snow promotes the increment of soil moisture values at the beginning of the vegetative period with consequences on community composition, species richness and the occurrence patterns of individual species (Körner 2003; le Roux et al. 2013).
Pauli et al. (2012) documented two different trends in European mountains regarding the relation between vascular plant species and climate warming in a short-term study (7 years). In particular, in the northern mountains, a rapid increase in species richness occurred, whereas in the Mediterranean areas, where both climatic conditions and local biodiversity are considerably different from temperate and boreal regions, species richness decreased. Moreover, Gottfried et al. (2012) identified a consistent thermophilization process on the high-mountain vegetation (7 years). Consistent changes in plant community composition (e.g. species richness and diversity) and ecology (e.g. the upward shifting of thermophilic plant species) have been described in many central European mountains, namely in the Alps through both short-term (∼7–10 years) (e.g. Pauli et al. 2007, 2012; Erschbamer et al. 2009, 2011) and long-term (∼50–100 years) (e.g. Körner 2003; Cannone et al. 2007; Holzinger et al. 2008; Parolo and Rossi 2008; Vittoz et al. 2008; Britton et al. 2009; Grabherr et al. 2010; Engler et al. 2011; Matteodo et al. 2013) vegetation analyses. Despite the peculiarities present in terms of the vegetation that characterize Mediterranean mountain habitats and their sensitivity to climatic variations, few studies have described the effects of climatic changes on Mediterranean high-mountain plant communities, and these studies mainly addressed a relatively short time period (Petriccione 2005; Fernández Calzado and Molero 2013; Stanisci et al. 2014). Instead, studies focussing on long-term changes in such fragile ecosystems are almost absent (Jiménez-Alfaro et al. 2014). Long-term studies represent a unique and valuable way for identifying large-scale vegetation patterns over time and for predicting how a changing climate affects the biodiversity of mountain habitats (Stöckli et al. 2011; Matteodo et al. 2013), and thus, these long-term studies represent an area of research that deserves attention, especially in the Mediterranean region.
In this work, we performed a re-visitation study using historical and newly collected vegetation data to explore which ecological and structural features have been most successful in coping with climatic changes during the last 42 years. The research site is part of the European Long-Term Ecological Research (LTER) network (http://www.lter-europe.net) and GLORIA project network (http://www.gloria.ac.at), where ecological research is carried out at regular intervals for bio-monitoring purposes.
We addressed the following questions. (i) Have the abundance and distribution of vascular plant species of high-mountain habitats changed during the last 42 years? (ii) Have certain plant structural or ecological characteristics been favoured under the ongoing climate change?
To address these issues, we used a set of ecological plant indicators (Landolt et al. 2010) and life form frequencies to compare historical and present-day re-survey data.
Methods
Study area
The study area includes the widest high-mountain zone with alpine vegetation of the Apennines and comprises the higher sectors (from ∼2400 up to 2790 m above sea level (a.s.l.)) of the Majella National Park (Fig. 1). The area is characterized by a large limestone summit plateau modelled by a periglacial phenomenon: tectonic-karst depressions all surrounded by slopes (Giraudi 1998). The land-use history of the study area is characterized by grazing activities (mainly by sheep) that peaked in the middle of the 19th century (Palombo et al. 2013). Grazing pressure decreased after the second world war, due to the abandonment of transhumance practices and still decreased in the last decades because of the establishment of a protected area, the Majella National Park (van Gils et al. 2012). Currently, grazing intensity is very low at high elevation habitats due to the limited accessibility and low vegetation cover.
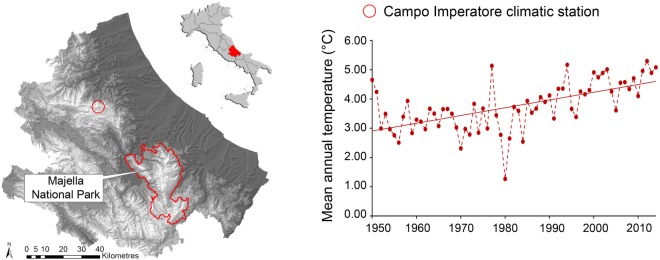
Figure 1.
Location of the study area in central Italy. Mean annual temperatures registered in the period 1950–2014 at Campo Imperatore climatic station (2125 m a.s.l.).
Temperature changes: 1950–2014
To identify the climatic trends of the last 60 years, we analysed annual mean temperature data from the Campo Imperatore weather station at 2125 m a.s.l. (∼50 km away from the study area but with similar environmental conditions). During the period between 1950 and 2014, we detected a significant increase in mean annual temperature of 1.7 °C (R2 = 0.3635, P < 0.001), amounting to 0.26 °C per decade (Fig. 1).
Data collection
We obtained data from 31 phytosociological relevés that had been sampled in 1972 (Feoli-Chiapella and Feoli 1977). We only selected data from relevés accompanied by an accurate description of the localities and mapped by the authors. In 2014, we re-visited the same areas and sampled 33 new phytosociological relevés [see Supporting Information—Table S1]. Since no permanent plots were marked in the first sampling period, in the 2014 fieldwork, we re-visited the same areas by following the description of the locations, which were marked in historical vegetation maps (Feoli-Chiapella and Feoli 1977), and collected detailed information on the morphology, soil, aspect and slope reported in the reference studies (Stöckli et al. 2011). Relevés were conducted in three habitat types widely distributed in the analysed mountains (Stanisci et al. 2011): dolines, gentle slopes and ridges (see Table 1). Relevés were performed following the same sampling protocol (considering plant community type, habitat type, plot size, previous species lists and dominant species cover estimations) (Chytrý et al. 2014) and in the same season of the previous study in order to remove the effects of phenological differences (Vymazalová et al. 2012). Sampling size varied according to the habitat type (from 15 to 100 m2) but was the same within each community type. The plant communities were sampled using the classic phytosociological approach using the Braun-Blanquet scale of abundance/dominance (Braun-Blanquet 1964; Westhoff and van der Maarel 1973). We used Conti et al. (2005) as a taxonomic reference.
Table 1.
Synthetic description of the environmental units (over 2000 m a.s.l.) of the Majella massif (central Apennines). Rel, number of relevés; Elev, elevation ± standard error (m a.s.l.); Slo, slope ± standard error (%); Veg, vegetation cover ± standard error (%); Area, reliefs area ± standard error (m2); Rich, species richness ± standard error (no. of species).
| Rel | Elev | Slo | Veg | Area | Rich | Characteristic plant species | |
|---|---|---|---|---|---|---|---|
| Dolines | 32 | 2493 ± 14 | 7 ± 1.1 | 87 ± 1.9 | 24 ± 3.9 | 18 ± 0.9 | Plantago atrata and Trifolium thalii |
| Gentle slopes | 19 | 2583 ± 36.5 | 13 ± 1.1 | 44 ± 5.9 | 58 ± 6.5 | 19 ± 1.5 | Carex kitaibeliana subsp. kitaibeliana and Festuca violacea subsp. italica |
| Ridges | 14 | 2659 ± 19.4 | 9 ± 1.3 | 65 ± 5.0 | 35 ± 7.6 | 22 ± 0.7 | Silene acaulis and Viola magellensis |
In order to investigate the changes in species composition, we analysed species abundance over time. Changes in the vegetation structure were examined using the life form categories of Raunkiaer (1934): chamaephytes (Ch), geophytes (G), hemicryptophytes (H), phanerophytes (Ph) and therophytes (Th). Plant species were classified according to flowering period in the following three groups: early (March–May), medium (June–August) and late (September–November) (Landolt et al. 2010). Finally, ecological features were measured using the Landolt indicator values (Landolt et al. 2010): temperature (T), moisture (F), soil nutrients (N) and dominance in situ (DG). These indicator values are based on the assumption that plants may serve as bioindicators (Landolt et al. 2010), as they reflect the species' requirements for environmental features. For each plant species, Landolt indices were expressed as a range of values from 1 to 5. Moreover, for the endemic Mediterranean flora, the Landolt indicator values were assigned by experts after checking the species requirements through literature data analysis (Feoli-Chiapella and Feoli 1977; Pignatti 1982; Conti 1998; Blasi et al. 2005; Pignatti et al. 2005; Stanisci et al. 2011). For comparing the ecological indicator values of the sampled flora for the different dates, we used weighted average (WA) values because they are reliable predictors of site conditions. Weighted average values were calculated using quantitative data (frequency of each plant group in each habitat type).
Data analysis
We analysed a matrix of 116 species × 64 relevés with detrended correspondence analyses (DCAs) using the Bray–Curtis distance. Then, we performed an analysis of similarities (ANOSIM) through a one-way ANOSIM test (9999 permutations) to search for significant differences between habitat types and temporal groups. Analysis of similarities is a non-parametric test of significant difference between two or more groups, based on any distance measure (Clarke 1993). The distances are converted to ranks. Then, the similarity percentage procedure (SIMPER—Clarke 1993) was performed to determine which species contribute most consistently to differences between the temporal groups.
For each relevé, we calculated the mean Landolt bioindicator values of temperature, moisture, soil nutrients and DG weighted according to species frequency as follows:
where rij is the frequency of the species i in the relevé j, and xi is the Landolt bioindicator value x for the species i. Moreover, for each relevé, the flowering period, life form and species richness values were calculated on presence/absence data. We performed a permutational multivariate analysis of variance (PERMANOVA, 9999 randomizations) based on Gower distances (Gower 1971), including the effect of the year (factor with two levels) and the habitat type (factor with five levels) as grouping variables. We also included the interaction between year and habitat types, allowing us to test whether the effect of year varied by habitat. The Mann–Whitney test was used post hoc to determine which variables (floristic, structural and ecological) were different between the old and new relevè. This is a non-parametric test, which means that the distributions can be of any shape. The two-tailed (Wilcoxon) Mann–Whitney U-test can be used to test whether the medians of two independent samples are different. All analyses were performed in the R statistical computing program (R Development Core Team 2014) using the Vegan package (Oksanen et al. 2013).
Results
The DCA revealed differences in the floristic composition of the relevés depending on the time and the habitat types in which they were collected. Eigenvalues for the DCA axes were 0.820 for DCA1 and 0.528 for DCA2, thus clearly discriminating between the two different time periods. The first axis mainly showed the separation of the relevés in habitat types (Fig. 2), whereas the second axis reflected the temporal split between the old (upper group) and new (lower group) samples (Fig. 2). The analysis of similarity confirmed these trends and revealed significant differences among the habitat types (ANOSIM R value = 0.714, ndoline = 32, ngentle slopes = 19, nridges = 14, P = 0.001) and between the temporal groups (ANOSIM R value = 0.302, nold = 31, nnew = 33, P = 0.001). In accordance with the similarity percentage analysis (Table 2), 27 of the 116 species contributed to 50 % of the observed temporal differences in vegetation composition. Of these, 16 increased and 11 decreased over time.
Figure 2.
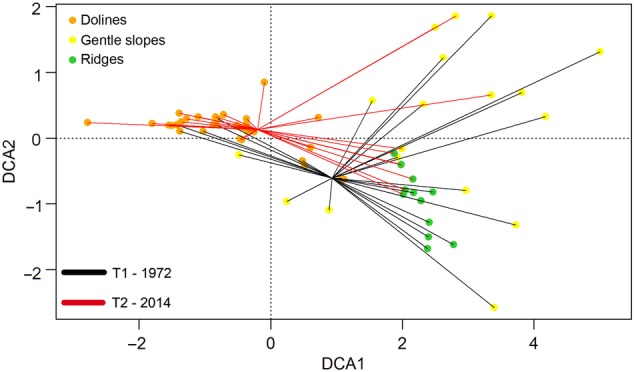
Detrended correspondence analysis scatter diagram of plots (grouped in doline, gentle slope and ridge habitat types), using species as the explanatory variables. Only the first two axes are represented. Black lines represent the relevés sampled in 1972; red lines represent the relevés sampled in 2014.
Table 2.
Plant species contribution (Species contrib. %) to the observed differences between plant communities in the two temporal groups assessed by similarity percentage procedure (SIMPER—Clarke 1993). Life forms (H, hemicryptophytes; Ch, chamaephytes; G, geophytes) and Landolt ecological indicators (T, temperature; F, moisture; N, soil nutrients) are also reported and mean abundance. Endemic species are indicated with asterisks.
| Species | Life form | T | F | N | Mean abundance (%) decrease |
Species contrib. (%) | |
|---|---|---|---|---|---|---|---|
| 1972 | 2014 | ||||||
| Viola magellensis* | H | 1.0 | 1.5 | 2.0 | 0.677 | 0.0909 | 2.5 |
| Myosotis ambigens* | H | 1.5 | 3.0 | 3.0 | 0.581 | 0.273 | 2.053 |
| Draba aizoides subsp. aizoides | H | 1.5 | 2.0 | 2.0 | 0.581 | 0.333 | 2.044 |
| Saxifraga oppositifolia subsp. oppositifolia* | Ch | 1.0 | 3.0 | 2.0 | 0.516 | 0.303 | 1.996 |
| Silene acaulis | Ch | 1.0 | 3.0 | 1.0 | 0.484 | 0.455 | 1.908 |
| Kobresia myosuroides | H | 1.5 | 2.0 | 2.0 | 0.484 | 0.212 | 1.75 |
| Salix retusa | Ch | 1.5 | 3.0 | 2.0 | 0.387 | 0.303 | 1.726 |
| Arenaria grandiflora subsp. grandiflora | Ch | 2.0 | 2.0 | 2.0 | 0.355 | 0.273 | 1.682 |
| Galium magellense* | H | 1.5 | 2.5 | 2.0 | 0.387 | 0.242 | 1.613 |
| Gentiana verna subsp. verna | H | 2.5 | 3.0 | 2.0 | 0.258 | 0.242 | 1.527 |
| Gentiana brachyphylla subsp. brachyphylla | H | 1.0 | 3.0 | 2.0 | 0.419 | 0.0303 | 1.518 |
| Species | Life form | T | F | N | Mean abundance (%) increase |
Species contrib. (%) | |
| 1972 | 2014 | ||||||
| Minuartia verna subsp. verna | Ch | 2 | 2 | 2 | 0.29 | 0.727 | 2.373 |
| Trifolium thalii | H | 1.5 | 3.5 | 3 | 0.161 | 0.606 | 2.329 |
| Plantago atrata subsp. atrata | H | 1.5 | 3.5 | 3 | 0.355 | 0.727 | 2.233 |
| Ranunculus pollinensis* | H | 2 | 3.5 | 4 | 0.387 | 0.697 | 2.153 |
| Gnaphalium hoppeanum subsp. magellense | H | 1.5 | 3.5 | 3 | 0.452 | 0.667 | 2.037 |
| Festuca violacea subsp. italica* | H | 1.5 | 3.0 | 3 | 0.419 | 0.606 | 1.999 |
| Carex kitaibeliana subsp. kitaibeliana | H | 2 | 2.5 | 3 | 0.516 | 0.758 | 1.962 |
| Armeria majellensis subsp. majellensis* | H | 2 | 2 | 2 | 0.484 | 0.545 | 1.952 |
| Cerastium thomasii* | Ch | 1.5 | 1.5 | 2 | 0.387 | 0.485 | 1.93 |
| Taraxacum glaciale* | H | 1.5 | 3.5 | 4 | 0 | 0.424 | 1.775 |
| Crepis aurea subsp. glabrescens | H | 2 | 3.0 | 4 | 0.0323 | 0.455 | 1.765 |
| Anthyllis vulneraria subsp. pulchella | H | 4 | 1.5 | 2 | 0.29 | 0.394 | 1.667 |
| Potentilla crantzii subsp. crantzii | H | 2 | 2.5 | 3 | 0.258 | 0.333 | 1.613 |
| Achillea barrelieri* | H | 1.5 | 3 | 2 | 0.29 | 0.333 | 1.554 |
| Leontodon montanus | H | 1.5 | 2 | 2 | 0.258 | 0.303 | 1.477 |
| Viola eugeniae subsp. eugeniae* | H | 2 | 3 | 2 | 0 | 0.394 | 1.474 |
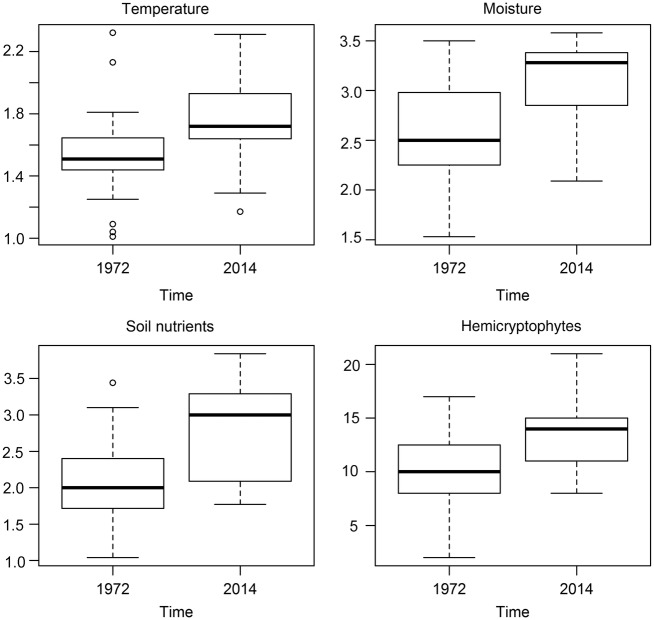
The PERMANOVA test showed that the ecological and structural variables (T, F, N, DG, life forms, species richness and flowering period) were significantly affected by time and habitat types but not by their interaction (Table 3). In particular, post hoc Mann–Whitney U comparisons noted major differences between the old and new data sets for T, F, N and hemicryptophyte frequencies. The T median value increased from 1.51 to 1.72 (Mann–Whitney z= 3.601, nold = 31, nnew = 33, P = 0.0001) (Fig. 3), variation that was due to both the decrease in frequencies of some cryophilic species (Landolt T values 1–1.5) and the increase in frequencies of some thermophilic species (Landolt T values 2–4). Ecologically, this implies an enhanced competition between slow-growing cold-adapted alpine plant species and lower elevational range species. Based on per cent similarity (Table 2), some cryophilic species, usually common in ridge habitat, have become less frequent, including Viola magellensis, Myosotis ambigens and Silene acaulis. Moreover, other cold-adapted species of gentle slopes, such as Kobresia myosuroides, Draba aizoides subsp. aizoides, Salix retusa and Gentiana verna subsp. verna, showed a decrease. During the same period, other species with an ecological optimum in lower vegetation belts (subalpine and treeline) and which are common in gentle slopes, such as Minuartia verna subsp. verna, Ranunculus pollinensis, Armeria majellensis subsp. majellensis and Carex kitaibeliana subsp. kitaibeliana, had increased in frequency (Table 2).
Table 3.
Permutational multivariate analysis of variance result. Time, comparison between old (1972) and new (2014) relevés. Habitat type, comparison between habitat types described in Table 1.
| Permutation N: 9999 | PERMANOVA (Gower) |
||||
|---|---|---|---|---|---|
| Source | Sum of squares | df | Mean square | F | P |
| Time | 0.1732 | 1 | 0.1732 | 6.496 | 0.0001 |
| Habitat type | 0.8434 | 2 | 0.4217 | 15.82 | 0.0001 |
| Interaction | −0.4466 | 2 | −0.2233 | −8.37 | 0.4432 |
| Residual | 1.5466 | 58 | 0.0267 | ||
| Total | 2.1166 | 63 | |||
Figure 3.
Box plots comparing the Landolt ecological values (temperature, moisture and soil nutrients) and hemicryptophyte (H) life form frequency between 1972 and 2014. All differences are significant according to a post hoc Mann–Whitney U-test.
The F median value increased from 2.50 to 3.28 (Mann–Whitney z = −3.393, nold = 31, nnew = 33, P = 0.0003) denoting a decrease of species that require soils with medium–low moisture content (fresh soils—Landolt F value 2.5) and an increase of species growing on soil with moderate moisture content (moderately moist soils—Landolt F value 3) (Fig. 3). Actually, some mesophilous species (Landolt F values 3–4) that are common in doline habitats, such as Plantago atrata subsp. atrata, Trifolium thalii, Viola eugeniae subsp. eugeniae and Gnaphalium hoppeanum subsp. magellense, had increased in frequency in the later relevés. The same species also grow optimally in medium fertile soils (Landolt N values 3–4) and contribute to an increase of the N median value from 2.00 to 3.00 (Mann–Whitney z = −3.807, nold = 31, nnew = 33, P = 0.0002) (Fig. 3).
Regarding life forms, only hemicryptophytes showed significant differences between the old and new relevés, increasing from 9.90 to 13.55 (median value, Mann–Whitney z = −4.187, nold = 31, nnew = 33, P = 0.0001), the greater part of the above-mentioned expanding species belong to this life form. However, changes in DG, species richness, length of flowering period and other life forms were not significant.
Discussion
The re-visitation study of alpine plant communities in the central Apennines showed consistent changes in floristic composition and in structural and ecological characteristics of high-mountain vegetation in doline, gentle slope and ridge habitats, along with significant increments in the Landolt indicators of temperature, moisture and soil nutrients and an increase in hemicryptophytes (herbaceous perennial plants). Although we cannot confirm with certainty that climate warming is the primary driver of these changes, the limited human impact in the area (e.g. grazing) and the increment on temperatures recorded in the central Apennines over the last 60 years (Fig. 1) lead us to suppose that the observed ecological changes are most likely related to climate change.
The long-term vegetation analysis clearly revealed an ongoing ‘thermophilization’ process (sensu Gottfried et al. 2012) in the central Apennines, which was related to an increase in thermophilic plant frequency and a clear decline in several cryophilic species. Similar to observations from other studies in the Alps (Grabherr et al. 1995, 2001; Theurillat and Guisan 2001; Walther et al. 2005; Pauli et al. 2007; Holzinger et al. 2008; Parolo and Rossi 2008; Vittoz et al. 2008; Erschbamer et al. 2009; Engler et al. 2011; Matteodo et al. 2013), we registered the local expansion of thermophilic species (e.g. A. majellensis subsp. majellensis, M. verna subsp. verna and C. kitaibeliana subsp. kitaibeliana). Furthermore, as described by Pauli et al. (2007) for the Alps, we also registered the local contraction of many cryophilic species, mainly in gentle slope habitats (e.g. M. ambigens, S. acaulis, V. magellensis and Saxifraga oppositifolia subsp. oppositifolia). Similar trends have been documented in other European mountains and have been related mainly to the effects of climate change (Britton et al. 2009; Engler et al. 2011; Gottfried et al. 2012; Fernández Calzado and Molero 2013).
We observed changes in other ecological characteristics with vegetation that, in 2014, had become more moisture and nutrient demanding. The increment of the nutrient and moisture ecological values could be related mainly to the expansion of species typical of medium fertile soils with good moisture content coming from doline habitats (e.g. P. atrata subsp. atrata and T. thalii). As observed in the tundra and temperate grasslands, the observed increment in nutrient ecological values could be related to the increase in the decomposition rates caused by the rise in temperatures (e.g. Gavazov 2010; Myers-Smith et al. 2011; Gong et al. 2015) and to the increase of atmospheric N deposition (Hättenschwiler and Körner 1997; Tørseth and Semb 1997). Nitrogen deposition is well known to affect species composition and richness on alpine grasslands over Europe (e.g. Bobbink et al. 2010). However, N deposition in Central Italy is low compared with those reported for Northern and Western Europe (Bonanomi et al. 2006; Ferretti et al. 2014). So far, due to a lack of local data, we can simply speculate that the increase in nutrient-demanding species could be related to the increase in N deposition. Similar processes towards mesic conditions were detected in montane grasslands in the western Alps, along with consistent changes in vegetation composition and structure (Theurillat and Guisan 2001). Notably, some species of medium fertile and humid soils have wide distribution ranges; that is, they are indiscriminately distributed from the montane to the alpine belt (e.g. high Landolt T values) (Landolt et al. 2010; Güsewell et al. 2012; Matteodo et al. 2013; Venn et al. 2014). On the other hand, cryophilic species of dry grasslands are usually poor moisture- and nutrient-demanding taxa, and high levels of soluble N deposition limit their growth (Vitousek and Howarth 1991; Hiltbrunner et al. 2005). The ecological changes (thermophilization and increase in the frequencies of nutrient- and moisture-demanding species) occurring in the Apennines are most likely a result of the reduction of cryophilous drought-tolerant and poor nutrient-demanding species and the expansion of lower elevational range species; both processes are probably triggered by the ongoing global change (Huber et al. 2007; Engler et al. 2011).
The long-term analysis of the Apennine's habitats revealed consistent changes in vegetation structure. Similar to that observed in long-term analysis of the Swiss Alps (Matteodo et al. 2013) and of the Iberian mountains (Jiménez-Alfaro et al. 2014), on the Italian Apennines, hemicryptophyte frequency increased over time. Jägerbrand et al. (2009) observed similar increments in subarctic-alpine grasslands in correspondence with nutrient addition and increasing temperatures under experimental conditions. Accordingly, recent evidence (e.g. Spasojevic and Suding 2012; Spasojevic et al. 2013) suggests that variations in nutrient availability, soil moisture and temperature led to changes in the functional composition of alpine plant communities with a shift towards more resource acquisitive functional traits (e.g. hemicryptophytes with well-developed leaves). Overall, our study revealed a clear long-term change in plant species abundance patterns, with an overall large increase in graminoids (e.g. Festuca violacea subsp. italica and C. kitaibeliana subsp. kitaibeliana) and other perennial herbs (e.g. G. hoppeanum subsp. magellense and R. pollinensis) and the consistent decrease of cryophilic cushions plants (e.g. S. acaulis and S. oppositifolia subsp. speciosa) and some steno-endemic species (e.g. V. magellensis and M. ambigens).
Despite the possible limitations of the applied re-visitation sampling approach to assess changes in vegetation composition structure and ecology due to the potential mismatch between old and new relevés (Chytrý et al. 2014), we are confident of the efficacy of our dedicated efforts to carry out the new sampling session in the same plant communities (and habitat types) as the historical one. Nevertheless, re-visitation (long-term) studies using historical records represent the only possibility for obtaining a reliable time perspective necessary to identify large-scale patterns over the last century (Stöckli et al. 2011; Matteodo et al. 2013) and for making predictions on the future assemblage of plant communities facing global changes.
Conclusions
Our results, based on long-term data obtained by the re-visitation of historical vegetation records, reinforce previous short-term observations for reporting changes on plant composition of high-mountain habitats related with climate in Mediterranean mountains. Furthermore, we observed a shift in the ecological values of high-mountain vegetation that showed a thermophilization process and an increase in nutrient-demanding and mesic species, typical of doline and gentle slope habitats. These changes are more likely attributable to the combined effect of higher temperatures and an increase in soil nutrients triggered by global change. This study responds to the crucial need of further long-term analysis describing vegetation changes over the last century. Such a gap in knowledge impels the development of additional research in other areas, in which, like the Mediterranean high mountains, the effect of climate changes is expected to be consistent. The herein adopted re-visitation approach might represent an adequate instrument to respond to the scarcity of a long-term series of ecological data describing natural ecosystems and the results can be gathered with existing short- to medium-term permanent observation networks (e.g. LTER). Since data from historical vegetation plots are generally available in many European countries, the re-visitation studies have a potential for application to other mountains in Italy and Europe at scales ranging from local to regional. With this in mind, we hope that several case studies will be analysed in the near future to provide long-term information for increasingly larger areas.
Sources of Funding
This work was partially supported by the NextData project (Data-LTER-Mountain) and the GLORIA-MEDIALPS project.
Contributions by the Authors
A.S. and M.L.C. conceived and designed the experiments. A.E., G.P. and L.F. collected the data. A.E. and L.F. analysed the data. A.E., A.S., L.F., M.L.C. and F.A. wrote the manuscript.
Conflict of Interest Statement
None declared.
Supporting Information
The following additional information is available in the online version of this article –
Table S1. Phytosociological table of new relevés.
Acknowledgement
We thank the Servizio Idrografico Regione Abruzzo for providing climatic data of the Campo Imperatore station.
Literature Cited
- Barthlott W, Lauer W, Placke A. 1996. Global distribution of species diversity in vascular plants: towards a world map of phytodiversity. Erdkunde 50:317–327. 10.3112/erdkunde.1996.04.03 [DOI] [Google Scholar]
- Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. 2012. Impacts of climate change on the future of biodiversity. Ecology Letters 15:365–377. 10.1111/j.1461-0248.2011.01736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniston M. 2003. Climatic change in mountain regions: a review of possible impacts. Climatic Change 59:5–31. 10.1023/A:1024458411589 [DOI] [Google Scholar]
- Blasi C, Di Pietro R, Pelino G. 2005. The vegetation of alpine belt karst-tectonic basins in the central Apennines (Italy). Plant Biosystems 139:357–385. 10.1080/11263500500350150 [DOI] [Google Scholar]
- Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman J-W, Fenn M, Gilliam F, Nordin A, Pardo L, De Vries W. 2010. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications 20:30–59. 10.1890/08-1140.1 [DOI] [PubMed] [Google Scholar]
- Bonanomi G, Caporaso S, Allegrezza M. 2006. Short-term effects of nitrogen enrichment, litter removal and cutting on a Mediterranean grassland. Acta Oecologica 30:419–425. 10.1016/j.actao.2006.06.007 [DOI] [Google Scholar]
- Braun-Blanquet J. 1964. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd edn Vienna: Springer. [Google Scholar]
- Britton AJ, Beale CM, Towers W, Hewison RL. 2009. Biodiversity gains and losses: evidence for homogenisation of Scottish alpine vegetation. Biological Conservation 142:1728–1739. 10.1016/j.biocon.2009.03.010 [DOI] [Google Scholar]
- Brunetti M, Maugeri M, Monti F, Nanni T. 2006. Temperature and precipitation variability in Italy in the last two centuries from homogenised instrumental time series. International Journal of Climatology 26:345–381. 10.1002/joc.1251 [DOI] [Google Scholar]
- Bruun HH, Moen J, Virtanen R, Grytnes J-A, Oksanen L, Angerbjörn A. 2006. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. Journal of Vegetation Science 17:37–46. 10.1111/j.1654-1103.2006.tb02421.x [DOI] [Google Scholar]
- Cannone N, Sgorbati S, Guglielmin M. 2007. Unexpected impacts of climate change on alpine vegetation. Frontiers in Ecology and the Environment 5:360–364. 10.1890/1540-9295(2007)5[360:UIOCCO]2.0.CO;2 [DOI] [Google Scholar]
- Chytrý M, Tichý L, Hennekens SM, Schaminée JHJ. 2014. Assessing vegetation change using vegetation-plot databases: a risky business. Applied Vegetation Science 17:32–41. 10.1111/avsc.12050 [DOI] [Google Scholar]
- Clarke KR. 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18:117–143. 10.1111/j.1442-9993.1993.tb00438.x [DOI] [Google Scholar]
- Conti F. 1998. An annotated checklist of the flora of the Abruzzo. Bocconea 10:1–276. [Google Scholar]
- Conti F, Abbate G, Alessandrini A, Blasi C. 2005. An annotated checklist of the Italian vascular flora. Rome: Palombi. [Google Scholar]
- Engler R, Randin C, Thuiller W, Dullinger S, Zimmermann NE, Araújo MB, Pearman PB, Le Lay G, Piedallu C, Albert CH, Choler P, Coldea G, De Lamo X, Dirnböck T, Gégout J-C, Gómez-García D, Grytnes J-A, Heegaard E, Høistad F, Nogués-Bravo D, Normand S, Puşcaş M, Sebastià M-T, Stanisci A, Theurillat J-P, Trivedi MR, Vittoz P, Guisan A. 2011. 21st century climate change threatens mountain flora unequally across Europe. Global Change Biology 17:2330–2341. 10.1111/j.1365-2486.2010.02393.x [DOI] [Google Scholar]
- Erschbamer B, Kiebacher T, Mallaun M, Unterluggauer P. 2009. Short-term signals of climate change along an altitudinal gradient in the South Alps. Plant Ecology 202:79–89. 10.1007/s11258-008-9556-1 [DOI] [Google Scholar]
- Erschbamer B, Unterluggauer P, Winkler E, Mallaun M. 2011. Changes in plant species diversity revealed by long-term monitoring on mountain summits in the Dolomites (northern Italy). Preslia 88:387–401. [Google Scholar]
- Feoli-Chiapella L, Feoli E. 1977. A numerical phytosociological study of the summits of the Majella Massive (Italy). Vegetatio 34:21–39. 10.1007/BF00119884 [DOI] [Google Scholar]
- Fernández Calzado MR, Molero J. 2013. Changes in the summit flora of a Mediterranean mountain (Sierra Nevada, Spain) as a possible effect of climate change. Lazaroa 34:65–75. 10.5209/rev_LAZA.2013.v34.n1.41523 [DOI] [Google Scholar]
- Ferretti M, Marchetto A, Arisci S, Bussotti F, Calderisi M, Carnicelli S, Cecchini G, Fabbio G, Bertini G, Matteucci G, De Cinti B, Salvati L, Pompei E. 2014. On the tracks of Nitrogen deposition effects on temperate forests at their southern European range—an observational study from Italy. Global Change Biology 20:3423–3438. 10.1111/gcb.12552 [DOI] [PubMed] [Google Scholar]
- Gavazov KS. 2010. Dynamics of alpine plant litter decomposition in a changing climate. Plant and Soil 337:19–32. 10.1007/s11104-010-0477-0 [DOI] [Google Scholar]
- Giraudi C. 1998. Nuovi dati sul glacialismo della montagna della Majella (Abruzzo, Italia centrale). Italian Journal of Quaternary Sciences 11:265–271. [Google Scholar]
- Gong S, Guo R, Zhang T, Guo J. 2015. Warming and nitrogen addition increase litter decomposition in a temperate meadow ecosystem. PLoS ONE 10:e0116013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried M, Pauli H, Futschik A, Akhalkatsi M, Barančok P, Benito Alonso JL, Coldea G, Dick J, Erschbamer B, Fernández Calzado M, Kazakis G, Krajči J, Larsson P, Mallaun M, Michelsen O, Moiseev D, Moiseev P, Molau U, Merzouki A, Nagy L, Nakhutsrishvili G, Pedersen B, Pelino G, Puscas M, Rossi G, Stanisci A, Theurillat J-P, Tomaselli M, Villar L, Vittoz P, Vogiatzakis I, Grabherr G. 2012. Continent-wide response of mountain vegetation to climate change. Nature Climate Change 2:111–115. 10.1038/nclimate1329 [DOI] [Google Scholar]
- Gower JC. 1971. A general coefficient of similarity and some of its properties. Biometrics 27:857–874. 10.2307/2528823 [DOI] [Google Scholar]
- Grabherr G, Gottfried M, Gruber A, Pauli H. 1995. Patterns and current changes in alpine plant diversity. In: Chapin FS III, Körner C, eds. Arctic and alpine biodiversity: patterns, causes and ecosystem consequences. Ecological Studies 113 Berlin: Springer, 167–181. [Google Scholar]
- Grabherr G, Gottfried M, Pauli H. 2001. Long-term monitoring of mountain peaks in the Alps. In: Burga CA, Kratochwil A, eds. Biomonitoring: general and applied aspects on regional and global scales. Dordrecht: Kluwer Academic Publishers, 153–177. [Google Scholar]
- Grabherr G, Gottfried M, Pauli H. 2010. Climate change impacts in alpine environments. Geography Compass 4:1133–1153. 10.1111/j.1749-8198.2010.00356.x [DOI] [Google Scholar]
- Güsewell S, Peter M, Birrer S. 2012. Altitude modifies species richness–nutrient indicator value relationships in a country-wide survey of grassland vegetation. Ecological Indicators 20:134–142. 10.1016/j.ecolind.2012.02.011 [DOI] [Google Scholar]
- Gutiérrez Girón A, Gavilán R. 2013. Plant functional strategies and environmental constraints in Mediterranean high mountain grasslands in Central Spain. Plant Ecology and Diversity 6:435–446. 10.1080/17550874.2013.783641 [DOI] [Google Scholar]
- Hättenschwiler S, Körner C. 1997. Annual CO2 budget of spruce model ecosystems in the third year of exposure to elevated CO2. Acta Oecologica 18:319–325. 10.1016/S1146-609X(97)80021-5 [DOI] [Google Scholar]
- Hiltbrunner E, Schwikowski M, Körner C. 2005. Inorganic nitrogen storage in alpine snow pack in the Central Alps (Switzerland). Atmospheric Environment 39:2249–2259. 10.1016/j.atmosenv.2004.12.037 [DOI] [Google Scholar]
- Holzinger B, Hülber K, Camenisch M, Grabherr G. 2008. Changes in plant species richness over the last century in the eastern Swiss Alps: elevational gradient, bedrock effects and migration rates. Plant Ecology 195:179–196. 10.1007/s11258-007-9314-9 [DOI] [Google Scholar]
- Huber E, Wanek W, Gottfried M, Pauli H, Schweiger P, Arndt SK, Reiter K, Richter A. 2007. Shift in soil–plant nitrogen dynamics of an alpine–nival ecotone. Plant and Soil 301:65–76. 10.1007/s11104-007-9422-2 [DOI] [Google Scholar]
- IPCC. 2013. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- IPCC. 2014. Climate change 2014: mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Jägerbrand AK, Alatalo JM, Chrimes D, Molau U. 2009. Plant community responses to 5 years of simulated climate change in meadow and heath ecosystems at a subarctic-alpine site. Oecologia 161:601–610. 10.1007/s00442-009-1392-z [DOI] [PubMed] [Google Scholar]
- Jiménez-Alfaro B, Gavilán RG, Escudero A, Iriondo JM, Fernández-González F. 2014. Decline of dry grassland specialists in Mediterranean high-mountain communities influenced by recent climate warming. Journal of Vegetation Science 25:1394–1404. 10.1111/jvs.12198 [DOI] [Google Scholar]
- Kazakis G, Ghosn D, Vogiatzakis IN, Papanastasis VP. 2007. Vascular plant diversity and climate change in the alpine zone of the Lefka Ori, Crete. Plant Conservation and Biodiversity 16:1603–1615. 10.1007/s10531-006-9021-1 [DOI] [Google Scholar]
- Körner C. 2003. Alpine plant life, 2nd edn Berlin: Springer. [Google Scholar]
- Landolt E, Bäumler B, Erhardt A, Hegg O, Klötzli F, Lämmler W, Nobis M, Rudmann-Maurer K, Schweingruber FH, Theurillat J-P, Urmi E, Vust M, Wohlgemuth T. 2010. Ecological indicator values and biological attributes of the flora of Switzerland and the Alps. Bern: Haupt. [Google Scholar]
- Le Roux PC, Aalto J, Luoto M. 2013. Soil moisture's underestimated role in climate change impact modelling in low-energy systems. Global Change Biology 19:2965–2975. 10.1111/gcb.12286 [DOI] [PubMed] [Google Scholar]
- Matteodo M, Wipf S, Stöckli V, Rixen C, Vittoz P. 2013. Elevation gradient of successful plant traits for colonizing alpine summits under climate change. Environmental Research Letters 8:024043 10.1088/1748-9326/8/2/024043 [DOI] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T, Blok D, Tape KD, Macias-Fauria M, Sass-Klaassen U, Lévesque E, Boudreau S, Ropars P, Hermanutz L, Trant A, Collier LS, Weijers S, Rozema J, Rayback SA, Schmidt NM, Schaepman-Strub G, Wipf S, Rixen C, Ménard CB, Venn S, Goetz S, Andreu-Hayles L, Elmendorf S, Ravolainen V, Welker J, Grogan P, Epstein HE, Hik DS. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environmental Research Letters 6:045509 10.1088/1748-9326/6/4/045509 [DOI] [Google Scholar]
- Nogués Bravo D, Araújo MB, Lasanta T, López Moreno JI. 2008. Climate change in Mediterranean mountains during the 21st century. Ambio 37:280–285. 10.1579/0044-7447(2008)37[280:CCIMMD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'hara RB, Simpson GL, Solymos P, Stevens MH, Wagner H. 2013. Vegan: community ecology package. R package version 2.0-10 http://CRAN.Rproject.org/package=vegan (25 May 2015).
- Palombo C, Chirici G, Marchetti M, Tognetti R. 2013. Is land abandonment affecting forest dynamics at high elevation in Mediterranean mountains more than climate change? Plant Biosystems 147:1–11. 10.1080/11263504.2013.772081 [DOI] [Google Scholar]
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37:637–669. 10.1146/annurev.ecolsys.37.091305.110100 [DOI] [Google Scholar]
- Parolo G, Rossi G. 2008. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology 9:100–107. 10.1016/j.baae.2007.01.005 [DOI] [Google Scholar]
- Pauli H, Gottfried M, Dirnböck T, Dullinger S, Grabherr G. 2003. Assessing the long-term dynamics of endemic plants at summit habitats. In: Nagy L, Grabherr G, Körner C, Thompson DBA, eds. Alpine biodiversity in Europe. Berlin: Springer, 195–207. [Google Scholar]
- Pauli H, Gottfried M, Reiter K, Klettner C, Grabherr G. 2007. Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Global Change Biology 13:147–156. 10.1111/j.1365-2486.2006.01282.x [DOI] [Google Scholar]
- Pauli H, Gottfried M, Dullinger S, Abdaladze O, Akhalkatsi M, Alonso JLB, Coldea G, Dick J, Erschbamer B, Calzado RF, Ghosn D, Holten JI, Kanka R, Kazakis G, Kollar J, Larsson P, Moiseev P, Moiseev D, Molau U, Mesa JM, Nagy L, Pelino G, Puscas M, Rossi G, Stanisci A, Syverhuset AO, Theurillat J-P, Tomaselli M, Unterluggauer P, Villar L, Vittoz P, Grabherr G. 2012. Recent plant diversity changes on Europe's mountain summits. Science 336:353–355. 10.1126/science.1219033 [DOI] [PubMed] [Google Scholar]
- Petriccione B. 2005. Short-term changes in key plant communities of Central Apennines (Italy). Acta Botanica Gallica 152:545–561. 10.1080/12538078.2005.10515513 [DOI] [Google Scholar]
- Pignatti S. 1982. Flora d'Italia. Bologna: Edagricole. [Google Scholar]
- Pignatti S, Menegoni P, Pietrosanti S. 2005. Bioindicazione attraverso le piante vascolari. Valori di indicazione secondo Ellenberg (Zeigerwerte) per le specie della Flora d'Italia. Braun-Blanquetia 39:1–97. [Google Scholar]
- Raunkiaer C. 1934. The life forms of plants and statistical plant geography. Oxford: The Clarendon Press. [Google Scholar]
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.Rproject.org/ (10 June 2015). [Google Scholar]
- Spasojevic MJ, Suding KN. 2012. Inferring community assembly mechanisms from functional diversity patterns: the importance of multiple assembly processes. Journal of Ecology 100:652–661. 10.1111/j.1365-2745.2011.01945.x [DOI] [Google Scholar]
- Spasojevic MJ, Bowman WD, Humphries HC, Seastedt TR, Suding KN. 2013. Changes in alpine vegetation over 21 years: are patterns across a heterogeneous landscape consistent with predictions? Ecosphere 4:1–18. 10.1890/ES13-00133.1 [DOI] [Google Scholar]
- Stanisci A, Carranza ML, Pelino G, Chiarucci A. 2011. Assessing the diversity pattern of cryophilous plant species in high elevation habitats. Plant Ecology 212:595–600. 10.1007/s11258-010-9849-z [DOI] [Google Scholar]
- Stanisci A, Frate L, Di Cella U, Pelino G, Petey M, Siniscalco C, Carranza ML. 2014. Short-term signals of climate change in Italian summit vegetation: observations at two GLORIA sites. Plant Biosystems; 10.1080/11263504.2014.968232. [DOI] [Google Scholar]
- Stöckli V, Wipf S, Nilsson C, Rixen C. 2011. Using historical plant surveys to track biodiversity on mountain summits. Plant Ecology and Diversity 4:415–425. 10.1080/17550874.2011.651504 [DOI] [Google Scholar]
- Theurillat JP, Guisan A. 2001. Potential impact of climate change on vegetation in the European Alps: a review. Climatic Change 50:77–109. 10.1023/A:1010632015572 [DOI] [Google Scholar]
- Toreti A, Desiato F. 2008. Changes in temperature extremes over Italy in the last 44 years. International Journal of Climatology 28:733–745. 10.1002/joc.1576 [DOI] [Google Scholar]
- Tørseth K, Semb A. 1997. Atmospheric deposition of nitrogen, sulfur and chloride in two watersheds located in southern Norway. Ambio 26:258–265. [Google Scholar]
- Van Gils H, Conti F, Ciaschetti G, Westinga E. 2012. Fine resolution distribution modelling of endemics in Majella National Park, Central Italy. Plant Biosystems 146:276–287. 10.1080/11263504.2012.685194 [DOI] [Google Scholar]
- Väre H, Lampinen R, Humphries C, Williams P. 2003. Taxonomic diversity of vascular plants in the European alpine areas. In: Nagy L, Grabherr G, Körner C, Thompson DBA, eds. Alpine biodiversity in Europe. Berlin: Springer, 133–148. [Google Scholar]
- Venn S, Pickering C, Green K. 2014. Spatial and temporal functional changes in alpine summit vegetation are driven by increases in shrubs and graminoids. AoB PLANTS 6: plu008; 10.1093/aobpla/plu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM, Howarth RW. 1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115. 10.1007/BF00002772 [DOI] [Google Scholar]
- Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecological Applications 7:737–750. [Google Scholar]
- Vittoz P, Bodin J, Ungricht S, Burga CA, Walther G-R. 2008. One century of vegetation change on Isla Persa, a nunatak in the Bernina massif in the Swiss Alps. Journal of Vegetation Science 19:671–680. 10.3170/2008-8-18434 [DOI] [Google Scholar]
- Vymazalová M, Axmanová I, Tichý L. 2012. Effect of intra-seasonal variability on vegetation data. Journal of Vegetation Science 23:978–984. 10.1111/j.1654-1103.2012.01416.x [DOI] [Google Scholar]
- Walther GR, Beißner S, Burga C. 2005. Trends in the upward shift of alpine plants. Journal of Vegetation Science 16:541–548. 10.1111/j.1654-1103.2005.tb02394.x [DOI] [Google Scholar]
- Westhoff V, Van der Maarel E. 1973. The Braun-Blanquet approach. In: Whittaker RH, ed. Classification of plant communities. The Netherlands: The Hague, 289–399. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.