Abstract
There has been continued interest in clinical objective measures of binaural processing. One commonly proposed measure is the binaural interaction component (BIC), which is obtained typically by recording auditory brainstem responses (ABRs)—the BIC reflects the difference between the binaural ABR and the sum of the monaural ABRs (i.e., binaural − (left + right)). We have recently developed an alternative, direct measure of sensitivity to interaural time differences, namely, a following response to modulations in interaural phase difference (the interaural phase modulation following response; IPM-FR). To obtain this measure, an ongoing diotically amplitude-modulated signal is presented, and the interaural phase difference of the carrier is switched periodically at minima in the modulation cycle. Such periodic modulations to interaural phase difference can evoke a steady state following response. BIC and IPM-FR measurements were compared from 10 normal-hearing subjects using a 16-channel electroencephalographic system. Both ABRs and IPM-FRs were observed most clearly from similar electrode locations—differential recordings taken from electrodes near the ear (e.g., mastoid) in reference to a vertex electrode (Cz). Although all subjects displayed clear ABRs, the BIC was not reliably observed. In contrast, the IPM-FR typically elicited a robust and significant response. In addition, the IPM-FR measure required a considerably shorter recording session. As the IPM-FR magnitude varied with interaural phase difference modulation depth, it could potentially serve as a correlate of perceptual salience. Overall, the IPM-FR appears a more suitable clinical measure than the BIC.
Keywords: electroencephalography, binaural processing, interaural time differences, auditory brainstem responses, auditory steady-state responses
Introduction
Binaural hearing confers considerable advantages in everyday listening environments. Specifically, differences in the timing and intensity of a sound at the two ears—interaural time and interaural level differences (ITDs and ILDs, respectively)—provide reliable cues as to the location of sound sources. Sensitivity to ITDs offers additional advantages in cocktail party conditions, enabling listeners to follow a conversation against a background of interfering noise from other talkers or from room reflections (Bronkhorst, 2000). Sensitivity to ITDs conveyed in the temporal fine structure (TFS) of sounds is limited to frequencies below about 1.4 kHz; above this frequency, phase locking in the auditory nerve apparently declines sharply, but sound wavelength decreases sufficiently to interact with the head to create useful localization cues based on ILD (Hartmann & Macaulay, 2014; Mills, 1958; see Stecker & Gallun, 2012 for a review). However, since many real-world sounds are modulated in amplitude, additional ITD cues conveyed in the modulated envelopes of high-frequency sounds also provide a potentially useful localization cue (Bernstein & Trahiotis, 1985; Henning, 1974). Given the benefits conferred by binaural hearing, particularly sensitivity to low-frequency ITDs, impairment to ITD processing is likely to be detrimental to auditory perception. ITD sensitivity declines with ageing (Abel, Giguere, Consoli, & Papsin, 2000; Babkoff et al., 2002; Herman, Warren, & Wagener, 1977; King, Hopkins, & Plack, 2014)—consistent with the notion that temporal processing might be impaired in older listeners (Gallun et al., 2014; Moore, Glasberg, Stoev, Füllgrabe, & Hopkins, 2012)—and in listeners with hearing loss, albeit with variability (Moore, Hutchings, & Meyer, 1991; Noble, Byrne, & Lepage, 1994; Smoski & Trahiotis, 1986). Such impairments in ITD processing, which are not necessarily predicted by the perceived sensation level of the stimulus, are interpreted as arising from some as-yet-undefined impairment in the processing of TFS information following cochlear damage (Lacher-Fougère & Demany, 2005).
A desire to understand how hearing impairment results in (or from) a loss of sensitivity to information conveyed in the TFS of sounds has led to an interest in developing clinically viable, objective measures of binaural processing. One commonly proposed electroencephalographic measure is the binaural interaction component (BIC). The analysis of the BIC is most commonly based on Wave V of the auditory brainstem response (ABR), since earlier response components do not show binaural interaction (e.g., Debruyne, 1984). The response of binaurally innervated neurons to binaural stimulation is not necessarily twice that of monaural stimulation. Hence, the summed magnitude of ABRs to sequential monaural stimulation of either ear alone can be larger than the magnitude of the ABR to simultaneous binaural presentation of the same stimulus (i.e., left-ear monaural ABR + right-ear monaural ABR > binaural ABR; Dobie & Berlin, 1979; Dobie & Norton, 1980). As such, the BIC can be attributed to simple binaural convergence at the level of the auditory brainstem but is not necessarily evidence of neural sensitivity to ITD (Ungan & Yagcioglu, 2002). Another, more practical, limitation of this measure is that the BIC derived from ABRs reflects only a small and subtle difference between ABR waveforms and can be difficult to detect against background noise in recordings, especially in neonates and children (Cone-Wesson, Ma, & Fowler, 1997; Stollman, Snik, Hombergen, Nieuwenhuys, & Koppel, 1996). The BIC calculated from middle- or late-latency responses, however, is usually somewhat larger (McPherson & Starr, 1993). Direct comparisons between studies are confounded partially by differing stimuli and recording techniques, but many authors have reported successfully observing the ABR BIC despite the relatively small magnitude of the component. For example, Ito, Hoke, Pantev, and Lütkenhöner (1988) and Levine (1981) reported clear BIC responses in over 90% of subjects, and Fowler and Leonards (1985) and Fowler and Broadard (1988) reported successfully recording a BIC in all subjects tested (see also Van Yper, Vermeire, De Vel, Battmer, & Dhooge, 2015). Several authors have reported high mean BIC amplitudes but without reporting individual variability; for example, Fowler and Horn (2012) reported a 0.30 μV BIC, Dobie and Norton (1980) reported a BIC amplitude of approximately 0.40 μV, and McPherson and Starr (1995) reported an exceptionally large 0.85 to 0.92 μV BIC. Nevertheless, despite previous studies reporting apparently robust BIC recordings, many studies did not provide an estimate of signal-to-noise ratio (SNR), and so a complete interpretation of findings is difficult.
A number of studies have failed to demonstrate a consistent or reliable influence of ITDs on the BIC, although it is generally accepted that BIC amplitude decreases, and latency increases, as the ITD is increased. Furst, Levine, and McGaffigan (1985) proposed that the BIC of the ABR is a physiological correlate of binaural fusion, with the BIC being relatively constant in amplitude for ITDs of up to 1 ms, but no BIC was observed for ITDs greater than 1.2 ms. Jones and Van der Poel (1990) calculated the BIC of middle-latency responses and demonstrated that the latency of the BIC was delayed by approximately half the magnitude of the ITD compared with the latency of the BIC latency observed for diotic presentation. Again, BIC amplitude varied little across the range of ITDs tested (0–800 μs; see also Brantberg, Hansson, Fransson, & Rosenhall, 1999). In contrast, McPherson and Starr (1995) reported that the magnitude of the ABR-derived BIC decreased progressively with increasing ITD or ILD, and that the BIC remained detectable for ITDs up to 1600 μs ITD. Riedel and Kollmeier (2002) reported that the ABR BIC decreased significantly for an ITD of 400 μs in comparison to a diotic (zero ITD) stimulus. However, such findings might be accounted for simply by the reduced overlap of the excitation from both ears arriving at binaurally sensitive neurons as the tone clicks are separated in time by the ITD.
More direct objective measures of ITD processing than the BIC can be evoked with salient changes to an ongoing stimulus and have been demonstrated with auditory-evoked P1-N1-P2 responses, which are attributed to multiple generator sites in the thalamus and auditory cortex (Hari, Aittoniemi, Järvinen, Katila, & Varpula, 1980; Liegeois-Chauvel, Musolino, Badier, Marquis, & Chauvel, 1994; Näätänen & Picton, 1987; Picton, Hillyard, Krausz, & Galambos, 1974). In particular, abrupt changes in either ITD (Halliday & Callaway, 1978; McEvoy, Picton, & Champagne, 1991) or interaural correlation (Chait, Poeppel, de Cheveigné, & Simon, 2005; Dajani & Picton, 2006) of a broadband noise can evoke P1-N1-P2 responses. Recording magnetoencephalographic responses from normal-hearing listeners, Ross, Tremblay, and Picton (2007b) demonstrated that an abrupt change in the interaural phase difference (IPD) at a minimum of an on-going amplitude-modulated low-frequency tone elicited P1-N1-P2 responses (see also Ross, Fujioka, Tremblay, & Picton, 2007a; Ross, 2008). In this study, an equal, diotic, rate of sinusoidal amplitude modulation (AM) was applied to both ears. The carrier was abruptly changed in IPD at an energy minimum in the modulation cycle, so as to minimize otherwise salient monaural phase-shift cues. Significant responses to the IPD transition were observed for carrier frequencies of 500 Hz and 1000 Hz but not for 1500 Hz. This was broadly consistent with subjects’ behavioral thresholds for detecting IPD changes (∼1200 Hz) and the upper frequency limit of IPD sensitivity (Garner & Wertheimer, 1951; Schiano, Trahiotis, & Bernstein, 1986; Zwislocki & Feldman, 1956). Steady-state responses, such as those elicited by AM, are thought to reflect the superposition of transient middle-latency responses (e.g., Galambos, Makeig, and Talmachoff, 1981). McAlpine, Haywood, Undurraga, and Marquardt (in press) demonstrated a steady-state response to periodic interaural phase modulations (IPMs) in an ongoing AM tone, in which IPM was applied periodically at minima in a 41-Hz diotic AM cycle. The abrupt IPD transitions occurred at a rate of 6.8 Hz or every six AM cycles (see also Dajani & Picton 2006 for a related study employing periodic changes in the interaural correlation of noise). In contrast to Ross et al. (2007b), the IPD switched between left- and right-ear leading, causing anti-phasic modulation in the activations of the left and right brain hemispheres, and a periodically changing stimulus lateralization percept. The periodic IPM evoked a steady-state response, termed the IPM following response, or IPM-FR. The magnitude of the IPM-FR varied with the depth of the IPM and was maximal in the ±90° IPM condition; corresponding to an IPM depth of 180°, the largest IPD transition is possible. Indeed, smaller or larger IPMs (e.g., ±67.5°, ±112.5°) elicited smaller magnitude responses. Furthermore, McAlpine et al. (in press) also provided psychophysical evidence that an IPM of ±90° (i.e., a 180° transition) was more perceptually salient than changes between smaller or larger IPDs, as expected (e.g., Garner & Wertheimer, 1951). This correlation between evoked responses and behavioral performance supports the notion that the IPM-FR can provide a meaningful index of IPD sensitivity.
The robust responses to IPD changes observed by McAlpine et al. (in press) suggest that the IPM-FR could be a suitable clinical measure of ITD sensitivity. Here, we compare the clinical utility of the IPM-FR and the BIC, in terms of the reliability of detecting binaural-evoked neural responses. The BIC was calculated in response to diotic stimuli only, using stimulus and recording parameters similar to those used by Riedel and Kollmeier (2002). As BIC amplitude is thought to either be unaffected or even marginally reduced by imposition of ITDs within the physiological range, the effect of ITD on the BIC was not investigated. A second aim of this study was to assess the best positioning of a single-channel electrode montage for IPM-FR recordings. To this end, responses were recorded from 16 electrode channels, and their magnitudes were compared. We observed that subjects typically showed a robust IPM-FR, whereas the BIC was not reliably observed. Additionally, a single IPM-FR was observed within a considerably shorter recording time than the BIC (5 min vs. 30 min). The IPM-FR provided a measure of ITD sensitivity that could be related directly to perceptual saliency—as larger IPMs evoked larger magnitude IPM-FRs. From these findings, it is concluded that the IPM-FR is a measure of binaural processing with greater overall clinical utility than the BIC for acoustically hearing subjects.
Method
Subjects
Ten normal-hearing subjects took part in the experiment (five female, mean age = 23 years; range 19–30 years). None reported any known hearing difficulties, and all demonstrated hearing thresholds of 20 dB HL or better for pure tones presented between 250 and 8000 Hz. The experiment lasted for approximately one and a half hours and was completed in a single session. The experiment was approved by the University College London ethics committee. All subjects provided their informed consent before beginning the experiment and were paid an honorarium for their time.
Stimuli: BIC
Rarefaction click stimuli were 100 µs rectangular voltage pulses presented at 95 dB peSPL. The interval between successive stimuli was in the range 55 to 65 ms, as the duration of the interstimuli interval was jittered randomly between ±5 ms from a base of 60 ms. Subjects were presented with an equal number of left-ear monaural, right-ear monaural, and binaural stimuli in a continuous sequence, and the presentation order of stimuli was randomized within the sequence (10,440 of each arrangement, 31,320 stimuli in total). The recording session lasted for approximately 30 min. Stimulus parameters were adapted from Riedel and Kollmeier (2002).
Stimuli: IPM-FR
All stimuli comprised a 520-Hz carrier tone with full sinusoidal AM at a rate of 41 Hz. The stimulus was presented continuously for 4 min and 48 s, at 80 dB SPL. Only the carrier was presented with an IPD, as the modulation envelope remained diotic at all times. Although the magnitude of the carrier IPD was held constant throughout the stimulus, its sign was periodically modulated between leading in the right and the left ear to create IPM. The sign inversion generating the IPM was applied instantaneously at a minimum in the modulation cycle. IPM occurred at a rate of 6.8 Hz (corresponded to an IPD transition every six AM cycles)—a value that indicates the total number of IPD transitions per second, irrespective of the direction of the transition (see Figure 1). IPM rate was described in this manner because the direction of the IPD transition was not predicted to affect the characteristics of the neural response—any directional effects would also be difficult to interpret due to the anticipated superpositioning of responses to successive IPD transitions.
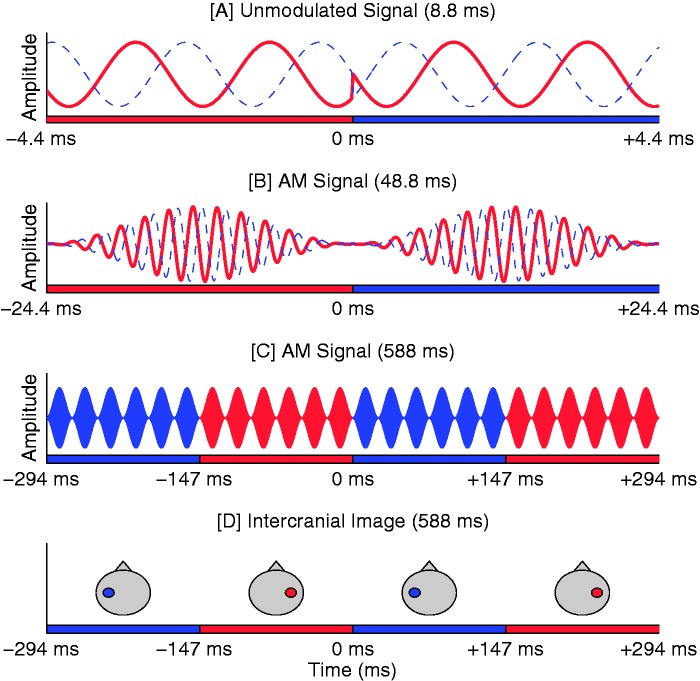
Figure 1.
Schematic illustration of the IPM-FR stimuli. Note that differing time scales are used in plots a, b, c, andd. (a) Red and blue traces correspond to the stimuli presented to the right and left ear, respectively. Here, an on-going +90° IPD is imposed on an unmodulated carrier. At 0 s, an IPD transition to −90° is imposed: The stimulus changes from right-ear leading to left-ear leading. (b) A 41-Hz AM is imposed on the carrier. The IPD transition occurs at a minima in the modulation cycle, reducing the perceptual salience of the monaural phase shifts. (c) Additional modulation cycles of the same stimulus are displayed. Here, the carrier is not illustrated; instead, red and blue shaded modulation cycles correspond to cycles in which the carrier IPD is leading in the right ear or left ear, respectively. Note that IPMs occurs every six modulation cycles, at a rate corresponding to 6.8 Hz. (d) Illustrative intercranial image locations associated with the stimulus shown in (c; if the IPM frequency would be <2 Hz).
Five different depths of IPM were assessed. These different conditions are referred to by the magnitude of the ongoing IPD and not by overall change in IPD at each IPD transition. To this end, for example, the descriptor of ±45° represents a condition in which carrier IPD was modulated between leading in the right ear by +45° and then leading in the left ear by −45°. Accordingly, the IPD traversed 90° at every IPD transition. Each IPD transition was created by concurrently alternating carrier phase at each ear. This means that, in the ±45° condition, the phase of the signal in the right ear would alternate between +22.5° and −22.5°, while that in the left ear would simultaneously alternate between −22.5° and +22.5°. In total, five different IPM conditions were tested; ±22.5°, ±45°, ±90°, ±112.5°, and ±135° (corresponding, respectively, to ITDs of 120, 240, 481, 601, and 721 μs for the 520-Hz carrier).
To verify that the evoked responses were not elicited by monaural phase cues, two diotic control conditions were assessed. In these conditions, both ears shared the same carrier phase at all times, but diotic phase transitions were imposed at a rate of 6.8 Hz. Phase changes of ±22.5° and ±56.25° were tested, corresponding to the size of the phase changes in the monaural signals of the ±45° and ±112.5° IPM conditions, respectively. Similar diotic control conditions were employed in the study by McAlpine et al. (in press), and the diotic phase changes did not evoke responses (see also Ross et al., 2007a, 2007b). However, the presentation level in the current experiment was higher than the 65 dB SPL used by McAlpine et al. (in press), and so these two diotic control conditions were included to ensure no monaural effects existed at 80 dB SPL.
Procedure
Subjects first completed the BIC recording session and then completed the IPM-FR session. Subjects were offered a short break between these two sessions. The BIC recording session comprised a single recording run of approximately 30 min, whereas the IPM-FR recording session comprised seven 5-min records (five IPM conditions and two diotic controls). Here, subjects were presented with a single repetition of each condition, in a random order, during a single testing session. During all recordings, subjects sat in a comfortable chair in an acoustically isolated sound booth, were encouraged to sit as still as possible, and watched a subtitled film.
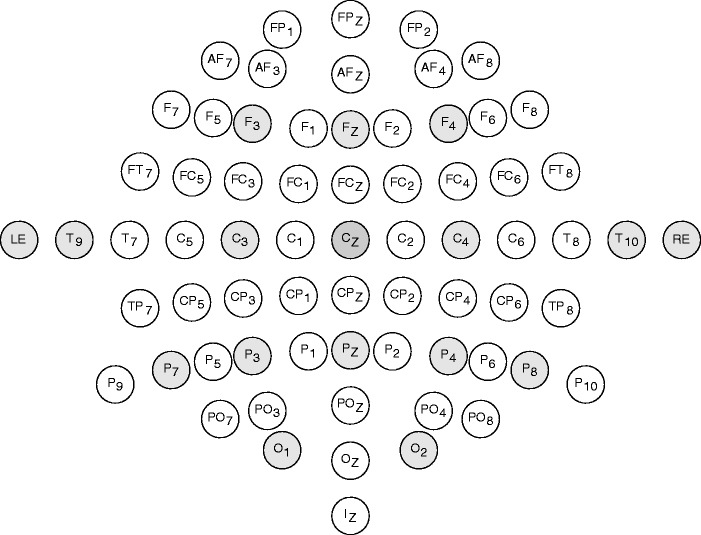
Responses were differentially recorded primarily from silver or silver chloride disc surface electrodes; the reference electrode was placed on the vertex (Cz) and the ground electrode was placed on the right clavicle. Fourteen recording electrodes were placed around the scalp (F3, Fz, F4, C3, C4, T9, T10, P7, P3, Pz, P4, P8, O1, and O2—see Figure 2). Two additional electrodes were placed in the left and right ear canal (gold-coated foam insert ear tips—Etymotic ER3–26A; Etymotic Research Inc., IL, USA). Electrode impedances were kept below 5 kΩ and monitored throughout the recording session. Stimuli were created in MATLAB 2013b and presented via an RME Fireface UC sound card (24 bits, 48 kHz sampling rate; RME, Haimhausen, Germany) connected to Etymotic Research ER-2 insert earphones (Etymotic Research Inc., IL, USA). Sound level was verified with a 2-cc ear simulator (Brüel & Kjaer 4157, Sound and Vibration Measurement A/S, Nærum, Denmark). Responses were amplified with a 20 × gain (RA-16LI; Tucker-Davis Technologies, FL, USA) and digitized with 16 bits per sample at a rate of 24.414 kHz (Medusa RA16PA; Tucker-Davis Technologies). The cutoff frequencies of the internal bandpass filters were 2.2 Hz to 7.5 kHz (6 dB per octave). Recordings were stored on a TDT RX5 Pentusa before being passed to the hard disk of the host computer via custom software. Subsequent off-line analysis was conducted in MATLAB 2013b.
Figure 2.
Schematic of the electrode positions used in the experiment. Electrode Cz served as a reference. Recording electrodes are shaded gray. RE and LE correspond to electrodes placed in the right and left ear canal (gold-coated foam inserts).
Data Analysis: BIC
ABR responses were first assessed for individual subjects and for each electrode channel separately. The entire ABR recording was divided into time windows (epochs) starting at stimulus onset and ending 10 ms after stimulus offset. Responses were then grouped according to whether they were evoked by left ear, right ear, or binaural stimulation. Responses to each of the three presentation types were averaged using a weighted averaging procedure (Don & Elberling, 1994; Elberling & Don, 1984; Elberling & Wahlgreen, 1985). For all conditions, the average Wave V amplitude—defined as the difference between the positive peak and the following trough—was identified by the experimenter. Earlier response components were not considered during analysis. The SNR was calculated using the method proposed by Don and Elberling (1994).
The BIC was computed from the established subtraction method—namely, by subtracting the binaural response waveform from the sum of the left-ear and right-ear monaural response waveforms (i.e., binaural − [left + right]). This was performed on the averaged response waveforms from each subject, and each recording channel individually. The BIC amplitudes were identified by the experimenter at time windows corresponding broadly to Wave V of the ABR response, defined as the difference between the positive peak and the following negative trough. For the purpose of SNR estimation, it was assumed that the residual noise in the three ABR recordings was uncorrelated, and so the estimate of residual noise in the BIC was calculated by summing the power of the variance from the three individual ABR recordings.
Data Analysis: IPM-FR
The IPM-FR analysis was conducted exclusively in the frequency domain. Each measurement was split into 75 epochs of 4.096 s. Epochs were transformed to the frequency domain (fast Fourier transform, 100,000 points, 0.24 Hz resolution). For individual responses, the frequency bins corresponding to the 6.8 Hz IPM rate and the 41 Hz AM rate were analyzed for significance using a two-dimensional, repeated measures Hotelling’s T2 test (Picton, John, Dimitrijevic, & Purcell, 2003).
Results
ABR Results
Mean Wave V amplitudes and SNRs for all 10 subjects are shown in Table 1 for each of the 16 electrode channels. It is apparent that frontal and central electrodes did not reliably record ABR responses. Accordingly, a one-way ANOVA—conducted on a subset of the binaural dataset for which electrodes in frontal (F3, Fz, and F4) and central (C3 and C4) positions were excluded from the analysis, and for which the dependent variable was the peak-to-peak amplitude—confirmed a significant effect of electrode position, F(10,90) = 32.13, p < .001. Bonferroni-corrected pairwise comparisons revealed that there were no significant differences between temporal, occipital, and in-ear electrodes. However, response amplitudes from centrally located parietal electrodes P3 and Pz were significantly lower than from temporal, occipital, and in-ear positions (p < .05 in all cases). Further analyses of electrode position were not pursued, as it is well established that a vertex-mastoid montage provides optimal signal representation for single-channel scalp-based positions (e.g., Picton et al., 1974). Finally, an analysis of the residual noise conducted on the entire dataset confirmed a significant effect of electrode position, F(15,135) = 9.20, p < .001, but not of stimulus type, monaural: left, right, or binaural: F(2,18) = 0.04, p = .96, and the interaction was also nonsignificant, F(30,270) = 1.27, p = .16. This indicates that the residual noise did not vary with stimulus condition.
Table 1.
Mean ABR Wave V Data From All 16 Electrode Positions.
| Right-monaural ABRs |
Left-monaural ABRs |
Binaural ABRs |
BIC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemisphere | Electrode | Position | Mean amplitude (nV) | Mean SNR (dB) | Number of responses ≥3.01 dB SNR | Mean amplitude (nV) | Mean SNR (dB) | Number of responses ≥3.01 dB SNR | Mean amplitude (nV) | Mean SNR (dB) | Number of responses ≥3.01 dB SNR | Mean amplitude (nV) | Mean SNR (dB) | Number of responses ≥3.01 dB SNR |
| Left | F3 | Frontal | 72.4 | −2.5 | 0 | 78.9 | −1.7 | 1 | 113.0 | 1.7 | 3 | 0.06 | −7.06 | 0 |
| C3 | Central | 63.7 | −1.7 | 2 | 70.2 | 0.0 | 0 | 75.1 | 2.6 | 2 | 0.07 | −5.09 | 0 | |
| P3 | Parietal (Medial) | 69.1 | 2.7 | 5 | 135.4 | 5.2 | 6 | 173.5 | 9.0 | 10 | 0.09 | −3.67 | 0 | |
| P7 | Parietal (Lateral) | 108.3 | 6.4 | 9 | 206.6 | 8.3 | 10 | 296.3 | 11.9 | 10 | 0.11 | −7.20 | 0 | |
| O1 | Occipital | 196.4 | 8.1 | 10 | 259.9 | 8.1 | 9 | 441.7 | 14.0 | 10 | 0.13 | −4.32 | 0 | |
| T9 | Mastoid | 194.8 | 7.7 | 9 | 278.0 | 9.5 | 10 | 433.4 | 13.5 | 10 | 0.16 | −4.08 | 0 | |
| Left ear | Ear canal | 182.8 | 8.3 | 9 | 270.9 | 11.1 | 10 | 443.6 | 15.0 | 10 | 0.13 | −6.37 | 0 | |
| Right | F4 | Frontal | 86.8 | 0.1 | 2 | 76.5 | −2.8 | 0 | 119.3 | 2.3 | 3 | 0.03 | −8.12 | 0 |
| C4 | Central | 97.1 | 1.4 | 3 | 52.3 | −5.4 | 0 | 97.3 | 1.6 | 4 | 0.05 | −16.22 | 0 | |
| P4 | Parietal (Medial) | 152.2 | 6.5 | 9 | 113.9 | 3.3 | 6 | 239.3 | 11.1 | 10 | 0.08 | −7.68 | 0 | |
| P8 | Parietal (Lateral) | 233.2 | 9.6 | 10 | 157.1 | 5.9 | 8 | 350.7 | 13.5 | 10 | 0.10 | −6.77 | 0 | |
| O2 | Occipital | 229.6 | 8.9 | 10 | 222.0 | 7.1 | 8 | 433.0 | 14.5 | 10 | 0.13 | −4.52 | 0 | |
| T10 | Mastoid | 316.1 | 11.6 | 10 | 254.4 | 8.8 | 9 | 516.9 | 15.7 | 10 | 0.12 | −6.54 | 0 | |
| Right ear | Ear canal | 287.3 | 12.5 | 10 | 212.1 | 8.8 | 9 | 471.5 | 16.3 | 10 | 0.10 | −10.83 | 0 | |
| Central | Fz | Frontal | 53.9 | −3.7 | 1 | 49.2 | −4.7 | 0 | 79.7 | 1.2 | 2 | 0.05 | −9.57 | 0 |
| Pz | Parietal | 72.5 | 1.4 | 1 | 83.9 | −0.3 | 4 | 129.6 | 7.0 | 10 | 0.06 | −9.77 | 0 | |
Note. Data are averaged across all 10 subjects, and separately displayed for right-ear monaural, left-ear monaural, and binaural presentations. Data corresponding to the BIC are also presented. Mean peak-to-peak amplitude, SNR (dB), and the number of significant responses (out of the 10 subjects) with an SNR of ≥3.01 dB (Don et al., 1984). Both significant and nonsignificant responses contribute to the mean values presented. ABR = auditory brainstem responses; BIC = binaural interaction component; SNR = signal-to-noise ratio.
A second ANOVA compared responses to binaural presentation from temporal-parietal (T9 and T10) and in-ear electrodes only. The two-way ANOVA with two factors (position vs. hemisphere) indicated no significant main effect of hemisphere, left = 0.44 μV, right = 0.49 μV: F(1,9) = 4.45, p = .064), or of position, in-ear = 0.46 μV, scalp = 0.47 μV: F(1,9) = 2.45, p = .15. A similar analysis of the SNR revealed it to be significantly higher for the in-ear position (15.63 dB) than for the scalp positions (14.56 dB), F(1,9) = 10.75, p = .009, and for the right hemisphere (15.98 dB) compared with the left hemisphere (14.21 dB), F(1,9) = 8.03, p = .019. The interaction between factors was nonsignificant, F(1,9) = 1.29, p > .284. Overall, this suggests that in-ear electrodes may provide a modest improvement in the SNR of ABR recordings in comparison to proximate scalp-placed electrodes. This is consistent with previous findings (Atcherson, Lim, Moore, & Minaya, 2012).
BIC Results
The BIC analysis focused exclusively on electrode channels T9, T10, right ear, and left ear, as these channels typically recorded the best ABR signals. A two-way ANOVA (position and hemisphere) assessed the BIC amplitude. The analysis revealed a significant main effect of hemisphere, left = 0.14 μV, right = 0.11 μV: F(1,9) = 6.55, p = .031, but not of position, in-ear = 0.11 μV, scalp = 0.14 μV: F(1,9) = 4.41, p = .065. A second ANOVA assessed how the SNR was affected by these factors. The interaction between factors was not significant, F(1,9) = 0.232, p = .641. This analysis again revealed a significant effect of hemisphere, right =−8.60 dB, left =−5.23 dB: F(1,9) = 7.73, p = .021, but not of position, in-ear = −8.60 dB, scalp = −5.13 dB: F(1,9) = 1.81, p = .082. The interaction between factors was not significant, F(1,9) = 1.37, p = 271. When considering the SNR of the BIC, the residual noise estimate was calculated from the sum of the power of the variance from the three individual ABR recordings. This established procedure arguably yields a relatively conservative estimate of SNR.
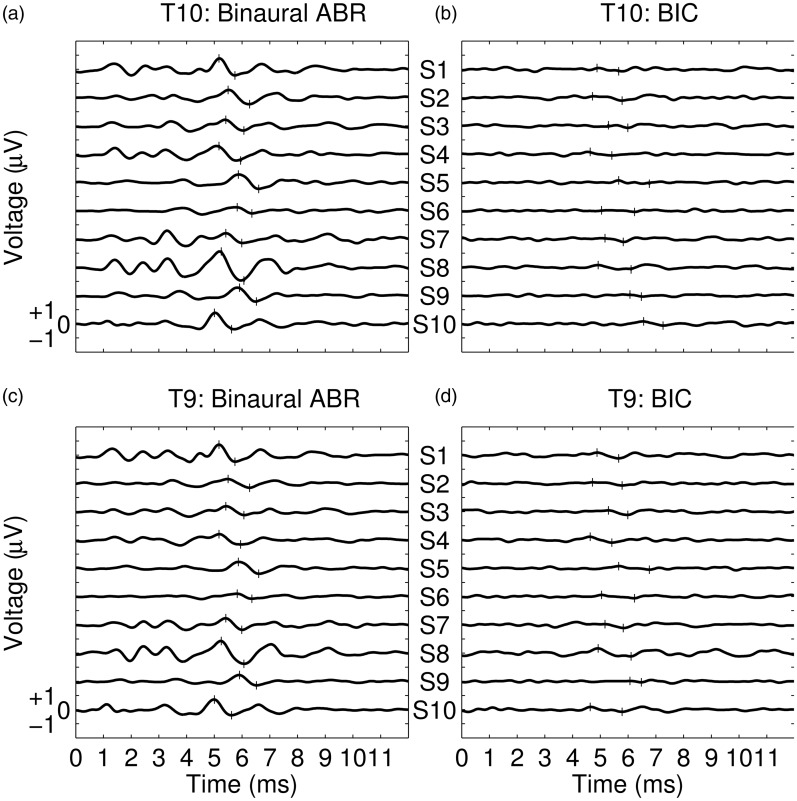
Given the lack of consistent differences between responses recorded at in-ear and scalp positions, we next assessed activity recorded at electrode T9 only—the electrode providing the highest mean amplitude (0.16 μV) and the best SNR (−4.08 dB). Individual values of SNR for this electrode are shown in Table 2, and individual BIC responses are shown in Figure 3(b); corresponding binaural ABR responses are shown in Figure 3(a). From Table 2 and Figure 3, it is apparent that the BIC was not reliably recorded from all subjects—indeed, in the majority of cases, it was ambiguous as to whether the waveform interpreted as being the BIC was, in actual fact, simply variability in the noise.
Table 2.
BIC amplitude and SNR Values for Each of the 10 Subjects (S1, S2, . . .) for Mastoid and In-Ear Electrodes Locations.
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | Mean | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BIC amplitude | |||||||||||
| T10 | 0.12 | 0.15 | 0.09 | 0.13 | 0.12 | 0.06 | 0.13 | 0.15 | 0.08 | 0.17 | 0.12 |
| T9 | 0.21 | 0.11 | 0.16 | 0.21 | 0.13 | 0.12 | 0.14 | 0.28 | 0.05 | 0.17 | 0.16 |
| Right ear | 0.10 | 0.16 | 0.09 | 0.09 | 0.11 | 0.07 | 0.10 | 0.14 | 0.09 | 0.07 | 0.10 |
| Left ear | 0.18 | 0.14 | 0.10 | 0.10 | 0.14 | 0.10 | 0.16 | 0.18 | 0.06 | 0.14 | 0.13 |
| BIC SNR | |||||||||||
| T10 | −5.93 | 1.64 | −11.13 | −3.91 | −11.55 | −12.29 | −7.79 | −0.40 | −8.02 | −6.03 | −6.54 |
| T9 | −0.56 | −0.94 | 0.67 | 0.27 | −13.71 | −3.50 | −13.11 | −0.54 | −6.93 | −2.46 | −4.08 |
| Right ear | −6.88 | −5.04 | −8.66 | −31.93 | −12.50 | −14.21 | −5.46 | −6.24 | −11.30 | −6.03 | −10.83 |
| Left ear | −0.97 | −2.27 | −−8.43 | −20.27 | −2.40 | −5.74 | −4.81 | −3.40 | −8.38 | −7.06 | −6.37 |
Note. Each subject’s highest amplitude or SNR value is indicated in italics. BIC = binaural interaction component; SNR = signal-to-noise ratio.
Figure 3.
(a) Individual binaural ABR responses recorded from electrode T10. Each trace corresponds to the average response from a single subject (as indicated by the labels S1–S10). Narrow vertical lines indicate the values in the waveform that were used by the experimenter to estimate Wave V amplitude. (b) Individual BIC responses recorded from electrode T9. Each trace corresponds to the BIC from a single subject; presented in the same subject order as used in (a). Narrow vertical lines indicate the values in the waveform to estimate BIC amplitude. (c and d) As for 3(a) and 3(b), respectively, except the plots represent responses observed from electrode T10.
IPM-FR Results
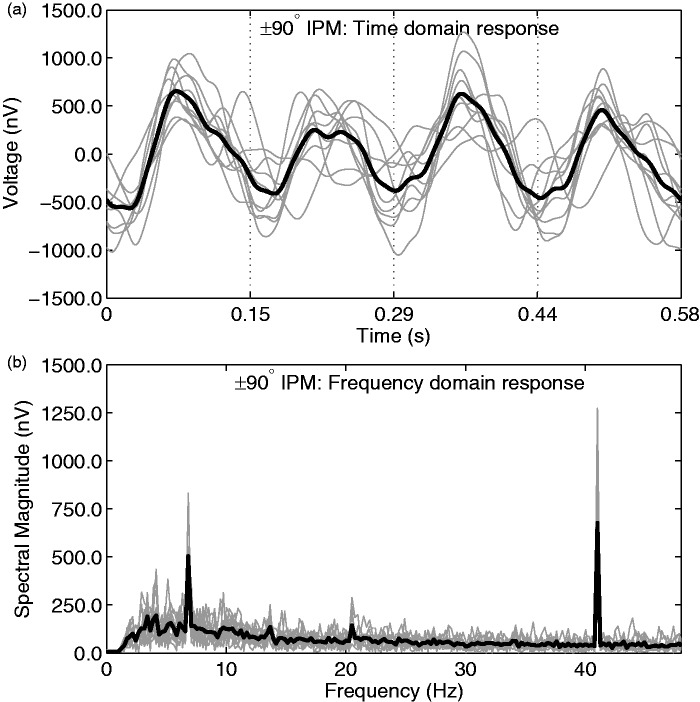
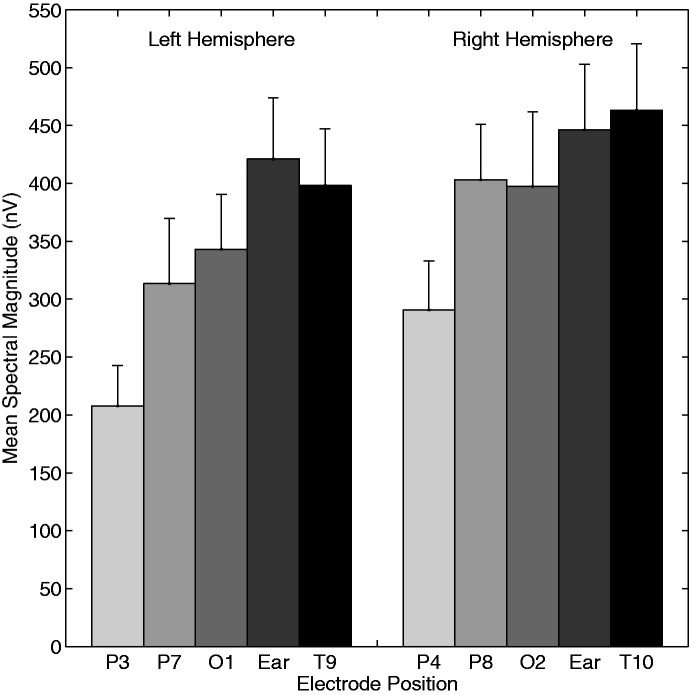
Responses were averaged across subjects to provide a mean response for each IPM condition. Illustrative group-averaged responses for the ±90° IPM condition, as recorded from electrode T9, are shown in Figure 4. Note that here, and in other sections of the IPM-FR analysis, electrode T9 was chosen despite other right-sided electrodes typically generating larger magnitude responses (see subsequent analyses). The choice of electrode T9 was so IPM-FR characteristics could be compared with the best-observed BIC—as this electrode recorded the largest mean amplitude and SNR for BIC responses. From Figure 4, a clear IPM-FR is evident, corresponding to the IPM rate (6.8 Hz), as well as a modulation-evoked auditory steady-state response (ASSR) at 41 Hz. In the first stage of analysis, data from the 16 electrode locations were averaged across all 10 subjects and the 5 IPM conditions. Mean spectral magnitude at 41 Hz and 6.8 Hz, SNR, and the number of significant responses from these recordings are summarized in Table 3. Note that because the data in Table 3 are averaged across all IPM conditions (±22.5° to ±135°), the systematic variation in IPM-FR magnitude with IPM depth is considered later in this section. Nevertheless, from Table 3, it is apparent that the IPM-FRs recorded from frontal and central electrodes (F3, Fz, F4, C3, and C4) had relatively low magnitudes and SNRs. As such, these electrodes were excluded from all further analysis. Group-averaged response amplitudes were compared by means of a three-way repeated measures ANOVA. Diotic control conditions were excluded from this analysis. We assessed the dependence of the IPM-FR on the electrode hemisphere (left vs. right), electrode position, and IPM condition. Electrode pZ was excluded from this analysis, owing to its mid-line position. We observed a main effect of electrode position, F(4,36) = 31.23, p < .001, and of IPM, F(4,36) = 4.11, p = .007. There was also a significant effect of hemisphere, with right-sided electrodes recording larger responses, F(1,9) = 26.93, p < .001. No interactions were significant (p > .05 in all cases). Concerning electrode position, Bonferroni-corrected post-hoc analysis indicated both mastoid (T9 and T10) and in-ear electrodes to generate significantly stronger responses than all parietal electrodes (P3, P4, P7, and P8; p < .05 in all comparisons). No other comparisons were significantly different from each other (p > .05 in all comparisons). Mean response magnitudes for different electrode positions are displayed in Figure 5.
Figure 4.
(a) Averaged responses from the ±90° IPM condition, as recorded from electrode T9. Time domain responses were filtered between 2 Hz and 20 Hz. The grand averaged response is highlighted with a thick black line, whereas individual responses are shown in gray. Dashed vertical lines indicate the time at which IPD transitions occurred. Note that for illustrative clarity, data in this panel are presented after being averaged into a 0.585 s epoch—for the main analysis and the spectrum below (b), a longer epoch was used (4.096 s). (b) Averaged responses in the frequency domain. The grand averaged response is highlighted with a thick black line, whereas individual responses are shown in gray. Data in this panel were not low-pass filtered. Note the clear peaks in the response at 6.8 Hz and 41 Hz, corresponding to the IPM rate and the amplitude modulation rate, respectively. As such, both forms of modulation are interpreted to have evoked a neural following response.
Table 3.
Mean IPM-FR Data for the 16 Electrode Positions.
| ASSR (41 Hz) |
IPM-FR (6.8 Hz) |
Diotic (6.8 Hz) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemisphere | Electrode | Position | Mean spectral magnitude (nV) | Mean SNR (dB) | Percent significant (Hotelling's T2) | Mean spectral magnitude (nV) | Mean SNR (dB) | Percent significant (Hotelling's T2) | Mean spectral magnitude (nV) | Mean SNR (dB) | Percent significant (Hotelling's T2) |
| Left | F3 | Frontal | 169.8 | 9.4 | 84.4 | 168.1 | −1.8 | 18 | 127.5 | −4.3 | 0 |
| C3 | Central | 75.7 | 10.0 | 91.1 | 88.0 | −2.7 | 6 | 62.5 | −2.3 | 0 | |
| P3 | Parietal (Medial) | 378.8 | 21.5 | 100 | 207.6 | 1.7 | 42 | 114.3 | −1.5 | 0 | |
| P7 | Parietal (Lateral) | 515.9 | 20.0 | 100 | 313.5 | 2.8 | 50 | 133.4 | −2.6 | 10 | |
| O1 | Occipital | 693.0 | 22.0 | 100 | 343.1 | 3.9 | 58 | 163.1 | −1.9 | 15 | |
| T9 | Mastoid | 667.6 | 21.2 | 100 | 398.2 | 6.1 | 80 | 144.2 | −2.1 | 10 | |
| Left ear | Ear canal | 652.1 | 22.0 | 100 | 421.0 | 6.1 | 78 | 154.1 | −1.8 | 0 | |
| Right | F4 | Frontal | 145.1 | 8.6 | 82.2 | 150.5 | −1.2 | 24 | 123.8 | −2.0 | 15 |
| C4 | Central | 106.7 | 13.7 | 100 | 107.1 | 1.3 | 38 | 54.2 | −4.7 | 5 | |
| P4 | Parietal (Medial) | 532.8 | 23.8 | 100 | 290.6 | 3.7 | 66 | 105.4 | −1.3 | 15 | |
| P8 | Parietal (Lateral) | 663.9 | 23.8 | 100 | 403.2 | 7.0 | 86 | 128.2 | −3.3 | 5 | |
| O2 | Occipital | 693.9 | 22.3 | 100 | 397.4 | −1.0 | 22 | 255.8 | −2.7 | 10 | |
| T10 | Mastoid | 792.3 | 24.9 | 100 | 463.2 | 7.9 | 84 | 145.4 | −0.9 | 10 | |
| Right ear | Ear canal | 728.6 | 24.3 | 100 | 446.3 | 7.1 | 80 | 141.9 | −1.8 | 10 | |
| Central | Fz | Frontal | 122.7 | 11.6 | 84.4 | 110.8 | −2.5 | 8 | 92.2 | −2.0 | 5 |
| Pz | Parietal | 365.6 | 23.9 | 100 | 184.3 | 1.6 | 52 | 78.2 | −1.3 | 10 | |
Note. Data are averaged across all 10 subjects. Mean spectral magnitude, SNR, and the percentage of significant responses (Hotelling’s T2 test) are displayed. Column (A) reflects the 41 Hz ASSR evoked from the amplitude modulation envelope; data from the five IPM conditions plus the two control conditions are averaged. Column (B) reflects the 6.8 Hz IPM-FR evoked from the periodic IPMs; data from the five (Dichotic) IPM conditions are averaged. Column (C) reflects the average 6.8 Hz evoked response observed from the two diotic control conditions. Concerning the group-averaged data, the mean SNR values indicate that the spectral magnitude at this frequency bin (6.8 Hz) was undistinguishable from the noise floor. ASSR = auditory steady-state response; IPM-FR = interaural phase modulation following response; SNR = signal-to-noise ratio.
Figure 5.
The spectral magnitude of the IPM-FR as observed from different electrode locations. Data are averaged across subjects and all IPM-FR conditions. Note that response magnitudes were comparatively larger in the right hemisphere in the left hemisphere. Largest magnitude responses were observed from the In-Ear and Mastoid positioned electrodes. Error bars represent +1 standard error. Frontally and centrally located electrodes failed to detect a reliable IPM-FR (Table 3) and so are not represented in this figure.
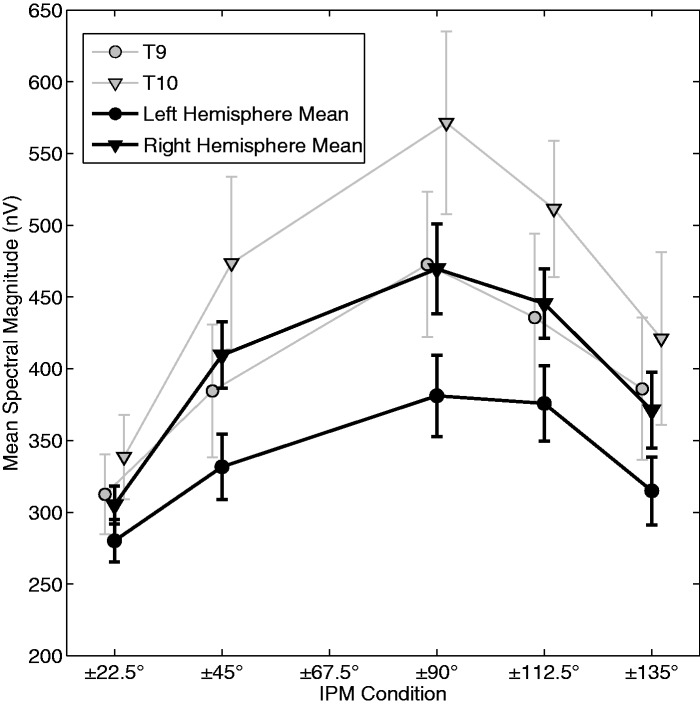
We next assessed the influence of the depth of IPM on the magnitude of the IPM-FR (Figure 6). In general, a broad tuning function was observed, with the±90° IPM condition evoking the largest IPM-FR. Bonferroni-corrected post-hoc analysis revealed that responses in the ±90° condition were significantly larger than either the ±22.5° or ±135° conditions (p < .05 in both cases), but not from either the ±45° or ±112.5° conditions (p > .05 in both cases). Both the ±45° and ±112.5° conditions evoked significantly larger responses than the ±22.5° condition (p < .05). No other differences were significant (p > .05 in all cases). To demonstrate that IPM-FR observed under optimal recording parameters is robust, Table 4 illustrates responses from the ±90° IPM condition as recorded from electrode T9. Nine of the 10 subjects displayed clear and significant IPM-FR responses. Only subject S2 showed a nonsignificant IPM-FR response and a poor SNR. Nonetheless, this subject did display a clear 41-Hz ASSR (Table 4, bottom panels), and so it is possible that a single earphone may have been partially blocked. This would have reduced interaural cues, but would have had less effect on the AM-evoked ASSR, which can also be evoked monaurally.
Figure 6.
The effect of IPM depth on the mean spectral magnitude of the IPM-FR. The gray traces indicate mean response magnitudes as observed from electrodes T9 (circles) and T10 (downward triangles). The black traces indicate mean data as averaged from all good electrodes sites. The left hemisphere dataset (circles) indicate the mean from electrodes P3, P7, O1, T9, and the left in-ear electrode. The right hemisphere dataset (downward triangles) indicate the mean from electrodes P4, P8, O2, T10, and the right in-ear electrode. Error bars indicate ±1 standard error.
Table 4.
IPM-FR and ASSR Response Characteristics for Each of the 10 Subjects (S1, S2, . . .) From Electrode Position T9 Only.
| Subject |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | |
| IPM-FR (6.8 Hz) | ||||||||||
| Spectral magnitude (nV) | 517.2 | 297.2 | 542.7 | 463.9 | 832.6 | 296.4 | 524.3 | 481.4 | 470.4 | 300.1 |
| SNR (dB) | 8.1 | −0.1 | 13.1 | 8.4 | 15.3 | 6.8 | 11.6 | 12.7 | 7.8 | 5.4 |
| p value (Hotelling's T2) | <0.005 | >0.05 | <0.0001 | <0.001 | <0.0001 | <0.005 | <0.0001 | <0.0001 | <0.005 | <0.05 |
| ASSR (41 Hz) | ||||||||||
| Spectral magnitude (nV) | 511.7 | 231.8 | 544.8 | 608.7 | 462.2 | 284.7 | 1169.7 | 974.8 | 953.2 | 419.5 |
| SNR (dB) | 18.4 | 20.5 | 20.0 | 23.4 | 16.6 | 16.3 | 23.7 | 26.8 | 25.4 | 21.1 |
| p value (Hotelling's T2) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Note. Data reflect the ±90° IPM condition only. ASSR = auditory steady-state response; IPM-FR = interaural phase modulation following response; SNR = signal-to-noise ratio.
Diotic controls were tested for the ±45° and ±112.5° conditions. In contrast to the IPM conditions, diotic responses at all recording electrodes had a negative SNR (Table 3). A two-way ANOVA concerning electrode T9 only investigated how response magnitude was affected by both phase transition sizes in the stimuli (45° and 112.5°, corresponding to ±45° and ±112.5° IPM conditions) and control (diotic) versus IPM (dichotic) presentation. This analysis did not find a significant main effect of IPM, F(1,9) = 2.96, p = .0631, but, most importantly, did reveal a significant effect interaural phase configuration (i.e., diotic vs. IPM), F(1,9) = 20.19, p = .001. The interaction was not significant. Overall, the data indicate that the IPM-FR evoked a substantially larger response than comparative diotic phase shifts (see also Table 3). This is evidence that the IPM-FR reflects primarily binaural processing.
Of less importance for the research question, but nevertheless interesting, is the analysis of the ASSR to the 41-Hz AM. Subjects typically displayed a strong ASSR alongside the IPM-FR (e.g., see Table 4). A three-way ANOVA was conducted on the hemisphere-separated dataset (i.e., F3, Fz, F4, C3, C4, and Pz excluded) and confirmed a significant effect of electrode position, F(4,36) = 16.62, p < .001, but not of IPM, F(4,36) = 1.99, p = .117. There was a significant main effect of hemisphere, F(1,9) = 20.14, p = .003, due to responses being larger for right-sided electrodes. The interaction between hemisphere and position was also significant, F(4,36) = 4.77, p = .03. The lack of an effect of IPM on ASSR magnitude was not due to the inclusion of poor-performing electrodes, as a one-way ANOVA, conducted for responses from electrode T9 only, indicated no significant effect of IPM condition on ASSR magnitude, F(4,36) = 0.33, p = .867.
Discussion
We recorded BIC and IPM-FR responses in the same group of normal-hearing subjects, with the primary aim of comparing the reliability of both measures. In general, the BIC proved difficult to detect, being of low amplitude and, in most cases, showed a negative SNR—even when recorded from electrode montages considered optimal for ABRs (King & Sininger, 1992; Sininger & Don, 1989). As such, it was difficult to interpret whether peaks in the residual waveform truly reflected a BIC. This was despite the fact that subjects’ ABR responses were reliably observed. In line with our experience reported here, several reports have concluded that the low amplitudes and high intersubject variability of the BIC limit its potential usefulness as a clinical measure of binaural function (Stollman et al., 1996; Van Yper et al., 2015; Wilson, Kelly-Ballweber, & Dobie, 1985). A primary interest of the current research was to develop an objective measure of ITD processing, not just of binaural convergence. Given that BIC amplitude is either not influenced or is reduced when an ITD is imposed (e.g., Brantberg et al., 1999; Riedel & Kollmeier, 2002), it would likely have been challenging to record BIC responses to stimuli with ITD in the current experiment. Nevertheless, any successful replication of previous findings concerning the effect of ITD on the BIC could potentially allow for further comparison between the two measures tested here by correlating, for example, how individual response properties change with increasing ITD or IPD. However, on the basis of the current data, clear challenges exist when measuring the BIC. Moreover, these challenges limit the clinical utility of the BIC for measuring binaural processing in normal-hearing listeners and, likely, in hearing-impaired listeners also. The measure may, however, have greater merit for assessing bilateral cochlear implant users, a consideration discussed later in this section.
The second measure tested in the current experiment, the IPM-FR, showed considerable advantages over the BIC. In the optimum ±90° IPM condition, as recorded at electrode T9, the mean response spectral magnitude was 0.47 μV, and 9 out of the 10 subjects displayed SNRs in excess of 9 dB (Table 4). A single subject failed to display an IPM-FR response. Direct comparisons between BIC and IPM-FR measures are made difficult due to the different nature of the two responses. Instead, one can consider the number of reliably observed responses for each measure. Electrode T9, on average, recorded the best BIC SNR. Even so, none of the 10 subjects displayed a significant BIC at this recording site (compare values in Table 2 with the criterion SNR of ≥3.01 dB, as suggested by Don & Waring, 1984). In contrast, for the ±90° IPM condition, 9 of the 10 subjects displayed a significant IPM-FR (as evaluated with a Hotelling’s T2 test suggested by Picton et al., 2003; see Table 4). A McNemar’s test conducted on these results confirmed that significantly more significant responses were observed with the IPM-FR measure than with the BIC (McNemar’s chi-squared = 7.11, df = 1, p < .01). On the basis of signal detection, the current results provide strong evidence that the IPM-FR is a more efficient measure than the BIC. The IPM-FR confers additional practical benefits over the BIC, particularly in terms of time efficiency—a single IPM-FR recording lasts around 5 min. Further investigations could assess whether even this short recording duration could be reduced further while preserving the reliably detectable response. In contrast, a single BIC measurement required 30 min in the current experiment, and a reduction in recording time seems impossible given the already poor SNR of the BIC response. A second benefit is that the IPM stimulus also captures an ASSR response to the modulation envelope that can serve as a monitor of the recording quality. In populations with impaired ITD processing, the relative strengths of the ASSR, which reflects neural sensitivity to the temporal envelope, and the IPM-FR, which reflects sensitivity to the TFS, may be insightful, and further study could compare the ratio of ASSR and IPM-FR magnitudes in subjects with normal and psychoacoustically assessed impairment in binaural processing.
The robust nature of the IPM-FR is consistent with the results of McAlpine et al. (in press). As for both McAlpine et al. (in press) and Ross et al. (2007a, 2007b), diotic phase-shifted stimuli did not evoke following responses, and so the IPM-FR is interpreted as an indication of dedicated ITD processing. Thus, unlike the BIC, the IPM-FR provides a direct measure of ITD processing, as the magnitude of the IPM-FR varied meaningfully with the magnitude of the IPM depth. In the current experiment, we observed a broad tuning in the magnitude of the IPM-FR to the ±90° condition. This condition corresponded to an IPM of 180°, which is the largest IPD difference possible, giving the cyclic nature of phase. Testing similar conditions, the study by McAlpine et al. (in press) also demonstrated tuning to IPM depth, and in addition showed a correlation between IPM-FR magnitude and IPM detection in a psychophysical task (an adaptive masking-based procedure). Such findings extend upon previous studies by Ross et al. (2007a, 2007b), as these authors tested only an IPD transition from diotic to anti-phasic, an arrangement which would unlikely cause variation in the hemispheric balance of brain activation. The current stimuli were modulated between symmetrical left- and right-leading IPDs, and so would likely modulate the activation of left and right brain hemispheres, as well as the stimulus lateralization percept.
There is considerable interest in developing an objective measurement of binaural processing in bilateral cochlear-implant users. An electrically evoked BIC has been observed from bilateral cochlear implant users, which is characterized by a shorter latency and larger amplitude than the acoustically derived BIC associated with normal hearing (Gordon, Valero, & Papsin, 2007; He, Brown, & Abbas, 2010; Pelizzone, Kasper, & Montandon, 1990). This likely reflects in part differing morphologies associated with acoustically and electrically evoked ABRs. Reduced evoked ABR latency is attributed to a reduced synaptic delay, resulting from the absence of a cochlear travelling wave and the synaptic excitation of afferent neurons (Van den Honert & Stypulkowski, 1986). Increased response amplitudes likely reflect increased synchronicity in peripheral responses to electrical stimulation (e.g., Shepherd & Javel, 1997). Evidence exists to suggests that the BIC might be a useful tool for matching implant electrodes across the ears (interaurally)—with stimulation to tonotopically matched electrode pairs evoking a larger amplitude BIC (He et al., 2010; Hu & Dietz, 2015). The electrically evoked BIC has also been observed in children, and those with a longer duration of unilateral CI use prior to bilateral implantation showed increased BIC latency (Gordon et al., 2007). Concerning the IPM-FR, it may be possible to translate the stimulus paradigm to electrical stimulation and so assess sound-source localization abilities objectively in subjects with bilateral cochlear implants. Such a measure could also be used for matching interaural electrode pairs. However, it remains to be established whether rapid changes in ongoing ITD would be salient to bilateral CI users.
One aim of the current study was to confirm the optimum electrode positions for single-channel IPM-FR recordings. For both ABR and modulation-evoked ASSR recordings, the data are consistent with the clinical convention that vertex-mastoid differential recording are optimal (e.g., see also Picton et al., 1974 for ABR; Tlumak, Rubinstein, & Durrant, 2007 for an ASSR review). Although IPM-FR responses have not previously been investigated, we also found that the electrode montages that best recorded the modulation ASSR (i.e., temporal and parietal positions) were also those that best recorded the IPM-FR component. Further research would be required to identify the exact generator site for the IPM-FR. It is plausible that the IPM-FR reflects a superpositioning of successive P1-N1-P2 responses. This is because Dajani and Picton (2006) demonstrated that periodic modulations to the correlation of interaural noise evoked P1-N1-P2 responses at slow modulation rates (<3 Hz), but at faster modulation rates (e.g., >6 Hz), the evoked response to this modulation became steady state in nature. If this finding extends to the IPM-FR paradigm used currently, P1-N1-P2 generators are thought to be located in lateral aspects of Heschl’s gyrus and the temporal plane (Eggermont & Ponton, 2002; Pantev et al., 1995). In contrast, the steady-state responses are thought to originate more medially in primary auditory cortex (Mäkelä & Hari, 1987; Pantev, Roberts, Elbert, Ross, & Wienbruch, 1996). As such, if the IPM-FR does indeed reflect a superpositioning of P1-N1-P2 responses occurring at a relatively slow rate (i.e., 6.8 Hz), it is conceivable that generators at either or both of these sites exist. Further study would be required to establish the exact nature of the IPM-FR and the associated generator sites. In practical terms, however, responses from primary auditory cortex will likely share the same optimum positioning of scalp-based electrodes, and so it is unsurprising that both the ASSR and the IPM-FR share similar optimum positions. Both IPM-FR and ASSR responses were asymmetrical, as larger responses were observed in electrodes placed on the right hemisphere. This is consistent with the notion that the right hemisphere may be more selective to spatial information (Palomäki, Tiitinen, Mäkinen, May, & Alku, 2005; Salminen, Tiitinen, Yrttiaho, & May, 2010; Tiitinen et al., 2006). Previous studies have also demonstrated ASSRs to be most strongly represented in the right hemisphere (Poelmans, Luts, Vandermosten, Ghesquière, & Wouters, 2012; Ross, Herdman, & Pantev, 2005). Despite this, the IPM-FR was also well observed from left-sided electrodes, and indeed, detailed single-channel IPM-FR analysis was conducted primarily from electrode T9. This electrode was chosen as it observed the best BIC response—albeit, even for this electrode the BIC was not reliably observed (see Table 2). Despite this criterion, the IPM-FR was observed clearly from this recording site. Finally, we demonstrated that in-ear electrodes offer little advantage to mastoid-placed surface electrodes.
In summary, our comparison of the magnitude and reliability of the IPM-FR compared with the BIC suggests that the IPM-FR could be a more clinically viable measure of ITD sensitivity in terms of signal detection. Further study could investigate the IPM-FR in clinical populations such as the elderly or the hearing impaired. For example, Ross et al. (2007a) demonstrated that both the N1-P2 response magnitude and psychophysical detection thresholds for a change in IPD both deteriorated as carrier frequency was increased. Using both measures, these authors demonstrated the upper frequency limit of ITD sensitivity decreased with aging. Moreover, the ITD-change-evoked response latency increased with ageing—but the evoked response to sound onset was consistent across groups. One might expect the IPM-FR to similarly be able to characterize reduced ITD sensitivity in elderly and potentially hearing impaired populations.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under ABCIT grant agreement number 304912.
References
- Abel S. M., Giguere C., Consoli A., Papsin B. C. (2000) The effect of aging on horizontal plane sound localization. The Journal of the Acoustical Society of America 108(2): 743–752. [DOI] [PubMed] [Google Scholar]
- Atcherson S. R., Lim T. J., Moore P. C., Minaya C. P. (2012) Comparison of auditory brainstem response peak measures using ear lobe, mastoid, and custom ear canal reference electrodes. Audiology Research 2(1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babkoff H., Muchnik C., Ben-David N., Furst M., Even-Zohar S., Hildesheimer M. (2002) Mapping lateralization of click trains in younger and older populations. Hearing Research 165(1): 117–127. [DOI] [PubMed] [Google Scholar]
- Bernstein L. R., Trahiotis C. (1985) Lateralization of sinusoidally amplitude-modulated tones: Effects of spectral locus and temporal variation. The Journal of the Acoustical Society of America 78(2): 514–523. [DOI] [PubMed] [Google Scholar]
- Brantberg K., Hansson H., Fransson P. A., Rosenhall U. (1999) The binaural interaction component in human ABR is stable within the 0-to 1-ms range of interaural time differences. Audiology and Neurotology 4(2): 88–94. [DOI] [PubMed] [Google Scholar]
- Bronkhorst A. W. (2000) The cocktail party phenomenon: A review of research on speech intelligibility in multiple-talker conditions. Acta Acustica United With Acustica 86(1): 117–128. [Google Scholar]
- Chait M., Poeppel D., de Cheveigné A., Simon J. Z. (2005) Human auditory cortical processing of changes in interaural correlation. The Journal of Neuroscience 25(37): 8518–8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone-Wesson B., Ma E., Fowler C. G. (1997) Effect of stimulus level and frequency on ABR and MLR binaural interaction in human neonates. Hearing Research 106(1–2): 163–178. [DOI] [PubMed] [Google Scholar]
- Debruyne F. (1984) Binaural interaction in early, middle and late auditory evoked responses. Scandinavian Audiology 13(4): 293–296. [DOI] [PubMed] [Google Scholar]
- Dajani H. R., Picton T. W. (2006) Human auditory steady-state responses to changes in interaural correlation. Hearing Research 219(1): 85–100. [DOI] [PubMed] [Google Scholar]
- Dobie R. A., Berlin C. I. (1979) Binaural interaction in brainstem-evoked responses. Archives of Otolaryngology 105(7): 391–398. [DOI] [PubMed] [Google Scholar]
- Dobie R., Norton S. J. (1980) Binaural interaction in human auditory evoked potentials. Electroencephalography and Clinical Neurophysiology 49(3): 303–313. [DOI] [PubMed] [Google Scholar]
- Don M., Elberling C. (1994) Evaluating residual background noise in human auditory brain-stem responses. The Journal of the Acoustical Society of America 96(5): 2746–2757. [DOI] [PubMed] [Google Scholar]
- Don M., Elberling C., Waring M. (1984) Objective detection of averaged auditory brainstem responses. Scandinavian Audiology 13(4): 219–228. [DOI] [PubMed] [Google Scholar]
- Eggermont J. J., Ponton C. W. (2002) The neurophysiology of auditory perception: From single units to evoked potentials. Audiology and Neurotology 7(2): 71–99. [DOI] [PubMed] [Google Scholar]
- Elberling C., Don M. (1984) Quality estimation of averaged auditory brainstem responses. Scandinavian Audiology 13(3): 187–197. [DOI] [PubMed] [Google Scholar]
- Elberling C., Wahlgreen O. (1985) Estimation of auditory brainstem response, ABR, by means of Bayesian inference. Scandinavian Audiology 14(2): 89–96. [DOI] [PubMed] [Google Scholar]
- Fowler C. G., Broadard R. S. (1988) Low-frequency activity in the binaural interaction: Component of the auditory brain stem response. Ear and Hearing 9(2): 65–69. [DOI] [PubMed] [Google Scholar]
- Fowler C. G., Horn J. H. (2012) Frequency dependence of binaural interaction in the auditory brainstem and middle latency responses. American Journal of Audiology 21(2): 190–198. [DOI] [PubMed] [Google Scholar]
- Fowler C. G., Leonards J. S. (1985) Frequency dependence of the binaural interaction component of the auditory brainstem response. International Journal of Audiology 24(6): 420–429. [DOI] [PubMed] [Google Scholar]
- Furst M., Levine R. A., McGaffigan P. M. (1985) Click lateralization is related to the β component of the dichotic brainstem auditory evoked potentials of human subjects. The Journal of the Acoustical Society of America 78(5): 1644–1651. [DOI] [PubMed] [Google Scholar]
- Galambos R., Makeig S., Talmachoff P. J. (1981) A 40-Hz auditory potential recorded from the human scalp. Proceedings of the National Academy of Sciences 78(4): 2643–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallun F. J., McMillan G. P., Molis M. R., Kampel S. D., Dann S. M., Konrad-Martin D. L. (2014) Relating age and hearing loss to monaural, bilateral, and binaural temporal sensitivity. Frontiers in Neuroscience 8: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner W. R., Wertheimer M. (1951) Some effects of interaural phase differences on the perception of pure tones. The Journal of the Acoustical Society of America 23(6): 664–667. [Google Scholar]
- Gordon K. A., Valero J., Papsin B. C. (2007) Auditory brainstem activity in children with 9–30 months of bilateral cochlear implant use. Hearing Research 233(1): 97–107. [DOI] [PubMed] [Google Scholar]
- Halliday R., Callaway E. (1978) Time shift evoked potentials (TSEPs): Method and basic results. Electroencephalography and Clinical Neurophysiology 45(1): 118–121. [DOI] [PubMed] [Google Scholar]
- Hari R., Aittoniemi K., Järvinen M. L., Katila T., Varpula T. (1980) Auditory evoked transient and sustained magnetic fields of the human brain localization of neural generators. Experimental Brain Research 40(2): 237–240. [DOI] [PubMed] [Google Scholar]
- Hartmann W. M., Macaulay E. J. (2014) Anatomical limits on interaural time differences: An ecological perspective. Frontiers in Neuroscience 8: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Brown C. J., Abbas P. J. (2010) Effects of stimulation level and electrode pairing on the binaural interaction component of the electrically evoked auditory brain stem response. Ear and Hearing 31(4): 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning G. B. (1974) Detectability of interaural delay in high-frequency complex waveforms. The Journal of the Acoustical Society of America 55(1): 84–90. [DOI] [PubMed] [Google Scholar]
- Herman G. E., Warren L. R., Wagener J. W. (1977) Auditory lateralization: Age differences in sensitivity to dichotic time and amplitude cues. Journal of Gerontology 32(2): 187–191. [Google Scholar]
- Hu H., Dietz M. (2015) Comparison of interaural electrode pairing methods for bilateral cochlear implants. Trends in Hearing 19: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Hoke M., Pantev C., Lütkenhöner B. (1988) Binaural interaction in brainstem auditory evoked potentials elicited by frequency-specific stimuli. Hearing Research 35(1): 9–19. [DOI] [PubMed] [Google Scholar]
- Jones S. J., Van der Poel J. C. (1990) Binaural interaction in the brain-stem auditory evoked potential: Evidence for a delay line coincidence detection mechanism. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section 77(3): 214–224. [DOI] [PubMed] [Google Scholar]
- King A., Hopkins K., Plack C. J. (2014) The effects of age and hearing loss on interaural phase difference discrimination. The Journal of the Acoustical Society of America 135(1): 342–351. [DOI] [PubMed] [Google Scholar]
- King A. J., Sininger Y. S. (1992) Electrode configuration for auditory brainstem response audiometry. American Journal of Audiology 1(2): 63–67. [DOI] [PubMed] [Google Scholar]
- Lacher-Fougère S., Demany L. (2005) Consequences of cochlear damage for the detection of interaural phase differences. The Journal of the Acoustical Society of America 118(4): 2519–2526. [DOI] [PubMed] [Google Scholar]
- Levine R. A. (1981) Binaural interaction in brainstem potentials of human subjects. Annals of Neurology 9(4): 384–393. [DOI] [PubMed] [Google Scholar]
- Liegeois-Chauvel C., Musolino A., Badier J. M., Marquis P., Chauvel P. (1994) Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section 92(3): 204–214. [DOI] [PubMed] [Google Scholar]
- Mäkelä J. P., Hari R. (1987) Evidence for cortical origin of the 40 Hz auditory evoked response in man. Electroencephalography and Clinical Neurophysiology 66(6): 539–546. [DOI] [PubMed] [Google Scholar]
- McAlpine, D., Haywood, N. R., Undurraga, J. A., & Marquardt, T. (in press). Objective measures of neural processing of interaural time differences. In van Dijk, P., Baskent, D., Gaudrain, E., de Kleine, E., Wagner, A., Lanting, C. (Eds.), Physiology, Psychoacoustics and Cognition in Normal and Impaired Hearing. New York, NY: Springer International Publishing.
- McEvoy L. K., Picton T. W., Champagne S. C. (1991) The timing of the processes underlying lateralization: Psychophysical and evoked potential measures. Ear and Hearing 12(6): 389–398. [DOI] [PubMed] [Google Scholar]
- McPherson D. L., Starr A. (1993) Binaural interaction in auditory evoked potentials: Brainstem, middle-and long-latency components. Hearing Research 66(1): 91–98. [DOI] [PubMed] [Google Scholar]
- McPherson D. L., Starr A. (1995) Auditory time-intensity cues in the binaural interaction component of the auditory evoked potentials. Hearing Research 89(1): 162–171. [DOI] [PubMed] [Google Scholar]
- Mills A. W. (1958) On the minimum audible angle. The Journal of the Acoustical Society of America 30(4): 237–246. [Google Scholar]
- Moore B. C., Glasberg B. R., Stoev M., Füllgrabe C., Hopkins K. (2012) The influence of age and high-frequency hearing loss on sensitivity to temporal fine structure at low frequencies (L). The Journal of the Acoustical Society of America 131(2): 1003–1006. [DOI] [PubMed] [Google Scholar]
- Moore D. R., Hutchings M. E., Meyer S. E. (1991) Binaural masking level differences in children with a history of otitis media. International Journal of Audiology 30(2): 91–101. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Picton T. (1987) The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology 24(4): 375–425. [DOI] [PubMed] [Google Scholar]
- Noble W., Byrne D., Lepage B. (1994) Effects on sound localization of configuration and type of hearing impairment. The Journal of the Acoustical Society of America 95(2): 992–1005. [DOI] [PubMed] [Google Scholar]
- Palomäki K. J., Tiitinen H., Mäkinen V., May P. J., Alku P. (2005) Spatial processing in human auditory cortex: The effects of 3D, ITD, and ILD stimulation techniques. Cognitive Brain Research 24(3): 364–379. [DOI] [PubMed] [Google Scholar]
- Pantev C., Bertrand O., Eulitz C., Verkindt C., Hampson S., Schuierer G., Elbert T. (1995) Specific tonotopic organizations of different areas of the human auditory cortex revealed by simultaneous magnetic and electric recordings. Electroencephalography and Clinical Neurophysiology 94(1): 26–40. [DOI] [PubMed] [Google Scholar]
- Pantev C., Roberts L. E., Elbert T., Ross B., Wienbruch C. (1996) Tonotopic organization of the sources of human auditory steady-state responses. Hearing Research 101(1): 62–74. [DOI] [PubMed] [Google Scholar]
- Pelizzone M., Kasper A., Montandon P. (1990) Binaural interaction in a cochlear implant patient. Hearing Research 48(3): 287–290. [DOI] [PubMed] [Google Scholar]
- Picton T. W., Hillyard S. A., Krausz H. I., Galambos R. (1974) Human auditory evoked potentials. I: Evaluation of components. Electroencephalography and Clinical Neurophysiology 36: 179–190. [DOI] [PubMed] [Google Scholar]
- Picton T. W., John M. S., Dimitrijevic A., Purcell D. (2003) Human auditory steady-state responses: Respuestas auditivas de estado estable en humanos. International Journal of Audiology 42(4): 177–219. [DOI] [PubMed] [Google Scholar]
- Poelmans H., Luts H., Vandermosten M., Ghesquière P., Wouters J. (2012) Hemispheric asymmetry of auditory steady-state responses to monaural and diotic stimulation. Journal of the Association for Research in Otolaryngology 13(6): 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel H., Kollmeier B. (2002) Auditory brain stem responses evoked by lateralized clicks: Is lateralization extracted in the human brain stem? Hearing Research 163(1): 12–26. [DOI] [PubMed] [Google Scholar]
- Ross B. (2008) A novel type of auditory responses: Temporal dynamics of 40-Hz steady-state responses induced by changes in sound localization. Journal of Neurophysiology 100(3): 1265–1277. [DOI] [PubMed] [Google Scholar]
- Ross B., Fujioka T., Tremblay K. L., Picton T. W. (2007a) Aging in binaural hearing begins in mid-life: Evidence from cortical auditory-evoked responses to changes in interaural phase. The Journal of Neuroscience 27(42): 11172–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B., Herdman A. T., Pantev C. (2005) Right hemispheric laterality of human 40 Hz auditory steady-state responses. Cerebral Cortex 15(12): 2029–2039. [DOI] [PubMed] [Google Scholar]
- Ross B., Tremblay K. L., Picton T. W. (2007b) Physiological detection of interaural phase differences. The Journal of the Acoustical Society of America 121(2): 1017–1027. [DOI] [PubMed] [Google Scholar]
- Salminen N. H., Tiitinen H., Yrttiaho S., May P. J. (2010) The neural code for interaural time difference in human auditory cortex. The Journal of the Acoustical Society of America 127(2): EL60–EL65. [DOI] [PubMed] [Google Scholar]
- Schiano J. L., Trahiotis C., Bernstein L. R. (1986) Lateralization of low-frequency tones and narrow bands of noise. The Journal of the Acoustical Society of America 79(5): 1563–1570. [DOI] [PubMed] [Google Scholar]
- Shepherd R. K., Javel E. (1997) Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hearing Research 108(1): 112–144. [DOI] [PubMed] [Google Scholar]
- Sininger Y. S., Don M. (1989) Effects of click rate and electrode orientation on threshold of the auditory brainstem response. Journal of Speech, Language, and Hearing Research 32(4): 880–886. [DOI] [PubMed] [Google Scholar]
- Smoski W. J., Trahiotis C. (1986) Discrimination of interaural temporal disparities by normal-hearing listeners and listeners with high-frequency sensorineural hearing loss. The Journal of the Acoustical Society of America 79(5): 1541–1547. [DOI] [PubMed] [Google Scholar]
- Stecker C., Gallun F. J. (2012) Binaural hearing, sound localization, and spatial hearing. In: Tremblay K. L., Burkard R. F. (eds) Translational perspectives in auditory neuroscience: Normal aspects of hearing, San Diego, CA, USA: Plural Publishing Inc, pp. 383–434. [Google Scholar]
- Stollman M. H. P., Snik A. F. M., Hombergen G. C. J. H., Nieuwenhuys R., Koppel P. T. (1996) Detection of the binaural interaction component in the auditory brainstem response. British Journal of Audiology 30(3): 227–232. [DOI] [PubMed] [Google Scholar]
- Tiitinen H., Salminen N. H., Palomäki K. J., Mäkinen V. T., Alku P., May P. J. (2006) Neuromagnetic recordings reveal the temporal dynamics of auditory spatial processing in the human cortex. Neuroscience Letters 396(1): 17–22. [DOI] [PubMed] [Google Scholar]
- Tlumak A. I., Rubinstein E., Durrant J. D. (2007) Meta-analysis of variables that affect accuracy of threshold estimation via measurement of the auditory steady-state response (ASSR). International Journal of Audiology 46(11): 692–710. [DOI] [PubMed] [Google Scholar]
- Ungan P., Yagcioglu S. (2002) Origin of the binaural interaction component in wave P4 of the short-latency auditory evoked potentials in the cat: Evaluation of serial depth recordings from the brainstem. Hearing Research 167(1): 81–101. [DOI] [PubMed] [Google Scholar]
- Van den, Honert C., Stypulkowski P. H. (1986) Characterization of the electrically evoked auditory brainstem response (ABR) in cats and humans. Hearing Research 21(2): 109–126. [DOI] [PubMed] [Google Scholar]
- Van Yper L. N., Vermeire K., De, Vel E. F., Battmer R. D., Dhooge I. J. (2015) Binaural interaction in the auditory brainstem response: A normative study. Clinical Neurophysiology 126(4): 772–779. [DOI] [PubMed] [Google Scholar]
- Wilson M. J., Kelly-Ballweber D., Dobie R. A. (1985) Binaural interaction in auditory brain stem responses: Parametric studies. Ear and Hearing 6(2): 80–88. [DOI] [PubMed] [Google Scholar]
- Zwislocki J., Feldman R. S. (1956) Just noticeable differences in dichotic phase. The Journal of the Acoustical Society of America 28(5): 860–864. [Google Scholar]