Abstract
Aim
Mature, differentiated enterocytes are essential for normal gut function and critical to recovery from pathological conditions. Little is known about the factors that regulate intestinal epithelial cell differentiation in the adult intestine. The transcription factor, Cdx2, involved in enterocytic differentiation, remains expressed in the adult. Since we have implicated Slfn3 in differentiation in vivo and in vitro, we examined whether it also mediated differentiation in the IEC-Cdx2-L1 cell model of differentiation.
Materials and Methods
IEC-Cdx2-L1 cells, permanently transfected with Cdx2 under the control of isopropyl-β-D-thiogalactoside (IPTG), were stimulated to differentiate by 16-day exposure to IPTG. Transcript levels of Cdx2, Slfn 3 and villin were determined by quantitative reverse transcriptase-polymerase chain reaction of mRNA isolated from IPTG-treated and control cells. Slfn3 expression was lowered with specific siRNA to investigate the role of Slfn3 in Cdx2-driven villin expression in IPTG-differentiated cells.
Results
Slfn3 and villin expression was significantly greater in IPTG-treated cells. Slfn3 siRNA lowered Slfn3 expression and abolished the IPTG-induced rise in villin expression (p<0.05 by ANOVA); Cdx2 expression was unaffected by Slfn3 siRNA.
Discussion
The data indicate that the presence of Slfn3 is required for Cdx2 to induce villin expression and thus, differentiation. However, Slfn3 must also promote differentiation independently of Cdx2 since IEC-6 cells that do not normally express Cdx2 can be differentiated by a variety of Slfn3-dependent mechanisms.
Keywords: Schlafen 3, Cdx2, intestinal epithelial cells, differentiation
Introduction
The intestinal epithelium exhibits great capacity for self-renewal and differentiation. Enterocytes differentiate as they migrate from the crypts to the villus tips where they are eventually shed [1]. Differentiated enterocytes are essential to the maintenance of normal absorptive function as well as to adaptation to various pathological states such as fasting, total parenteral nutrition, ileus and short gut syndrome [2]. However, the intracellular signaling pathways responsible for differentiation along the crypt-villus axis of the small intestine remain largely unknown. Several transcription factors influence differentiation during embryonic development and homeostasis of the adult intestine [3, 4]. Among them, Cdx2, a gene of the paraHox cluster, encodes for a homeodomain transcription factor that plays an important role in gut patterning in the embryo [5]. In adult animals, Cdx2 expression is restricted to the intestinal epithelium where it regulates diverse genes that define a functionally differentiated phenotype [6].
Schlafen3 (Slfn3) is a member of a family of growth regulatory genes first described in mice in 1999 that influence T-cell development, differentiation in haematopoietic cell lines, and the inflammatory response to HIV infection [7–11]. Our laboratory has demonstrated that Slfn3 promotes differentiation in rat intestinal epithelial cells (IEC). In vitro, disparate differentiation stimuli upregulate Slfn3 expression in IEC-6 cells; Slfn3-specific siRNA decreases both basal and stimulated dipeptidyl dipeptidase 4 (DPPIV) activity, a canonical marker of differentiation [12]. In vivo, jejunal Slfn3 levels are low in atrophic adult rat mucosa expression and increase with gestational age [13, 14]. Finally, intraluminal instillation of a Slfn3 adenovirus enhances mucosal mRNA and protein levels of several differentiation markers (villin, sucrase-isomaltase, DPPIV, and Glut2) while treatment with silencing RNA lowers their expression [15].
How Slfn3 regulates the expression of these differentiation markers also awaits elucidation. Schlafens have been shown to affect cell cycle proteins like cyclin D1 and p27Kip1 [16, 17] but their interactions with homeodomain proteins have not been investigated. We hypothesized that Cdx2 might induce and act through Slfn3 to drive differentiation. We tested our hypothesis in a well-accepted model of enterocytic differentiation, IEC-6 cells permanently transfected with Cdx2 (IEC-Cdx2-L1) under IPTG control [18–20]. Although our previous observations suggest that Slfn3 can induce differentiation independently of Cdx2 in native IEC-6 cells that lack Cdx2 [12], our results here also indicate that Slfn3 acts downstream of Cdx2 and mediates at least some of the Cdx2 effects on rat enterocytic differentiation.
Materials and Methods
Cell Culture
The stable Cdx2-transfected IEC-6 cell line (Cdx2-L1), developed and characterized by Suh and Traber [18] was a gift of Dr. J.Y. Wang (University of Maryland, Baltimore, MD). Cells were maintained in Dulbecco’s Modified Medium supplemented with 0.1 U/ml bovine insulin and 5% heat-inactivated FBS in a humidified 5% CO2 atmosphere. Isopropyl-β-D-thiogalactoside (IPTG; 4 mM) treatment for 16 days was used to induce gene expression; non-IPTG-treated cells served as controls. To lower Slfn3 expression, 16-day-differentiated cells were trypsinized and replated at 30% confluence and then treated with specific Slfn3 siRNA (50–200 nM), or a non-targeting control (NT1) (Ambion, Thermo Scientific, Pittsburgh, PA) using Oligofectamine or Lipofectamine RNAiMAX (Life Technologies, Grand Island, NY). Cells were lysed after 18–48 hours for assay of Cdx2, Slfn3 and villin expression by RT-PCR; changes in Slfn3 protein expression were confirmed by Western blot.
Quantitative RT-PCR
RNA was extracted with the RNeasy kit (QIAGEN, Valencia, CA). Complementary DNA was then amplified with SYBR Green Real-Time PCR Master Mix on an Applied Biosystems 7500 Real-Time PCR (Invitrogen, Grand Island, NY) using the following rat primers: Slfn3: for-attctgctgtgcagtgttcg/rev-ttgcttggagaaacatgctg; villin: for-caacttctatgagggagactgctac/rev-tagtcatccagctgtgtggtataga; Cdx2: for-cgatacatcaccaccaggagg/rev-tggctctgcggttctgaaa; and 18S: for-agttccgaccataaacgatgc/rev-cccttccgtcaattcctttaa, all from Integrated DNA Technologies (Coralville, IA). Expression levels were calculated from the threshold cycle (Ct) as 2−ΔΔCt using 18S as the reference.
Western blot
Control and IPTG-induced IEC-Cdx2-L1 cells were lysed 48 hours after exposure to Slfn3 siRNA. Equal amounts of protein, determined by the bicinchoninic acid method (BCA, Thermo Fisher, Rockford, IL), were separated by SDS-Page electrophoresis and the proteins transferred to nitrocellulose membranes. Membranes were exposed to Slfn3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA); GAPDH (glyceraldehyde 3-phosphate dehydrogenase; Meridian Life Sciences, Memphis, TN) served as control. Bands were detected on an Odyssey Infrared Imaging System using Li-Cor Biosciences secondary antibodies (Lincoln, NB) and densitometry analyzed on a Kodak 440CF Image Station (Rochester, NY).
Statistical analysis
At least three experiments were performed with similar results. Data from representative experiments are presented as X±SE; one-way ANOVA was used to calculate significance, seeking 95% confidence.
Results
IPTG treatment induces differentiation in IEC-Cdx2-L1 cells
We first determined whether 16 days of IPTG treatment enhanced Slfn3 and villin expression, used as an index of differentiation. Cdx2, Slfn3, and villin expression were significantly elevated after exposure to IPTG (Fig. 1, *p<0.05).
Figure 1.
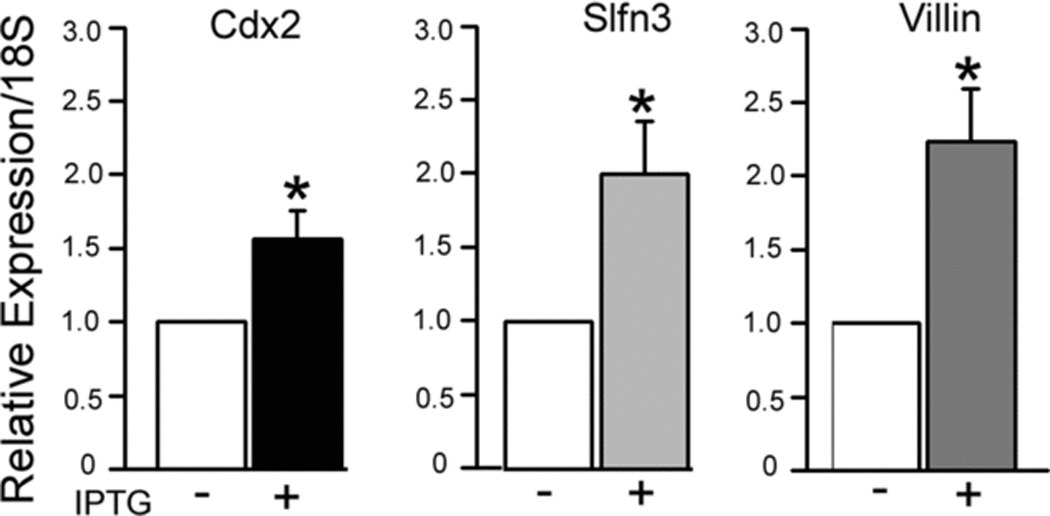
Treatment of IEC-Cdx2-L1 enterocytes with 4 uM IPTG for 16 days significantly induces Cdx2 expression. This is accompanied by significant increases in expression of Schlafen3 (Slfn3) and villin, a marker of differentiation (*p<0.05 vs control cells without IPTG).
Lowering Slfn3 levels via specific siRNA decreases villin expression in differentiated IEC-Cdx2-L1 cells
Since both Slfn3 and villin expression were elevated in IPTG-treated IEC-Cdx2-L1 cells, we asked whether the increase in villin mRNA was a consequence of the cells’ differentiation status or more directly correlated to the higher levels of Slfn3. Slfn3 siRNA decreased baseline expression by 30% and completely ablated the IPTG-induced increase in Slfn3 (Fig 2A; p<0.05 by one-way ANOVA). As shown in Fig 2B, Slfn3 protein levels paralleled RNA expression, higher with IPTG and significantly lower after siRNA exposure (p<0.05 by ANOVA). Slfn3 siRNA reduced basal and significantly blunted the IPTG-driven increase in villin expression (Fig 3; p<0.05 by ANOVA).
Figure 2.
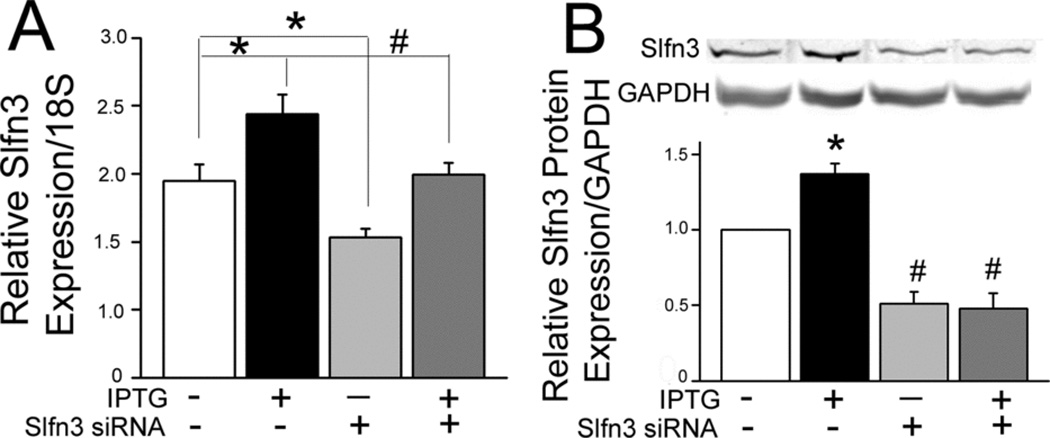
A) Slfn3 specific siRNA lowers basal and ablates IPTG-stimulated Slfn3 expression in IEC-Cdx2-L1 cells when compared to the non-targeting control siRNA (NT1) (*p<0.05 vs NT1 control, #p<0.05 vs NT1-IPTG by one-way ANOVA, representative of 3 similar). B) IPTG exposure increases Slfn3 protein levels in NT1-treated cells (*p<0.05); specific siRNA significantly lowers both basal and IPTG-stimulated Slfn3 protein expression (#p<0.05 vs NT1 and NT1-IPTG by ANOVA). Bars: densitometric analysis of n=5; a representative Western blot is shown above the bars.
Figure 3.
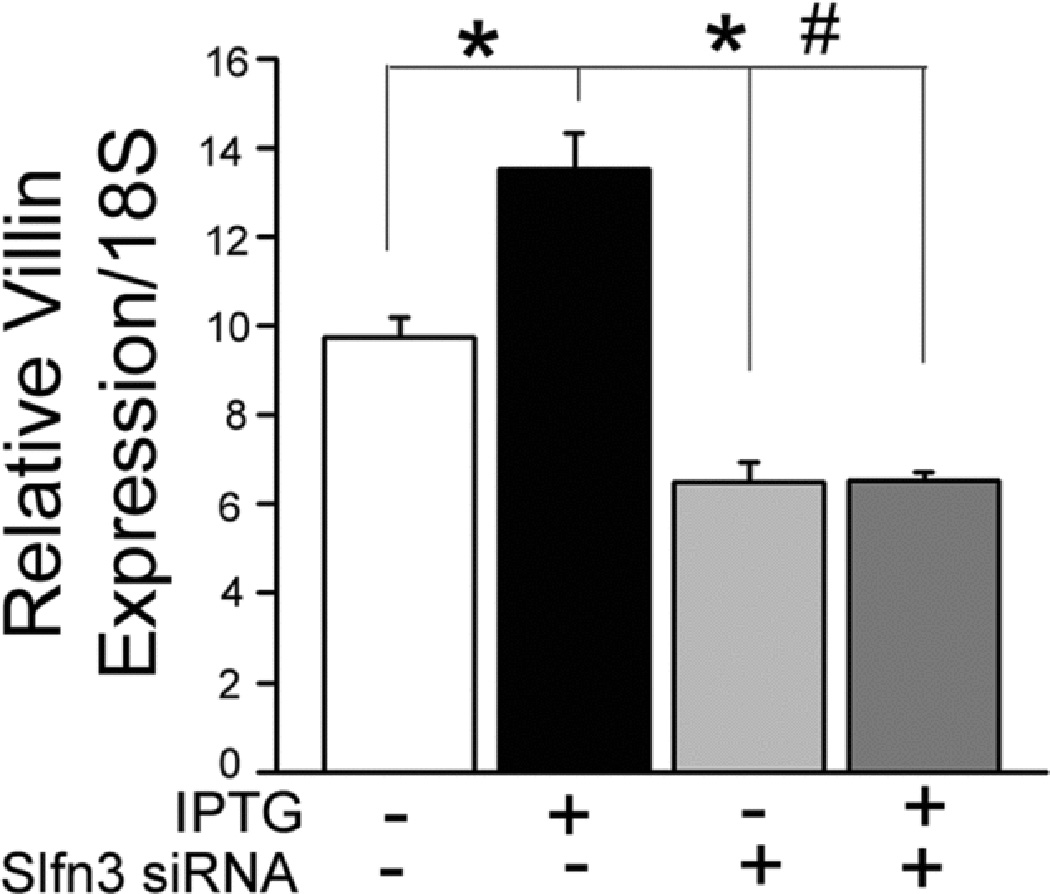
Lowering Slfn3 expression with Slfn3 specific siRNA significantly decreases both basal and IPTG-induced villin mRNA levels in IEC-Cdx2-L1 cells (*p<0.05 vs NT1 control, #p<0.05 vs NT1 and NT1-IPTG, representative of 3 similar).
Cdx2 expression is not decreased in IEC-Cdx2-L1 cells after lowering Slfn3 levels via siRNA
We confirmed that Cdx2 expression was elevated in IPTG-differentiated cells. However, Cdx2 expression appeared somewhat elevated in the face of decreased Slfn3 (Fig 4), suggesting that Slfn3 acts downstream of Cdx2 to regulate villin expression. Results shown in Figs 2A, 3 and 4 were all obtained from the same experimental samples while results depicted in Fig 2B were derived from samples in separate parallel studies.
Figure 4.
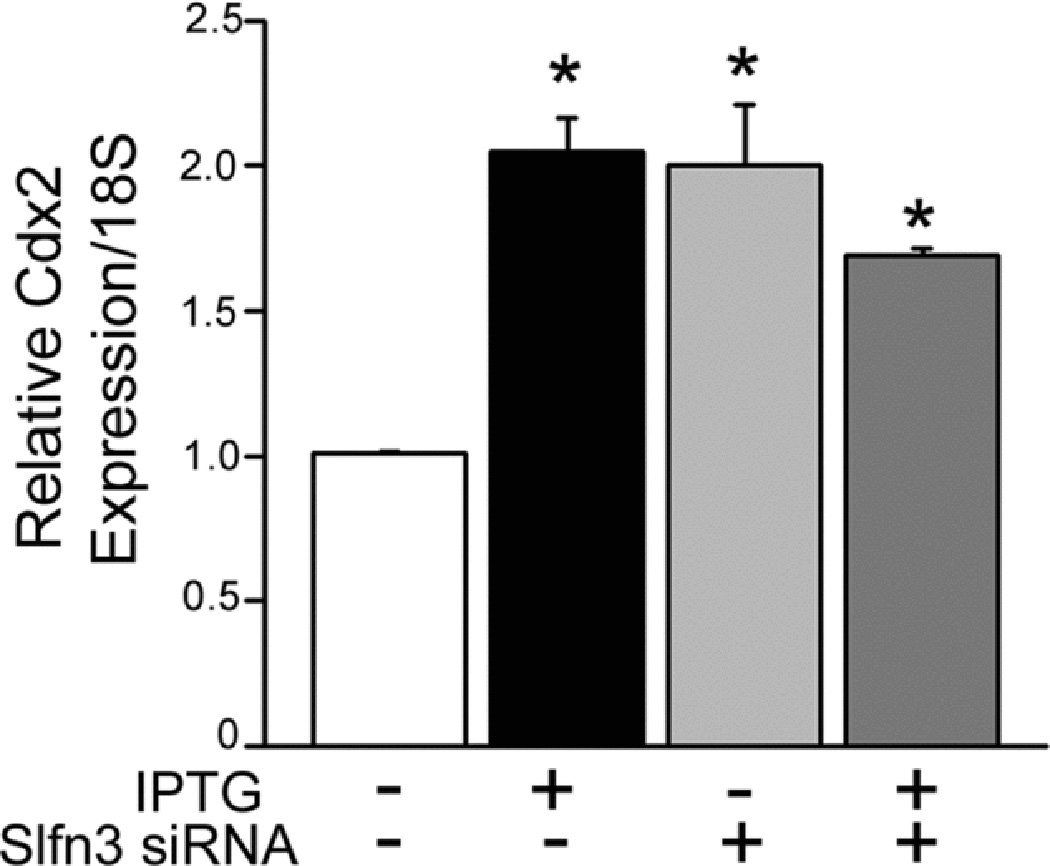
Cdx2 expression levels, elevated by IPTG-treatment, are not affected by Slfn3-specific siRNA, suggesting that Cdx2 lies upstream of Slfn3 in IEC-Cdx1-L1 enterocytes (*p<0.05 vs control cells without IPTG, representative of 4 similar).
Discussion
While we have previously implicated Slfn3 in the induction of differentiation by extracellular stimuli such as repetitive deformation, butyrate and TGF-beta [12], our present results further suggest that Slfn3 is required for at least some aspects of differentiation mediated by the intracellular trigger Cdx2. Cdx2 is a member of a regulatory network that controls intestinal differentiation, proliferation, adhesion and apoptosis [3, 21, 22]. Mouse embryos lacking Cdx2 develop a foregut type of mucosa while loss of Cdx2 in adult mice alters intestinal gene expression and villus morphology, leading to chronic diarrhea and death [6]. Conversely, mice overexpressing Cdx2 exhibit reduced post-natal growth, perhaps because of early epithelial maturation and decreased nutrient absorption [5].
IEC-Cdx2-L1 cells express elevated Slfn3 and villin levels when stimulated to differentiate by IPTG. Villin is a well-described marker for differentiation [23] and we have previously reported that adenoviral overexpression of Slfn3 increases villin expression in human Caco-2 intestinal epithelial cells in vitro and in rat intestinal mucosa in vivo while lowering Slfn3 with siRNA decreased villin expression [12, 13, 15], establishing a correlation between Slfn3 and villin abundance. How Slfn3 promotes enterocytic differentiation or villin expression remains unknown. Two Cdx2 binding sites have been identified in the villin promoter, and Cdx2 regulates villin expression in the human SW480 cell line [24]. Increasing Cdx2 by IPTG induction increased Slfn3 expression in IEC-Cdx2-L1 cells, while Cdx2 expression under the admittedly artificial conditions of the exogenous IPTG promoter remained elevated after treatment with Slfn3 siRNA, suggesting that Cdx2 lies upstream of Slfn3. That villin expression was lower in the presence of persistently elevated Cdx2 levels further suggests that Slfn3 is required for the Cdx2 effect on villin expression. Basal Cdx2 expression in these cells may reflect some “leakiness” of the promoter, an effect that may account for our observation that villin expression is significantly lower in response to modestly decreased Slfn3 levels in the absence of IPTG. Conversely, these results may support a Cdx2-independent action of Slfn3 on villin expression.
Figure 5 delineates a hypothesized relationship between Cdx2, Slfn3, and villin based upon our current observations and previously published data. Exogenous physiologic stimuli are known to induce both Slfn3 [12] and Cdx2 [3]. In this manuscript, we demonstrate that specifically overexpressing Cdx2 under the control of an IPTG-driven promoter can itself induce Slfn3 in IEC-6 rat enterocytes, and that this induction of Slfn3 is required for the effect of Cdx2 on villin expression.
Figure 5.
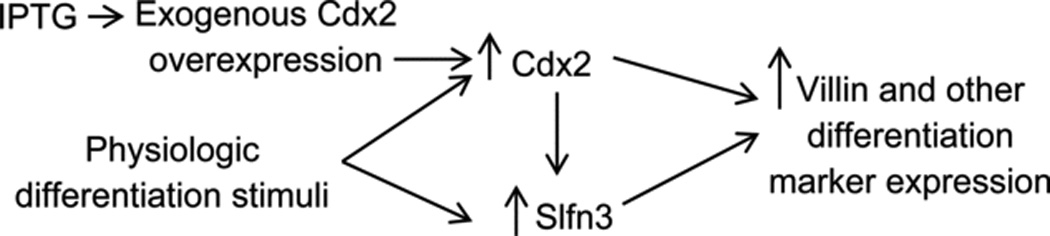
Cartoon illustrates the hypothesized relationship between Cdx2, Schlafen 3, and enterocytic differentiation markers such as villin, based upon current and previously published observations.
Little is known about the intracellular signaling pathways that regulate Cdx2 activity, particularly in response to extracellular signals. Cdx2 may affect differentiation via interactions with other transcription factors, such as HNF1α/β, GATA4–6, or ETS and may bind to either promoter or enhancer elements in its target genes [4, 21, 25]. Cdx2 binding and co-operation with individual transcription factors are also dictated by the differentiation status of the cell [26]. In addition, Cdx2 can alter differentiation through non-transcriptional mechanisms including inhibition of DNA repair, interactions with Smad3 or β-catenin and stabilization of p27Kip1 [27]. Our results indicate that absence of Slfn3 interferes with Cdx2-dependent villin expression in differentiated IEC-Cdx2-L1 cells. Thus, although Cdx2 binds the villin promoter directly, Slfn3 may act as a necessary co-factor to facilitate Cdx2 co-operation with a specific transcription factor or to affect one of its non-transcriptional functions.
Native IEC-6 cells do not express Cdx2 [18]. However, villin is expressed when IEC-6 cells are grown beyond confluence [28] and butyric acid stimulates differentiation in those cells [17]. Our previous results also support a direct role for Slfn3 in IEC-6 differentiation in response to several exogenous stimuli [12]. Furthermore, when Slfn3 is expressed in IEC-6 by adenoviral infection, DPPIV activity is significantly elevated when compared to the non-transfected controls (unpublished observations Yuan and Basson). Thus, although Cdx2 promotes rat intestinal epithelial cell differentiation in part through Slfn3, Slfn3 can mediate differentiation independently of Cdx2.
A well-differentiated small intestinal mucosa is essential for proper function as it supports optimal nutrient absorption and barrier function. Highly differentiated IEC-Cdx2-L1 cells migrate faster than parental IEC-6 controls [20], also underscoring the importance of a differentiated mucosa to wound healing. The mucosa must simultaneously act as an absorptive surface that can actively translocate nutrients to supplement passive diffusion and a barrier to toxins and bacteria. We have previously traced in vitro influences on intestinal epithelial differentiation by luminal contents such as butyrate [29–31] and glutamine [32], growth factors such as TGF-β [33] or somatostatin [34], chemical factors like pH [35], and even physical forces like repetitive deformation during peristalsis or villous motility [36, 37]. In short gut syndrome, when there is inadequate mucosal capacity to maintain nutrition [2], attention has chiefly focused on the use of mitogens such as teduglutide to stimulate enterocytic proliferation to increase mucosal mass, but such mitogenic stimuli may actually reduce differentiation [38]. Conversely, malignancy represents an obvious loss of differentiation while proliferation increases. Mucosal atrophy and dedifferentiation in the absence of luminal nutrients [39] may also be important for the loss of barrier function that occurs in this setting.
It therefore becomes important to elucidate the pathways by which intestinal epithelial differentiation is regulated as well as those that regulate intestinal epithelial proliferation. Cdx2 is well-described to be one of the key transcription factors that turn on a gene program for enterocytic differentiation [3–6]. Such transcription factors are generally thought to act directly on promoters for gene transcriptions, and this is certainly true. However, the present results suggest that the induction of Slfn3 by Cdx2 may be essential to Cdx2 promotion of at least one differentiation marker, villin. This is particularly interesting since we have recently reported that Slfn3 is a predominantly cytosolic protein, and its activity is not affected by blocking its entry into the nucleus [40]. Precisely how Slfn3 works in this setting is not yet known. We have previously demonstrated that other cytoplasmic signals such as tyrosine kinases [41], PKC [42], and cyclic nucleotides [43] can modulate intestinal epithelial differentiation.
Certainly transcriptional activity is ultimately affected, as we demonstrate here by showing an increase in villin promoter activity. Slfn3 and its human ortholog(s) likely bind to other proteins, either sequestering them from nuclear entry, protecting them from degradation so that they are available for subsequent activity, or manipulating them in some other way that ultimately results in some third class of proteins being available within the nucleus. However, taken together with our present results, this both suggests a previously unsuspected complexity to the actions of Cdx2 and raises the possibility that manipulation of the pathway by which Slfn3 promotes enterocytic differentiation may ultimately be a desirable clinical pharmacological target. Enhancing enterocytic differentiation should be considered in any effort at improving function in conditions such as fasting, total parenteral nutrition or adaptation to massive bowel resection. To that end, Cdx2-Slfn3 interactions, at the transcriptional or non-transcriptional level, remain an important topic for further study.
Acknowledgments
The study was funded by NIH R56DK096137 (M. D. Basson).
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
No commercial relationships exist between any of the authors or any commercial entity.
Literature Cited
- 1.de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60(7):1322–1332. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw D, Gohil K, Basson MD. Intestinal mucosal atrophy and adaptation. World J Gastroenterol. 2012;18(44):6357–6375. doi: 10.3748/wjg.v18.i44.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richmond CA, Breault DT. Regulation of gene expression in the intestinal epithelium. Prog Mol Biol Transl Sci. 2010;96:207–229. doi: 10.1016/B978-0-12-381280-3.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudreau F, Rings EH, van Wering HM, Kim RK, Swain GP, Krasinski SD, et al. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277(35):31909–31917. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 5.Crissey MA, Guo RJ, Funakoshi S, Kong J, Liu J, Lynch JP. Cdx2 levels modulate intestinal epithelium maturity and Paneth cell development. Gastroenterology. 2011;140(2):517–528. doi: 10.1053/j.gastro.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verzi MP, Shin H, Ho LL, Liu XS, Shivdasani RA. Essential and redundant functions of caudal family proteins in activating adult intestinal genes. Mol Cell Biol. 2011;31(10):2026–2039. doi: 10.1128/MCB.01250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity. 1998;9(5):657–668. doi: 10.1016/s1074-7613(00)80663-9. [DOI] [PubMed] [Google Scholar]
- 8.Bustos O, Naik S, Ayers G, Casola C, Perez-Lamigueiro MA, Chippindale PT, et al. Evolution of the Schlafen genes, a gene family associated with embryonic lethality, meiotic drive, immune processes and orthopoxvirus virulence. Gene. 2009;447(1):1–11. doi: 10.1016/j.gene.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geserick P, Kaiser F, Klemm U, Kaufmann SH, Zerrahn J. Modulation of T cell development and activation by novel members of the Schlafen (slfn) gene family harbouring an RNA helicase-like motif. Int Immunol. 2004;16(10):1535–1548. doi: 10.1093/intimm/dxh155. [DOI] [PubMed] [Google Scholar]
- 10.Katsoulidis E, Carayol N, Woodard J, Konieczna I, Majchrzak-Kita B, Jordan A, et al. Role of Schlafen 2 (SLFN2) in the generation of interferon alpha-induced growth inhibitory responses. J Biol Chem. 2009;284(37):25051–25064. doi: 10.1074/jbc.M109.030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Kao E, Gao X, Sandig H, Limmer K, Pavon-Eternod M, et al. Codon-usage-based inhibition of HIV protein synthesis by human schlafen 11. Nature. 2012;491(7422):125–128. doi: 10.1038/nature11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan L, Yu Y, Sanders MA, Majumdar AP, Basson MD. Schlafen 3 induction by cyclic strain regulates intestinal epithelial differentiation. Am J Physiol Gastrointest Liver Physiol. 2010;298(6):G994–G1003. doi: 10.1152/ajpgi.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovalenko PL, Basson MD. The correlation between the expression of differentiation markers in rat small intestinal mucosa and the transcript levels of schlafen 3. JAMA Surg. 2013;148(11):1013–1019. doi: 10.1001/jamasurg.2013.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh MF, Hermann R, Sun K, Basson MD. Schlafen 3 changes during rat intestinal maturation. Am J Surg. 2012;204(5):598–601. doi: 10.1016/j.amjsurg.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovalenko PL, Yuan L, Sun K, Kunovska L, Seregin S, Amalfitano A, et al. Regulation of epithelial differentiation in rat intestine by intraluminal delivery of an adenoviral vector or silencing RNA coding for Schlafen 3. PLoS One. 2013;8(11):e79745. doi: 10.1371/journal.pone.0079745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brady G, Boggan L, Bowie A, O'Neill LA. Schlafen-1 causes a cell cycle arrest by inhibiting induction of cyclin D1. J Biol Chem. 2005;280(35):30723–30734. doi: 10.1074/jbc.M500435200. [DOI] [PubMed] [Google Scholar]
- 17.Patel VB, Yu Y, Das JK, Patel BB, Majumdar AP. Schlafen-3: a novel regulator of intestinal differentiation. Biochem Biophys Res Commun. 2009;388(4):752–756. doi: 10.1016/j.bbrc.2009.08.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16(2):619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, Rao JN, Liu L, Rizvi M, Turner JD, Wang JY. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;288(6):G1159–G1169. doi: 10.1152/ajpgi.00407.2004. [DOI] [PubMed] [Google Scholar]
- 20.Rao JN, Platoshyn O, Li L, Guo X, Golovina VA, Yuan JX, et al. Activation of K(+) channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol Cell Physiol. 2002;282(4):C885–C898. doi: 10.1152/ajpcell.00361.2001. [DOI] [PubMed] [Google Scholar]
- 21.Boyd M, Hansen M, Jensen TG, Perearnau A, Olsen AK, Bram LL, et al. Genome-wide analysis of CDX2 binding in intestinal epithelial cells (Caco-2) J Biol Chem. 2010;285(33):25115–25125. doi: 10.1074/jbc.M109.089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang R, Santiago NA, Olds LC, Sibley E. The homeodomain protein Cdx2 regulates lactase gene promoter activity during enterocyte differentiation. Gastroenterology. 2000;118(1):115–127. doi: 10.1016/s0016-5085(00)70420-3. [DOI] [PubMed] [Google Scholar]
- 23.Khurana S, George SP. Regulation of cell structure and function by actin-binding proteins: villin's perspective. FEBS Lett. 2008;582(14):2128–2139. doi: 10.1016/j.febslet.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamichi N, Inada K, Furukawa C, Sakurai K, Tando T, Ishizaka A, et al. Cdx2 and the Brm-type SWI/SNF complex cooperatively regulate villin expression in gastrointestinal cells. Exp Cell Res. 2009;315(10):1779–1789. doi: 10.1016/j.yexcr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Krasinski SD, Van Wering HM, Tannemaat MR, Grand RJ. Differential activation of intestinal gene promoters: functional interactions between GATA-5 and HNF-1 alpha. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G69–G84. doi: 10.1152/ajpgi.2001.281.1.G69. [DOI] [PubMed] [Google Scholar]
- 26.Verzi MP, Shin H, He HH, Sulahian R, Meyer CA, Montgomery RK, et al. Differentiation-specific histone modifications reveal dynamic chromatin interactions and partners for the intestinal transcription factor CDX2. Dev Cell. 2010;19(5):713–726. doi: 10.1016/j.devcel.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renouf B, Soret C, Saandi T, Delalande F, Martin E, Vanier M, et al. Cdx2 homeoprotein inhibits non-homologous end joining in colon cancer but not in leukemia cells. Nucleic Acids Res. 2012;40(8):3456–3469. doi: 10.1093/nar/gkr1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Serrano F, Rios A, Marchal JA, Caba O, Martinez-Amat A, Prados J, et al. Differentiation of intestinal epithelial cells mediated by cell confluence and/or exogenous nucleoside supplementation. Cells Tissues Organs. 2010;191(6):478–488. doi: 10.1159/000276593. [DOI] [PubMed] [Google Scholar]
- 29.Basson MD, Emenaker NJ, Hong F. Differential modulation of human (Caco-2) colon cancer cell line phenotype by short chain fatty acids. Exp Biol Med. 1998;217(4):476–483. doi: 10.3181/00379727-217-44261. [DOI] [PubMed] [Google Scholar]
- 30.Emenaker NJ, Basson MD. Short chain fatty acids inhibit human (SW116) colon cancer cell invasion by reducing urokinase plasminogen activator activity and stimulating TIMP-1 and TIMP-2 activities rather than via MMP modulation. J Surg Res. 1998;76(1):41–46. doi: 10.1006/jsre.1998.5279. [DOI] [PubMed] [Google Scholar]
- 31.Basson MD, Lin YW, Hanly AM, Emenaker NJ, Shenoy SG, Rothberg BEG. Identification and comparative analysis of human colonocyte short-chain fatty acid response genes. J Gastrointest Surg. 2000;4(5):501–512. doi: 10.1016/s1091-255x(00)80093-1. [DOI] [PubMed] [Google Scholar]
- 32.Murnin M, Kumar A, di Li G, Brown M, Sumpio BE, Basson MD. Effects of glutamine isomers on human (Caco-2) intestinal epithelial proliferation, strain-responsiveness, and differentiation. J Gastrointest Surg. 4(4):435–442. doi: 10.1016/s1091-255x(00)80025-6. 200. [DOI] [PubMed] [Google Scholar]
- 33.Walsh MF, Ampasala DR, Hatfield J, Vander Heide R, Suer S, Rishi AK. Transforming growth-factor-β stimulates intestinal epithelial focal adhesion kinase synthesis via Smad-and p38-dependent mechanisms. Am J Pathol. 2008;173(2):385–399. doi: 10.2353/ajpath.2008.070729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sgambati SA, Zrif A, Basson MD. Octreotide differentially modulates human Caco-2 intestinal cell proliferation and differentiation by decreasing intracellular cAMP. Regulatory Peptides. 1996;61(3):219–227. doi: 10.1016/0167-0115(95)00163-8. [DOI] [PubMed] [Google Scholar]
- 35.Perdikis DA, Davies R, Zhukarov A, Brenner B, Etter L, Basson MD. Differential effects of mucosal pH on human (Caco-2) intestinal epithelial cell motility, proliferation and differentiation. Dig Di Sci. 1998;43(7):1537–1546. doi: 10.1023/a:1018871016691. [DOI] [PubMed] [Google Scholar]
- 36.Han O, di Li G, Sumpio BE, Basson MD. Strain induces Caco-2 intestinal epithelial proliferation and differentiation via PKC and tyrosine kinase signals. Am J Physiol Gastro and Liver Physiol. 1998;275(3):G534–G541. doi: 10.1152/ajpgi.1998.275.3.G534. [DOI] [PubMed] [Google Scholar]
- 37.Basson MD, di Li G, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol. 1996;162(2):476–488. doi: 10.1002/(SICI)1097-4652(199608)168:2<476::AID-JCP26>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Chaturvedi LS, Basson MD. Glucagon-like peptide 2 analogue teduglutide: stimulation of proliferation but reduction of differentiation in human Caco-2 intestinal epithelial cells. JAMA Surg. 2013;148(11):1037–1042. doi: 10.1001/jamasurg.2013.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovalenko PL, Basson MD. Changes in morphology and function in small intestinal mucosa after Roux-en-Y surgery in a rat model. J Surg Res. 2012;177(1):63–69. doi: 10.1016/j.jss.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaturvedi L, Sun K, Walsh MF, Kuhn LA, Basson MD. The P-loop region of Schlafen 3 acts within the cytosol to induce differentiation of human Caco-2 intestinal epithelial cells. Biochim Biophys Acta. 2014;1843(12):3029–3037. doi: 10.1016/j.bbamcr.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basson MD, Emenaker NJ, Rashid Z. Effects of modulation of tyrosine phosphorylation on brush border enzyme activity in human Caco-2 intestinal epithelial cells. Cell Tissue Res. 1998;292(3):553–562. doi: 10.1007/s004410051084. [DOI] [PubMed] [Google Scholar]
- 42.Basson MD, Hong F. Modulation of human Caco-2 intestinal epithelial cell phenotype by protein kinase C inhibitors. Cell Biol Int. 1995;19(12):1025–1032. doi: 10.1006/cbir.1995.1045. [DOI] [PubMed] [Google Scholar]
- 43.Basson MD, Hong F. Regulation of human Caco-2 intestinal epithelial brush border enzyme activity by cyclic nucleotides. Cancer Lett. 1996;99(2):155–160. doi: 10.1016/0304-3835(95)04058-7. [DOI] [PubMed] [Google Scholar]


