Abstract
Background: The zinc content of maize, a major global food staple, is generally insufficient alone to meet the requirements of young children.
Objectives: The primary objective of this study was to determine whether substitution of biofortified maize (34 μg zinc/g grain) for control maize (21 μg zinc/g) was adequate to meet zinc physiologic requirements in young children for whom maize was the major food staple. A secondary objective was to compare total daily zinc absorption when maize flour was fortified with zinc oxide to a total concentration of 60 μg zinc/g.
Methods: Participants included 60 rural Zambian children with a mean age of 29 mo who were randomly assigned to receive 1 of 3 maize types (control, biofortified, or fortified) all of which were readily consumed (>100 g on 1 d). Total daily zinc intake (from maize and low-zinc relish) was determined from duplicate diet collections. Multiplication by fractional absorption of zinc, measured by a dual isotope ratio technique, determined the total daily zinc absorption on the day the test meals were given.
Results: The mean ± SD total daily zinc intake (milligrams per day) from the biofortified maize (5.0 ± 2.2) was higher (P < 0.0001) than for the control maize (2.3 ± 0.9). Intake of zinc from the fortified maize (6.3 ± 2.6) did not differ from the biofortified maize. Fractional absorption of zinc from control maize (0.28 ± 0.10) did not differ from the biofortified maize (0.22 ± 0.06). Total daily absorption of zinc (milligrams per day) from the biofortified maize (1.1 ± 0.5) was higher (P = 0.0001) than for the control maize (0.6 ± 0.2), but did not differ from the fortified maize (1.2 ± 0.4).
Conclusions: These results indicate that feeding biofortified maize can meet zinc requirements and provide an effective dietary alternative to regular maize for this vulnerable population. This trial was registered at clinicaltrials.gov as NCT02208635.
Keywords: zinc absorption, biofortification, maize, Zambia, young children, fortified, maize, physiologic zinc requirement
Introduction
Maize is one of the 3 most important cereals worldwide. It provides an estimated 15% of the world’s protein and 20% of the world’s calories and is a dietary staple for >200 million people. Unfortunately, even though maize kernels supply many macro- and micronutrients for human metabolic needs, the amounts of some essential nutrients, including zinc, are inadequate for consumers who rely on maize as a major food staple (1). Zinc is essential for the growth and health of young children (2). In sub-Saharan Africa, maize is typically provided to young children as a watery maize porridge for breakfast or a thicker gruel for other meals of the day. The latter is often accompanied by vegetables or fruit that contain little zinc content (1).
Genetic variation for zinc concentration in maize kernels, as well as environmental and genotype by environment interaction effects, have been reported (3, 4). The mean zinc concentration in the kernels is ∼2 mg zinc/100 g grain (5). However, the concentration is lower than in animal foods and bioavailability is thought to be substantially less favorable. In addition, the quantity of maize consumed to meet energy and protein needs is insufficient to avoid a major risk of zinc deficiency, especially in young children (2). One of the biofortification strategies to alleviate zinc deficiency in maize-consuming populations is the development of zinc-biofortified (high-kernel zinc) maize (6). However, no information is available regarding the absorption of zinc by young children who are fed zinc-biofortified maize.
This study was located in southern Zambia in one of numerous rural communities that are notably dependent on maize as their only food staple. Maize production far exceeds that of any other grain in Zambia (7), which has been identified as one of the African countries of greatest concern for micronutrient deficiencies including zinc (8). As applies in many African countries, young children in poor rural Zambian families are dependent on maize grown on limited family land as their major food staple (9). Typically, breakfast consists of watery maize porridge (phala); a stiffer maize porridge (nshima), accompanied by relishes, is consumed for other meals. This study did not include formal dietary histories. However, it is evident that maize provides the only important source of zinc, even though this is inadequate. This monotonous diet is especially evident during the period of complementary feeding.
The primary objective of this research was to determine whether zinc biofortification of maize developed with conventional breeding methods was effective in increasing the zinc absorbed from a maize-based diet, and to determine whether it would sufficiently meet estimated physiologic requirements for zinc in young children consuming typical quantities of this cereal grain in rural Zambia.
Because the increase in the zinc content of the biofortified maize was relatively modest compared with the zinc content of at least one other grain recently studied (10), a secondary objective was to compare the quantity of zinc absorbed from the zinc-biofortified maize with that from the control diet when the flour was fortified with zinc to an amount in excess of that achieved with biofortification.
Methods
Study design.
The study was a randomized cross-sectional observational study measuring zinc absorption in rural Zambian children aged 1–5 y whose major food staple was maize. Participants, 20 per group, were randomly assigned to receive ∼100 g of control, biofortified, or fortified maize flour for the 3 main meals of a 1-d period. The endogenous zinc in the flour was extrinsically labeled with a 70Zn stable isotope preparation. Fractional absorption of zinc for the entire test day was measured with the use of a dual isotope tracer ratio technique (11, 12), which compares the ratio of urine enrichment of orally vs. intravenously administered isotope (67Zn). Total dietary zinc for the day was determined with the use of the measured zinc content of duplicate diets from the test day.
Subjects.
The participants were all residents of the Ngwerere Clinic catchment area, Chongwe District, Zambia, where local records indicated that maize contributed a minimum of 75% energy intake.
Mothers learned of the study by word of mouth, and those interested in having their child participate came to the local health center where the study was conducted. All of these poor rural Zambian children met the inclusion criteria, and mothers gave their informed consent. Inclusion criteria included maize as the single food staple (supplemented with a limited mix of vegetables, but no animal products) and no longer breastfeeding. Other inclusion criteria included being clinically healthy, resident of the target community, and had not received food or micronutrient supplements during the previous month.
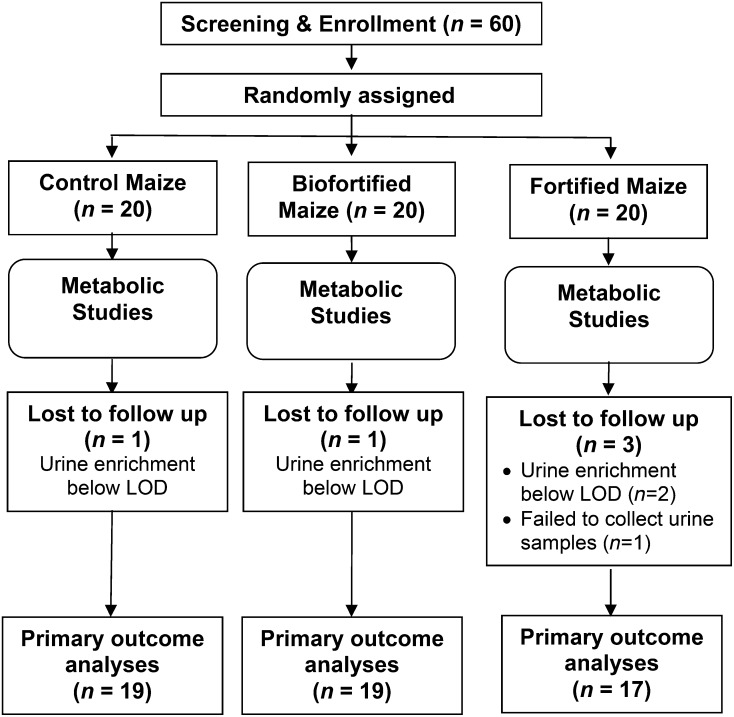
Participants were randomly assigned to the control, biofortified, or fortified maize group. Random assignment was by sequential numbers and order of recruitment, with study numbers of 1–60 randomly matched to a color (red, blue, or yellow); each color corresponded to a type of maize treatment group. Participants were assigned a study subject number, and all subsequent data were collected and analyzed with the use of this number. The color code was blinded to field and laboratory personnel. There were 20 participants randomly assigned to each group (Figure 1). This number was determined from previous experience (10) and by the quantity of biofortified grain available.
FIGURE 1.
Consort diagram of study participants including reasons for losses to follow-up, e.g., urine samples not collected. LOD, limit of detection.
The project was approved by the University of Zambia Biomedical Research Ethics Committee and the Colorado Multiple Institute Review Board of the University of Colorado. Locally trained health personnel obtained informed consent from parents if the child was eligible and they were interested in participating. The study was explained to parents in the local language and consent was indicated by signature or thumbprint.
Source and preparation of study grain.
Biofortified and control whole grain maize were provided by the International Maize and Wheat Improvement Center in Mexico and HarvestPlus Zambia, respectively. One experimental high-kernel zinc (biofortified) hybrid from the HarvestPlus breeding program at the International Maize and Wheat Improvement Center was used in this study. It was grown in Agua Fria, Puebla, Mexico, located at 20°32´ N, 97°28´ W, 110 meters above sea level, which has a mean annual temperature of 22°C and precipitation of 1200 mm. Ears were harvested by hand without removing the husks and subsequently dried and shelled before the kernels were shipped to Zambia. The control and biofortified maize kernels, together with 0.5 kg highly zinc-fortified flour prepared from the control maize, were stored temporarily in clearly labeled bags at the University of Zambia Teaching Hospital before being transferred to a secure room at Ngwerere Health Clinic. A total of 15 kg of both control and biofortified maize were roller-milled by a local commercial miller in the Ngwerere catchment area under the supervision of one of the investigators (CM Westcott) and the flour collected into clean prelabeled bags. After the mill was cleaned, an additional 15 kg control maize was roller-milled and the ZnO-fortified premix was slowly added as the control flour was conveyed to the prelabeled storage bag. Samples of all 3 flours were collected for subsequent determination of zinc content. All zinc concentrations in the control and zinc-biofortified maize kernels were measured at the University of Colorado Denver. Analyses of multiple samples from the zinc-fortified flour confirmed excellent mixing of the ZnO fortificant (<5% CV). Throughout the study period, all grain and flour were stored in vermin-proof and waterproof containers in a locked room.
Preparation of zinc stable isotope solutions.
67Zn and 70Zn stable isotope dose solutions were prepared from enriched ZnO (Trace Science International) as previously described (13) in the Pediatric Nutrition Laboratory at the University of Colorado School of Medicine and delivered to the University Teaching Hospital in Zambia. The 67Zn was prepared under sterile conditions and subsequently checked for pyrogens and sterility by the University of Colorado Hospital before use. Solutions were stored in a sterile medical vial in Zambia at 4°C until administration.
Human study.
As with other studies of biofortified grains undertaken by this group (10, 14), the administration of study grains was limited to one study day and provided ∼100 g (dry weight) of maize to each participant. A reason for this study design was the limited quantity of biofortified grain available for this project, which was undertaken at an early stage in the evaluation of these grains. The study grains replaced the local maize normally consumed on that day. This potential limitation was not considered to be of concern, because the differences in zinc concentrations of the grains, although nutritionally important, were modest and current evidence does not favor previous and current zinc status as a major factor in determining the efficiency of zinc absorption (12, 15).
On this test day, mothers brought their children to the Ngwerere Health Center before breakfast, where they remained until completion of the third and final main meal of the day. The 3 test meals were fed by the mother under close supervision by a member of the research staff assigned to each participating family who was, in turn, supervised by the field director (CM Westcott). The breakfast provided was a preweighed portion of maize porridge (phala, cooked from the appropriately labeled research flour); plate waste was weighed immediately after the child finished eating and duplicate samples were collected for laboratory analyses of zinc content. Any porridge that was drooled from the participant’s mouth was collected on a preweighed zinc-free filter paper that was reweighed at the end of the meal and subtracted from the amount consumed by child. After breakfast, anthropometric measurements were obtained.
Labeled test meals were repeated at approximately 1200 and 1700 with the use of the same procedures. These meals were derived from the same research maize flour prepared as nshima and accompanied by a relish consisting of cabbage, oil, onion, and salt. The quantities of zinc provided by the relish accounted for ∼5% of the zinc in the test meals; a further 5% of the labeled daily intake was contributed by the zinc stable isotope doses. Duplicates of each meal were weighed and saved.
At approximately halfway through each of these meals, administration of an accurately weighed quantity of 70Zn oral stable isotope solution was commenced; the solution was carefully dispensed into the mouth with the use of a syringe. The syringe and dose vial were rinsed 2 times with sterile water, which was also administered to the participant by the end of the meal. All losses of doses from drooling or spits were collected on ashless filter paper during the dose administration and analyzed for isotope content. This amount was subtracted from the calculated dose to give a final administered isotope dose.
At 1400, the sterile solution of 67Zn was infused over 2 min into an antecubital vein with the use of a 3-way stopcock to allow infusions through one port and the rinsing of the isotope syringe and tubing through the other. Any dose losses during administration were collected on ashless filter paper, analyzed, and subtracted from the administered dose. After isotope administration, participants were offered a small banana (zinc content ∼200 μg), which was the only nonlabeled food for the test day. Immediately before the intravenous infusion, a 5 mL blood sample was collected from the same venipuncture for plasma zinc and inflammatory marker data.
At the end of the dinner meal, families returned home equipped with oral instructions and prelabeled containers (nos. 1–8) for collecting morning and afternoon urine samples (≥10 mL). The urine samples were collected for 4 consecutive d commencing 4 d after the study day and were collected from the home daily by one of the research assistants. Samples were kept frozen until shipment to the University of Colorado Denver for laboratory analyses.
The duplicate diets, adjusted for plate waste, were combined in a plastic container and stored at −20°C for <1 d before homogenization and frozen storage of 4 weighed aliquots.
Sample analyses.
All laboratory analyses except that for phytate were undertaken at the University of Colorado. Duplicate diets were dried in an electric drying oven before ashing at 450°C; samples were reconstituted in 6M HCl, and total zinc concentration was measured with the use of atomic absorption spectrophotometry fitted with a deuterium arc background lamp (day-to-day variation <2%; model AAnalyst 400, Perkin-Elmer Corporation) (13). Plasma zinc concentrations were measured against standards prepared in glycerol solution by direct aspiration with the use of atomic absorption spectrophotometry (CV <3%). Urine samples were digested with the use of a MARS microwave sample preparation system (CEM) as previously described (13). Isotope enrichment was determined by measurement of the isotope ratios 67Zn:66Zn and 70Zn:66Zn by inductively coupled plasma MS (<0.5% CV for inter- and intra-assay results; Agilent 7700× inductively coupled plasma MS, Agilent Technologies) and conversion of ratios to enrichment was determined with the use of a mathematic matrix. Tracer enrichment was defined as all of the zinc in the sample that was derived from the isotopically enriched tracer preparation divided by the total zinc in the sample (16). Phytate analyses were performed at the University of Flinders (Adelaide, Australia) by J Stangoulis with the use of an HPLC technique. Plasma α-glycoprotein was measured with the use of a standard ELISA kit (5.3% intra-assay precision and 6.6% inter-assay precision, R&D Systems Quantikine ELISA).
Data processing.
Fractional absorption of zinc was determined by our dual isotope tracer technique based on isotopic enrichments in urine from orally and intravenously administered isotopes (11) administered on 1 d. Total absorbed zinc (milligrams per day) from the test meals for this day was calculated by multiplying total intake of zinc in the test meals by the fractional absorption of zinc.
Data analyses.
Group data sets were examined for normality and means for dietary intakes and total absorbed zinc were compared by 2-factor ANOVA and Tukey’s honest significant difference multiple comparisons test across groups with the use of GraphPad Prism version 6.00 for Windows (GraphPad Software). Data are presented as means ± SDs unless otherwise noted. All comparisons were considered significant at P < 0.05.
Results
Fifty-five children aged 17–44 mo completed the study. One provided no urine and 4 others had urine with zinc isotope ratios below the detection limit (Figure 1). Growth variables were unremarkable with weight proportional to length, the average for the latter being only mildly below WHO standards. Demographic and anthropometric data by maize flour group are given in Table 1. There were no significant differences among groups.
TABLE 1.
Characteristics of study population, by maize random assignment group1
| Maize random assignment group |
||||
| Control (n = 19) | Biofortified (n = 19) | Fortified (n = 17) | P (ANOVA) | |
| Age, mo | 30.2 ± 9.3 | 30.5 ± 6.6 | 27.7 ± 7.0 | 0.50 |
| Male | 30 | 45 | 45 | |
| Weight, kg | 11.8 ± 1.7 | 12.2 ± 1.7 | 11.6 ± 2.0 | 0.59 |
| Height, cm | 86.6 ± 6.9 | 89.4 ± 5.9 | 86.5 ± 7.4 | 0.34 |
| WAZ | −0.67 ± 1.09 | −0.57 ± 1.00 | −0.63 ± 1.06 | 0.96 |
| WAZ/HAZ | −1.16 ± 1.89 | −0.73 ± 1.45 | −1.00 ± 1.25 | 0.70 |
| WLZ/WHZ | −0.03 ± 1.12 | −0.25 ± 0.85 | −0.24 ± 1.20 | 0.78 |
| BMIZ | 0.15 ± 1.22 | −0.16 ± 0.91 | −0.03 ± 1.27 | 0.70 |
Values are means ± SDs or percentages. BMIZ, BMI-for-age z score; HAZ, height-for-age z score; WAZ, weight-for-age z score; WHZ, weight-for-height z score; WLZ, weight-for-length z score.
Grain zinc concentration was 21 μg/g in the control maize and 34 μg/g in the biofortified maize. There was no loss of zinc during roller-milling to produce the zinc flours. The zinc concentration of the fortified flour was 60 μg/g flour.
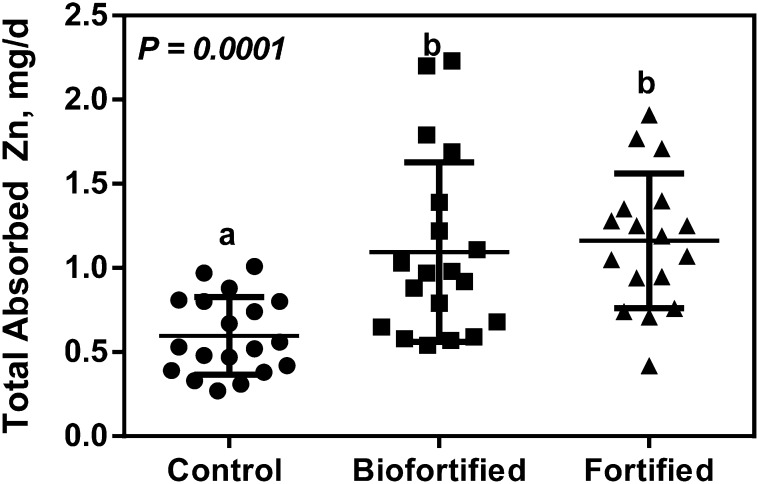
The estimated quantities of maize consumed from the 3 test meals were 118 ± 41, 130 ± 44, and 118 ± 49 g for the control, biofortified, and fortified maize groups, respectively (P = 0.62). Fractional absorption of zinc for the study day was significantly higher in the control group than in the fortified group (Table 2). The quantity of zinc absorbed from the 3 test meals was significantly greater for the biofortified group than the control group (P = 0.0001). Daily zinc absorption for the fortified group did not differ significantly from that for the biofortified group. The quantities of zinc absorbed are summarized in Table 2 with individual values depicted in Figure 2.
TABLE 2.
Absorption of zinc from test meals consumed for 1 d by study participants, by maize group1
| Maize random assignment group |
||||
| Control (n = 19) | Biofortified (n = 19) | Fortified (n = 17) | P | |
| Total dietary zinc, mg/d | 2.3 ± 0.9a | 5.0 ± 2.2b | 6.3 ± 2.6b | <0.0001 |
| Fractional absorption of zinc | 0.28 ± 0.10b | 0.22 ± 0.06a,b | 0.20 ± 0.07a | 0.015 |
| Total absorbed zinc, mg/d | 0.6 ± 0.2a | 1.1 ± 0.5b | 1.2 ± 0.4b | 0.0001 |
Values are means ± SDs. Different superscript letters within the same row denote significant group differences (ANOVA).
FIGURE 2.
Total absorbed zinc from test meals consumed by young, rural Zambian children who received control, zinc-biofortified, or zinc-fortified maize for 1 d. Lines and bars represent means ± SDs, n = 17 or 19 (zinc-fortified). Means without a common letter differ, P < 0.05.
Phytate intakes from the test meals were 848 ± 290, 1569 ± 495, and 992 ± 422 mg/d for the control, biofortified, and fortified maize groups, with the phytate intake significantly higher in the biofortified group (P < 0.0001). Because of the higher zinc concentrations in the biofortified and fortified flours, these groups both had significantly lower phytate:zinc molar ratios (34:1 and 15:1, respectively; P < 0.0001) than the control group (38:1).
Biomarker results were unremarkable with the exception of the higher mean plasma zinc concentration in the fortified group (Table 3) than in the control or biofortified groups.
TABLE 3.
Biochemical data for study participants, by maize random assignment group1
| Maize random assignment group |
||||
| Control (n = 19) | Biofortified (n = 19) | Fortified (n = 17) | P | |
| Plasma zinc (60–100),2 μg/dL | 58 ± 13a | 59 ± 13a | 74 ± 10b | 0.003 |
| Plasma α-glycoprotein (50–120),2 mg/dL | 107 ± 45 | 104 ± 51 | 97 ± 46 | 0.81 |
| EZP, mg | 56.8 ± 23.1 | 53.7 ± 14.7 | 58.1 ± 18.9 | 0.77 |
| EZP (3.0),3 mg/kg | 4.9 ± 2.7 | 4.4 ± 1.1 | 5.1 ± 1.9 | 0.56 |
Values are means ± SDs. Different superscript letters within the same row denote significant group differences (ANOVA). EZP, exchangeable zinc pool.
Normal range is denoted in parentheses.
Estimated lower cut-off value for this age group (L Miller, NF Krebs, KM Hambidge, unpublished results, 2014).
Discussion
The quantity of zinc absorbed from the biofortified maize meals on the test day was not only highly significantly greater than that from the control maize but was substantially higher than physiologic zinc requirements for this age group as estimated by the Institute of Medicine (17). This amount of absorption appears to provide a comfortable margin to account for possible excessive endogenous zinc losses in impoverished communities. The greater the contribution of the biofortified food staple to the daily diet, the greater will be the benefits of biofortification. This has been well illustrated by the results of this trial, in which maize typically provides the only food, apart from some vegetable relishes, at all 3 meals of the day for young children in poor rural Zambian communities.
This favorable result occurred despite the high phytate concentration and high dietary phytate:zinc molar ratio of the biofortified grain.
Because of uncertainty whether the zinc in the biofortified maize would be sufficient to meet the physiologic requirement, a third study arm was included in which the control maize was fortified with zinc to an amount that doubled the increase achieved with biofortification. There was very little difference between the daily quantity of zinc absorbed from the fortified and biofortified maize (Table 2). This was despite the higher zinc concentration of the zinc-fortified flour and corresponding reduction in phytate:zinc molar ratio of the zinc-fortified flour group. However, as illustrated with saturation response modeling (18, 19), the small difference in total absorbed zinc between the biofortified and fortified groups was to be expected, because the physiologic requirement had been met without fortification by the zinc-biofortified grain. Mean plasma zinc (but not exchangeable zinc pool) in the fortified group was significantly higher than that for the biofortified group. This was an unexpected finding because subjects were recruited from the same community and randomly assigned to each group; there was no prestudy stabilization on assigned maize group and no laboratory evidence of inflammation in any group. Should it have resulted from a chance and unexplained difference in zinc status, the latter also conceivably could have contributed to the small difference in total absorbed zinc between these 2 groups (the plasma zinc was significantly higher in the fortified group, but with no difference in exchangeable zinc pool). However, the weight of present evidence is that zinc intake rather than zinc status has the primary role in regulating zinc absorption (12, 15, 18).
In a recent report of maize flour fortification in 4 African countries (20), Zambians were the most likely to consume maize as a grain rather than flour. This, however, does not apply to young children for whom, in poor rural communities, maize flour is the primary food source. For those who can afford to purchase maize flour, only 20% were reported to purchase roller-milled flour. In contrast, hammer-milled maize flour is used extensively in rural areas in which there is widespread availability of hammer mills, of which there are 600 used commercially in the country. An open hammer mill, in our experience, effectively removes zinc and iron from the grain. In a pilot study, use of this procedure resulted in flour from which zinc, iron, and phytate were substantially decreased (2 and 5 μg zinc/g control and biofortified flour, respectively); analysis of the chaff from this procedure revealed very high concentrations of zinc (65 μg/g) and iron. If, however, the mill is “closed” to function as a roller mill, all of the zinc is retained in the flour. The hammer mill is popular because of the preference for white flour. Behavioral change communication will be necessary to encourage the use of whole flour, for which there are major benefits from iron (21) and other micronutrient intakes, as well as those for zinc. Indeed, major efforts at a national level are worthwhile and, if effective, would greatly enhance the value of zinc-biofortification of Zambian maize. Education should include appropriate milling procedures, specifically avoiding the use of the popular and widely distributed hammer mill in the “open” position. The form in which maize is prepared for feeding varies widely even within African countries. For example, in Kenya, one-third of the population has been reported to purchase sifted (roller-milled) flour. In comparison, in Central America and Mexico, considered the original home of maize, the process of nixtamalization does not deplete the zinc content. In view of the role of maize as one of the staple cereal grains most widely used globally, including its popularity in rural areas in which more energy per unit of land is supplied by maize than any other grain, a case can be made for including maize as a priority grain for biofortification.
In conclusion, the results of this study of zinc absorption from zinc-biofortified maize are encouraging for further human research in rural populations dependent on maize as their major food staple. This applies especially for very young children in whom the effects of zinc deficiency are most notable. The benefits of biofortification in some countries, including Zambia, will depend on behavioral change in the preparation of the flour from the zinc-biofortified grain, which in turn will require acceptance of food that is less white than is customary at this time. Such changes have been observed in Western industrialized countries over the past half-century, giving encouragement that they can also be achieved quite readily in other populations. The ready acceptance of less refined flour by young children participating in this study suggests that behavioral change is quite feasible.
Finally, we note that the concentration of zinc in the biofortified grain was substantially lower than in some other grains (10) but was still adequate to meet goals. This favorable result occurred despite a very high dietary phytate:zinc molar ratio.
Acknowledgments
We thank the following for their contributions: Kennedy Mulenga of Ngwerere Rural Health Centre; F Mwila, traditional birth attendant coordinator; Mate Nasilele and Hamunjele Ng’andu, nutritionists; Raphael Mutale from HarvestPlus and Kevin Pixley from the International Maize and Wheat Improvement Center for helping in grain selection and production; Leland Miller (University of Colorado) for providing unpublished data regarding estimated lower cutoff value for exchangeable zinc pool; Jennifer Kemp and Lei Sian (University of Colorado) for laboratory support in sample analyses; Claire Willing (University of Colorado) for technical support in preparing for the studies; and Andrée and Martin Schouten for their gracious hospitality in Lusaka. EC, JEW, NFK, and KMH designed the research protocol; EC and KMH presented to the International Atomic Energy Agency as a component of the Coordinated Research Project “Food fortification and biofortification to improve micronutrient status during early life." CMW was primarily responsible for field studies after preparatory visits with EC and EMM; NP provided the high-kernel zinc maize for the project and details about the maize in the manuscript; ZWP provided sample analyses; JEW analyzed the data and performed the statistical analysis; EC, CMW, and KMH wrote the paper; and KMH had primary responsibility for the final content. All authors read and approved the final manuscript.
References
- 1.Nuss ET, Tanumihardjo SA. Maize: A paramount staple crop in the context of global nutrition. Compr Rev Food Sci Food Saf 2010;9:417–36. [DOI] [PubMed] [Google Scholar]
- 2.Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab 2013;62: Suppl 1:19–29. [DOI] [PubMed] [Google Scholar]
- 3.Banziger M, Long J. The potential for increasing the iron and zinc density of maize through plant-breeding. Food Nutr Bull 2000;21:397–400. [Google Scholar]
- 4.Maziya-Dixon B, Kling JG, Menkir A, Dixon A. Genetic variation in total carotene, iron, and zinc contents of maize and cassava genotypes. Food Nutr Bull 2000;21:419–22. [Google Scholar]
- 5.Ortiz-Monasterio JI, Palacios-Rojas N, Meng E, Pixley K, Trethowan R, Pena RJ. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. J Cereal Sci 2007;46:293–307. [Google Scholar]
- 6.Saltzman A, Birol E, Bouis HE, Boy E, De Moura FF, Islam Y, Pfeiffer WH. Biofortification: Progress toward a more nourishing future. Global Food Security 2013;2:9–17. [Google Scholar]
- 7.FAO. GIEWS Country Briefs: Zambia [Internet]. [cited 2014 Sep 9]. Available from: http://www.fao.org/giews/countrybrief/country.jsp?code=ZMB.
- 8.Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012;7:e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanjra MA, Culas RJ. The political economy of maize production and poverty reduction in Zambia: analysis of the last 50 years. J Asian Afr Stud 2011;46:546–66. [DOI] [PubMed] [Google Scholar]
- 10.Kodkany BS, Bellad RM, Mahantshetti NS, Westcott JE, Krebs NF, Kemp JF, Hambidge KM. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J Nutr 2013;143:1489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friel JK, Naake VL, Jr, Miller LV, Fennessey PV, Hambidge KM. The analysis of stable isotopes in urine to determine the fractional absorption of zinc. Am J Clin Nutr 1992;55:473–7. [DOI] [PubMed] [Google Scholar]
- 12. Krebs NF, Hambidge KM: Zinc metabolism and homeostasis: The application of tracer techniques to human zinc physiology. Biometals 2001, 2001:397–412. [DOI] [PubMed]
- 13.Krebs NF, Westcott JE, Culbertson DL, Sian L, Miller LV, Hambidge KM. Comparison of complementary feeding strategies to meet zinc requirements of older breastfed infants. Am J Clin Nutr 2012;96:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosado JL, Hambidge KM, Miller LV, Garcia OP, Westcott J, Gonzalez K, Conde J, Hotz C, Pfeiffer W, Ortiz-Monasterio I, et al. The quantity of zinc absorbed from wheat in adult women is enhanced by biofortification. J Nutr 2009;139:1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung CS, Stookey J, Dare D, Welch R, Nguyen TQ, Roehl R, Peerson JM, King JC, Brown KH. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am J Clin Nutr 2008;87:1224–9. [DOI] [PubMed] [Google Scholar]
- 16.Krebs N, Miller LV, Naake VL, Lei S, Westcott JE, Fennessey PV, Hambidge KM. The use of stable isotope techniques to assess zinc metabolism. J Nutr Biochem 1995;6:292–307. [Google Scholar]
- 17.Food and Nutrition Board Institute of Medicine: Dietary Reference Intakes for Vitamin A, Vitamin K, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Washington (DC): National Academy Press; 2001. [PubMed]
- 18.Miller LV, Krebs NF, Hambidge KM. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J Nutr 2007;137:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller LV, Krebs NF, Hambidge KM. Mathematical model of zinc absorption: effects of dietary calcium, protein and iron on zinc absorption. Br J Nutr 2013;109:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiedler JL, Afidra R, Mugambi G, Tehinse J, Kabaghe G, Zulu R, Lividini K, Smitz MF, Jallier V, Guyondet C, et al. Maize flour fortification in Africa: markets, feasibility, coverage, and costs. Ann N Y Acad Sci 2014;1312:26–39. [DOI] [PubMed] [Google Scholar]
- 21.Moretti D, Biebinger R, Bruins MJ, Hoeft B, Kraemer K. Bioavailability of iron, zinc, folic acid, and vitamin A from fortified maize. Ann N Y Acad Sci 2014;1312:54–65. [DOI] [PubMed] [Google Scholar]