Abstract
Staphylococcus aureus is a gram-positive bacterium responsible for a wide range of infections. Host cell cycle alteration is a sophisticated mechanism used by pathogens to hijack the defense functions of host cells. We previously demonstrated that S. aureus MW2 (USA400) bacteria induced a G2/M phase transition delay in HeLa cells. We demonstrate here that this activity is triggered by culture supernatant compounds. Using size exclusion chromatography of the MW2 supernatant, followed by mass spectroscopy analysis of corresponding peaks, we identified phenol-soluble modulin α (PSMα) peptides as the likely candidates for this effect. Indeed, synthetic PSMα1 and PSMα3 caused a G2/M phase transition delay. The implication of PSMα in cell cycle alteration was confirmed by comparison of S. aureus Los Angeles County clone (LAC) wild-type with the isogenic mutant LAC∆psmα, which lacks the psmα operon encoding PSMα1–4. PSMα-induced G2/M transition delay correlated with a decrease in the defensin genes expression suggesting a diminution of antibacterial functions of epithelial cells. By testing the supernatant of S. aureus human clinical isolates, we found that the degree of G2/M phase transition delay correlated with PSMα1 production. We show that PSMs secreted by S. aureus alter the host cell cycle, revealing a newly identified mechanism for fostering an infection.—Deplanche, M., Filho. R. A. E.–A., Alekseeva, L., Ladier, E., Jardin, J., Henry, G., Azevedo, V., Miyoshi, A., Beraud, L., Laurent, F., Lina, G., Vandenesch, F., Steghens, J.-P., Le Loir, Y., Otto, M., Götz, F., Berkova, N. Phenol-soluble modulin α induces G2/M phase transition delay in eukaryotic HeLa cells.
Keywords: Staphylococcus aureus, cell cycle alteration, defensins, toxin, innate immune response
Staphylococcus aureus, a highly versatile gram-positive bacterium, can cause a multitude of diseases ranging from mild superficial skin infections to life-threatening disseminated infections such as pneumonia, osteomyelitis, meningitis, endocarditis, and sepsis (1, 2). Intermittent S. aureus colonization occurs in 30–50% of healthy adults (3), 10% of whom harbor S. aureus in the gastrointestinal tract (4).
The occurrence of antibiotic-resistant strains and the absence of an effective vaccine complicate the treatment of staphylococcal infections. To colonize and propagate within the host, S. aureus expresses a wide range of virulence factors, such as surface proteins, that govern adhesion to and invasion of host cells, evasion of immune responses (5), and biofilm formation (5). Other types of virulence factors, such as toxins, induce host cell lysis or elicit inflammatory responses (6, 7).
The host epithelium is in perpetual contact with numerous microorganisms, resulting in a multiplicity of the host’s defense mechanisms. The integrity of the epithelial barrier is dependent on a regeneration of epithelial cells (8). Pathogens use specialized strategies to disrupt key cell functions and invade the epithelium to establish persistent colonization. Some of those strategies rely on cell cycle alteration. This cycle comprises the G1 phase characterized by cell growth, the S phase characterized by DNA replication, the G2 phase in which cells are prepared for division, the M phase during which mitosis occurs, and the G0 phase during which cells can enter a quiescent state. Bacterial toxins may interfere with the host cell cycle machinery, e.g., cytolethal distending toxin of Escherichia coli or Helicobacter species, which induces the DNA-damage signaling pathways together with alteration of the host cell cycle (9, 10)
We recently found that S. aureus USA400 MW2 induces a G2/M phase transition delay in epithelial cells. The delay was associated with the accumulation of inactive cyclin-dependent kinase 1 and unphosphorylated histone [3H]. We also showed that bacteria preferred the G2 phase for intracellular replication (11). However, the nature of the bacterial factor that delays cell cycle phase transition was not identified.
We show here that the cell cycle is altered by S. aureus compounds that were secreted into the culture supernatant. It was determined that they belonged to the cytolytic phenol-soluble modulin α (PSMα) peptides. PSMα-induced G2/M-transition delay was related to the decrease of the antibacterial functions of the epithelial cells. The expression level of antibacterial peptides such as human β-defensins (hBDs), the first line of defense against staphylococcal infection (12), was lower in G2/M-phase cells compared with cells in the G1 phase. We also tested clinical human S. aureus isolates and found that cell cycle delay activity was associated with PSMα1 production.
MATERIALS AND METHODS
Reagents
N-formylated PSMα1 and PSMα3 peptides were provided by CecoLabs (Tübingen, Germany). Lactate dehydrogenase (LDH) was quantified using the Pierce LDH Cytotoxicity Assay (Thermopierce, Rockford, IL, USA). All other reagents were provided by Sigma-Aldrich (Saint-Quentin Fallavier, France).
Eukaryotic cells
The human cervix cancer HeLa cells (American Type Culture Collection, Manassas, VA, USA) were cultured in complete DMEM (cDMEM) [GlutaMax; Life Technologies, Carlsbad, CA, USA; 10% fetal calf serum (FCS)] supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin sulfate at 37°C with 5% CO2. Trypsin/EDTA (Gibco, Saint Aubin, France) was used to release adherent cells for subculturing.
Staphylococcus aureus strains and culture conditions
Staphylococcus aureus clinical isolates were obtained from patients diagnosed with staphylococcal enterocolitis. The methicillin-resistant S. aureus USA400 MW2 strain, S. aureus USA300 [Los Angeles County clone (LAC) wild-type (WT)], and its isogenic mutant LAC∆psmα, which lacks the psmα operon encoding PSMα1–4 (13), were obtained from the Laboratory of Human Bacterial Pathogenesis, National Institutes of Health (Bethesda, MD, USA).
All S. aureus cultures were performed as follows: aliquots from overnight cultures on brain heart infusion (BHI) broth were diluted (1:50) in DMEM. The mutant (LAC∆psmα) in which the psmα operon was exchanged for a spectinomycin-resistant cassette was grown in BHI containing 250 µg/ml spectinomycin before inoculating into DMEM.
Strains were incubated at 37°C under anaerobic conditions until cultures reached an optical density of 0.6 at 600 nm, corresponding to 108 colony-forming units (CFUs)/ml (14). The staphylococci were harvested by centrifugation, washed twice with PBS, and resuspended in the interaction medium (DMEM). Bacterial concentrations were estimated spectrophotometrically and confirmed by plate counts.
Preparation of bacterial supernatants
Bacteria were grown in DMEM and harvested at an OD600nm of 0.6. The culture supernatants (pH adjusted to 7.4) were concentrated 10-fold in a SpeedVac (SpeedVac Concentrator SVC11 and Refrigerated Condensation Trap Savant, Thermo Fisher Scientific, Waltham, MA, USA), sterile filtered with a 0.22 µm filter (Millipore, Billerica, MA, USA), and stored at −20°C. The total protein concentrations of the concentrated supernatants did not differ >10% between samples. Concentrated DMEM was used as a control.
Double thymidine block
The double thymidine block (DTB) was used to synchronize cells at the G1/S border (9, 11). Briefly, cells were grown in a 25 ml flask up to 30% confluence. After washing with PBS, the cells were cultivated in cDMEM containing 2 mM thymidine for 18 hours. Then, thymidine was removed by washing with PBS. The cells were cultivated in fresh cDMEM for 9 hours to release cells and then cultivated in cDMEM containing 2 mM thymidine for 17 hours.
Cell culture infection
HeLa cells (30% confluence at the beginning of DTB) were infected with S. aureus with a multiplicity of infections (MOIs, number of bacteria per cell at the onset of infection) of 100:1 at the periods indicated after DTB release (infection medium: DMEM). HeLa cell concentrations were determined using 1 of the 4 samples (11). The remaining samples were used for the analysis in triplicate. The low HeLa cell density at the beginning of the experiment was used to ensure cell proliferation during the entire experiment because cells cease proliferating when they reach confluence and enter a state of quiescence (15).
Bacterial concentrations were estimated spectrophotometrically and were confirmed by CFU determination. Unbound bacteria were removed 2 h after infection by washing the wells with PBS, followed by incubation in cDMEM with 3% FCS containing 20 μg/ml of lysostaphin and 100 μg/ml gentamicin for 2 hours, which eliminates the extracellular bacteria (16), followed by incubation in cDMEM containing 25 μg/ml of gentamicin for the periods indicated. The incubation time was chosen in agreement with the recognized evaluation of the phases of the HeLa cell cycle and on the basis of our results (11).
Exposure of HeLa cells to the bacterial supernatants or to PSMα1 and PSMα3
Three hours after DTB release, cells were exposed to concentrated S. aureus supernatants. The concentrated DMEM was used as a control because bacteria were grown in DMEM for the preparation of bacterial supernatants. To analyze the role of PSMα, HeLa cells were exposed to N-formylated synthetic PSMα1 or PSMα3 ranging from 0.1 to 10 µg/ml. Exposure of HeLa cells to the culture supernatants or synthetic PSMα was performed in DMEM without FCS (17).
Flow cytometry analysis
Detached cells were combined with adherent cells, which were collected by trypsin/EDTA treatment and fixed in 70% ethanol overnight. Cells were then stained with propidium iodide (PI) and analyzed with an Accuri C6 flow cytometer (Becton Dickinson, Le Pont de Claix, France) (11). Data were collected from 20,000 cells, and analysis was performed with CFlow software (Becton Dickonson).
Mitotic index evaluation
To estimate the degree of HeLa cell synchronization, mitotic indexes (MIs; the percentage of cells in mitosis from the total number of cells) were evaluated as previously described (11). Briefly, cells were grown on coverslips in 12-well plates (Nunc, NuclonTM Surface; Thermo Scientific, Langenselbold, Germany) followed by DTB synchronization. Plates were centrifuged to avoid loss of mitotic cells during washing, and the cells were then fixed with 4% paraformaldehyde/PBS for 1 hour. The coverslips were then mounted on slides with DAPI-containing ProLong antifade Vectashield medium (Vector Laboratory, Les Ulis, France). The MIs were determined from 400 cells per assay. The cells were counted with a fluorescence microscope using ×400 magnification (Nikon, Tokyo, Japan). The cell cycle phases were determined at the same time by flow cytometry.
Size-exclusion chromatography
Samples concentrated 10-fold (either S. aureus culture supernatants or DMEM) were applied to size-exclusion chromatography (SEC) through a 200 μl sample loop, using a Superdex 75 HR 10/30 column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Elution with PBS buffer at a flow rate of 0.3 ml/min was performed at room temperature. The elution was monitored at 280 nm, and fractions were collected (150 µl/tube) and tested for their capacity to modify the HeLa cell cycle. Samples that demonstrated cell cycle modulation activity were characterized by mass spectrometry.
Tandem mass spectrometry analysis of bioactive fractions
Mass spectrometry experiments were performed using a nanoRSLC Dionex U3000 system fitted to a Q Exactive mass spectrometer (Thermo Scientific, San Jose, CA, USA) equipped with a nanoelectrospray ion source as previously described (18). Briefly, a preliminary sample concentration step was performed on a µ-precolumn C18 pepMap100 (C18 column, 300 µm internal diameter, 5 mm length, 5 µm particle size, 100 Å pore size; Dionex, Amsterdam, The Netherlands) followed by separation on a C18 reversed-phase column (PepMap100, 75 µm inner diameter, 150 mm length, 3 µm particle size, 100 Å pore size; Dionex) using solvent A [2% (v/v) acetonitrile, 0.08% (v/v) formic acid, and 0.01% (v/v) triflouroacetic acid in deionized water] and solvent B [95% (v/v) acetonitrile, 0.08% (v/v) formic acid and 0.01% (v/v) triflouroacetic acid in deionized water]. Peptides were separated using a gradient of 4–20% solvent B for 15 min and 20–50% solvent B for 10 minutes at a flow rate of 0.3 µl/min. Electrospray ionization of eluted peptides occurred at a voltage of 2 kV, and spectra were recorded in positive mode. A data-dependent acquisition mode was used to fragment the 10 most intense ions in the MS spectrum, and the instrument was externally calibrated according to the supplier’s instructions.
Peptides were identified from MS/MS spectra using X! Tandem (TANDEM) pipeline software (Plateforme d'Analyze Protéomique de Paris Sud-Ouest, Institut National de la Recherche Agronomique INRA, Jouy-en-Josas, France, http://pappso.inra.fr). To perform a database search with nonspecific cleavage rules, a small in-house database composed of 485 proteins from www.uniprot.org was used. Phosphorylation of serine and N-formylation of methionine was selected as variable modifications, and a minimum score corresponding to an e-value <0.05 was considered a prerequisite for valid peptide identification.
Detection of PSMα in the culture supernatants
Culture supernatants of the human isolates were concentrated 10-fold, precipitated by cold methanol [dilution: 1/5 (v/v)], and left for 10 minutes on ice. After centrifugation, the supernatants were analyzed by reversed-phase HPLC mass spectrometry on an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA, USA) using the C12 Uptisphere Strategy 100 Å, 2.2 µm (50 mm) column (Interchim, Montluçon Cedex, France).
A gradient from 42% to 90% of acetonitrile for 13 minutes in the presence of 0.1% formic acid was used. Conditions for PSMα detection were as follows.
The injection volume was 100 µl with a flow rate of 0.220 ml/min at 45°C. Peptide masses were calculated from multiply charged ions obtained by the coupled electrospray ionization-equipped LC/MSD Trap SL mass spectrometer using Agilent LC/MSD 5.2 software. The PSMα peptide standard curve of the respective synthetic PSMα peptide was obtained for concentrations ranging from 0.1 to 40 µg/ml. The quantity of PSMα in the sample was proportional to the surface of the specific peak corresponding to the PSMα on a chromatogram. This allowed the detection of PSMα peptides at concentrations as low as 0.1 µg/ml.
Analysis of cytotoxic effects of PSMα
HeLa cells were grown to 80% confluence in 96-well culture plates. The cells then were exposed to PSMα for 24 hours in DMEM. PSMα concentration ranged from 0.1 to 10 µg/ml. The cytotoxic activity of PSMα was estimated by release of LDH (Pierce LDH Cytotoxicity Assay Kit; Pierce, Rockford, IL, USA), a reliable biochemical indicator of cell injury (19). For controls, untreated cells were included for measurements of spontaneous LDH release and maximum LDH release, which was induced by lysis reagent. Background control was performed by measuring the LDH activity in DMEM medium at A492 nm.
Cytotoxicity levels were calculated as follows: LDH release of treated cells (%) = (LDH PSM-treated cells – LDH control untreated cells)/(maximum LDH release – LDH control untreated cells) × 100.
Lysostaphin protection assay
A protection assay was performed as previously described (20) with the following modifications. HeLa cells were grown in 12-well plates overnight, confluent monolayers were infected with LAC WT strain or its isogenic mutant (LAC∆psmα) at a MOI of 100:1 in DMEM medium. Infection of host cells was synchronized by centrifugation (500 g; 10 minutes). Cells were then incubated for 50 minutes. Afterward, cells were incubated for 1 hour in DMEM supplemented with 20 µg/ml of lysostaphin and 100 µg/ml of gentamicin. At the end of incubation (T2) and 6 h after infection (T6), cells were collected after trypsinization. Cell concentrations were determined by a Trypan blue exclusion assay from 1 sample. The remaining samples were used for the CFU analysis in triplicate: cells were lysed with 0.05% Triton X-100 in PBS, and CFUs were determined after overnight incubation on BHI agar.
Defensin expression analysis
Exposure of the synchronized HeLa cells to PSMα was performed as described above. Expression of hBD-1,-3, and -9 in PSMα-treated cells was evaluated by RT-qPCR. Total RNA was isolated with Qiazol Reagent (#79306; Qiagen, Germantown, MD, USA). The RNA concentration was measured by spectroscopy (NanoDrop, Wilmington, DE, USA), and cDNA was synthesized from 1 µg of RNA, using a qScript cDNA Synthesis kit (Quanta Biosciences, Gaithersburg, MD, USA). Reactions devoid of reverse transcriptase and reactions containing H2O instead of cDNA were used as a negative control.
Primers for hBD-1,-3, and -9 were designed according to the sequences available at the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/). To avoid genomic DNA amplification, primer sequences were designed to cover ≥2 subsequent exons of hBD-1, -3, and -9 (NCBI accession #NM_005218.3, NM_018661, and NM_001037380, respectively; Supplemental Table S1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, NCBI accession #NM2046.3) and peptidylproyl isomerase A (PPIA; NCBI accession #NM_001300981) were used as housekeeping genes. Relative quantification relates the PCR signal of the target transcript in a treatment group to that of an untreated control. Each 13 µl PCR mixture included 2 µl of cDNA, 200 nM of primers, 12.5 µl of IQ Sybr Green supermix (2×) (Bio-Rad, Marne la Coquette, France), and H2O. Amplification was carried out on a CFX96 Real Time System (Bio-Rad) for 3 minutes at 95°C and 40 cycles of 2 steps consisting of 5 seconds at 95°C and 30 seconds at 60°C. The relative quantification of the mRNA levels of the target genes was determined using CFX Manager based on the ΔCT method (21). The amount of target was normalized to PPIA and GAPDH genes: ΔCT = CT(target gene) – CT (housekeeping gene), where CT represents the cycle number required to reach a defined threshold target abundance. The relative mRNA level was calculated as xΔCT (x = primer efficiency).
Statistical analysis
At least 4 different assays were performed per experiment. The differences among the groups were assessed by ANOVA. P < 0.05 was considered significant. Tukey’s honestly significant difference test was applied for comparison of means between groups. The values are expressed as mean ± sd.
RESULTS
Evaluation of the degree of HeLa cells synchronization
The MI of the asynchronous cells was 2.8 ± 0.5% (Table 1). There was a variation in MIs during progression through the cell cycle (Table 1). The duration of the cell cycle phases was in agreement with the recognized evaluation of the phases of the HeLa cell cycle (10, 8, 3, and 1 hour, for G1, S, G2, and M, respectively). Three hours after DTB release, the MI of the majority of cells in the S phase was 0.9 ± 0.5%. Eleven hours after DTB release, when the majority of cells were in G2/M phases (>85%), the MI increased to 19.5 ± 2.4%. The MI decreased to 1.9 ± 1.1% at 24 hours after DTB release with the majority cells in the G1 phase (55 ± 3.8%). Finally, we observed a faint increase of the MI (up to 4.7 ± 1.1%) 33 hours after DTB release, when 25 ± 2.3% of the cells were in G2/M phases. Our findings were in the agreement with the observation of others (22). The degree of the synchronization was sufficient for the analysis of the impact of S. aureus on host cell cycle.
TABLE 1.
Evaluation of the degree of HeLa cells synchronization
| Experimental conditions | G1% | S% | G2/M% | Mitotic index% |
|---|---|---|---|---|
| Asynchronous cells | 69 ± 6.4 | 17 ± 4.5 | 14 ± 3.8 | 2.8 ± 0.5 |
| Synchronous cells | ||||
| Time after DTB release (h) | ||||
| 3 | 2.6 ± 1.3 | 95.4 ± 3.9 | 2.0 ± 1.6 | 0.9 ± 0.5 |
| 6 | 6 ± 1.8 | 76 ± 7.2 | 18 ± 3.9 | 2.0 ± 1.4 |
| 9 | 10 ± 3.8 | 30 ± 4.5 | 40 ± 3.9 | 13.3 ± 2.1 |
| 11 | 7 ± 3.7 | 8 ± 3.5 | 85.3 ± 7.1 | 19.5 ± 2.4 |
| 13 | 45 ± 4.7 | 15 ± 1.8 | 40 ± 4.8 | 9.4 ± 2.3 |
| 16 | 75 ± 6.2 | 5 ± 1.4 | 20 ± 2.3 | 2.4 ± 1.6 |
| 18 | 55 ± 4.3 | 25 ± 2.8 | 20 ± 2.3 | 2.2 ± 1.6 |
| 21 | 50 ± 5.1 | 35 ± 3.4 | 15 ± 1.8 | 2.0 ± 1.4 |
| 24 | 55 ± 3.8 | 30 ± 2.8 | 15 ± 1.6 | 1.9 ± 1.1 |
| 27 | 55 ± 4.6 | 35 ± 4.1 | 10 ± 1.6 | 1.7 ± 0.9 |
| 30 | 57 ± 5.3 | 28 ± 2.6 | 20 ± 2.5 | 3.3 ± 1.6 |
| 33 | 50 ± 4.4 | 25 ± 2.9 | 25 ± 2.3 | 4.7 ± 1.1 |
| 36 | 65 ± 5.2 | 20 ± 1.8 | 15 ± 1.5 | 1.8 ± 1.2 |
Staphylococcus aureus culture supernatants modulate host cell cycle similarly to S. aureus bacteria
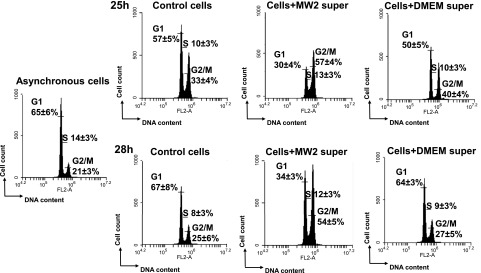
We previously showed that cells of strain S. aureus MW2 induced a G2/M phase transition delay. To verify whether the cells secreted the active molecules, concentrated MW2 culture supernatant was compared with concentrated DMEM for its capacity to effect the cell cycle. Exposure time was similar to the period of bacterial infection (11). After 25 hours of exposure of synchronized HeLa cells to concentrated MW2 supernatants, the percentage of cells in the G2/M phase was higher (57 ± 4%) compared with untreated cells (33 ± 4%), whereas only 40 ± 4% of HeLa cells exposed to concentrated DMEM were in the G2/M phase. This proportion was stable because the number of cells exposed to concentrated MW2 supernatants in the G2/M phase was still higher (54 ± 3%) after 3 h compared with untreated cells (25 ± 6%), where 27 ± 7% of the cells were exposed to concentrated DMEM (Fig. 1). This result suggests that the MW2 culture supernatant contains substances that interfere with the induction of G2/M transition.
Figure 1.
Culture supernatants of S. aureus MW2 strain induced G2/M transition delay. Synchronized HeLa cells were exposed either to MW2 supernatants or DMEM concentrated 10-fold. After 25 and 28 h of incubation, detached and adherent cells were fixed in 70% ethanol overnight, stained with PI, and analyzed by flow cytometry. Data were collected from 20,000 cells, and analysis was performed with CFlow software. The average percentage of cell cycle phase ± sd is indicated. The values of the 1 representative assay of the 4 is shown. Exposure of the cells to MW2 supernatant induced a G2/M phase transition delay in a time-dependent manner. *P < 0.05.
Detection of PSMα peptide derivatives in S. aureus supernatant chromatography fractions, which induce the G2/M phase transition delay
Concentrated MW2 culture supernatants and concentrated DMEM were fractionated using SEC. The fractions were pooled (2–4 fractions) and tested for their activities 28 hours post-treatment. The activities of S. aureus culture supernatant fractions were compared with those of DMEM for their capacity to alter the HeLa cell cycle. The pool of culture supernatants containing fractions 23–26 induced the strongest G2/M delay (53 ± 8% of cells), in contrast to the corresponding pool of DMEM fractions (22 ± 5% of cells; P < 0.05). The majority of the cells exposed to that fraction pool of the MW2 supernatant were still in the G2/M phase, whereas the cells exposed to the corresponding pool of DMEM fractions had completed the G2/M phase and progressed to the G1 phase (Fig. 2).
Figure 2.
The pool of chromatography fractions 23–26 of MW2 supernatant induces G2/M transition delay. Synchronized HeLa cells were exposed to 100 µl of size-exclusion chromatography fractions of concentrated MW2 supernatants or concentrated DMEM. After 25 hours, HeLa cells were analyzed by flow cytometery. The average percentage of HeLa cell cycle phase ± sd is indicated on the histograms.
We then analyzed fractions 23–26 by tandem mass spectrometry analysis. A number of molecules were detected in the active fractions compared with the concentrated DMEM control, including several peptides derived from those of the PSMα peptide family. The majority of these peptides were derivatives of PSMα1 or PSMα3. However, PSMα2 and PSMα4 derivatives were also identified (Table 2). Based on these results, we considered PSMα1 and PSMα3 as the most likely candidates responsible for the G2/M transition delay.
TABLE 2.
Detection of PSMα peptide derivatives in S. aureus chromatography fractions
| Uniprot entry | Gene name | Description | Peptide sequence | X!Tandem e-value | SEC Fractions identification |
|---|---|---|---|---|---|
| PSMA1_STAAW | psmA1 | Phenol-soluble modulin α 1 | GIIKVIKS | 1,7E-3 | 23 |
| PSMA1_STAAW | psmA1 | Phenol-soluble modulin α 1 | IIAGIIKV | 1,2E-2 | 23, 24 |
| PSMA1_STAAW | psmA1 | Phenol-soluble modulin α 1 | IIKVIKS | 1,8E-2 | 23, 24 |
| PSMA1_STAAW | psmA1 | Phenol-soluble modulin α 1 | LIEQFTGK | 1,1E-2 | 23, 24, 25 |
| PSMA1_STAAW | psmA1 | Phenol-soluble modulin α 1 | IIAGIIKVIKS | 3,2E-5 | 23, 25 |
| PSMA3_STAAW | psmA3 | Phenol-soluble modulin α 3 | FKDLLGKF | 8,8E-4 | 23, 24, 25 |
| PSMA3_STAAW | psmA3 | Phenol-soluble modulin α 3 | AKLFKF | 4,7E-2 | 24, 25, 26 |
| PSMA3_STAAW | psmA3 | Phenol-soluble modulin α 3 | FFKDLLGK | 3,6E-2 | 25 |
| PSMA3_STAAW | psmA3 | Phenol-soluble modulin α 3 | FVAKLF | 3,6E-2 | 25 |
| PSMA3_STAAW | psmA3 | Phenol-soluble modulin α 3 | FVAKLFKF | 4,7E-3 | 26 |
| PSMA2_STAAW | psmA2 | Phenol-soluble modulin α 2 | GIIKFIKG | 4,6E-2 | 23 |
| PSMA2_STAAW | psmA2 | Phenol-soluble modulin α 2 | IIAGIIKF | 1,1E-3 | 23 |
| PSMA2_STAAW | psmA2 | Phenol-soluble modulin α 2 | IIKFIKGL | 5,1E-3 | 23 |
| PSMA4_STAAW | psmA4 | Phenol-soluble modulin α 4 | IIDIFAK | 4,2E-4 | 23, 24, 26 |
| PSMA4_STAAW | psmA4 | Phenol-soluble modulin α 4 | IDIFAK | 8,2E-3 | 24 |
PSMα1 and PSMα3 alter HeLa cell cycles to a different extent
Synthetic PSMα1 and PSMα3 at concentrations ranging from 0.1 to 10 µg/ml were tested for their capacity to affect the cell cycle. Cytofluorometric analysis 25 hours after treatment revealed that PSMα1 and PSMα3 slowed down HeLa cell cycle progression. The untreated control cells completed the first cycle and progressed to the G2/M phase of the second cycle, whereas PSMα1- and PSMα3-treated (at concentrations of 0.1 and 1 µg/ml) cells progressed to the G1 phase of the first cell cycle (Table 3). This resulted in a lower percentage of PSM-treated cells in the G2/M phase compared with the untreated control. Increasing the concentration of PSMα1 to 10 µg/ml resulted in an even stronger effect: the cell cycle progression was greatly slowed down and the majority of the treated cells were in the G2/M phase of the first cycle. The percentage of host cells in the G2/M phase was twice as high as untreated cells (53 ± 6.6 vs. 18 ± 4.1%). PSMα3 showed lower activity compared with PSMα1 under the same conditions.
TABLE 3.
PSMα1 and PSMα3 induces the G2/M phase transition delay
| Experimental conditions | G1% | S% | G2/M% |
|---|---|---|---|
| Controla | 53 ± 7.9a | 29 ± 5.8a | 18 ± 4.1a |
| Cells + 0.1 µg/ml PSMα1 | 85 ± 9.8 | 9 ± 5.2 | 6 ± 3.8* |
| Cells + 1 µg/ml PSMα1 | 81 ± 9.7 | 9 ± 4.9 | 10 ± 4.9* |
| Cells + 10 µg/ml PSMα1 | 30 ± 5.6 | 17 ± 5.6 | 53 ± 6.6* |
| Cells + 0.1 µg/ml PSMα3 | 81 ± 8.6 | 14 ± 4.8 | 5 ± 4.1* |
| Cells + 1 µg/ml PSMα3 | 79 ± 7.9 | 12 ± 4.2 | 9 ± 4.8* |
| Cells + 10 µg/ml PSMα3 | 51 ± 6.8 | 18 ± 4.8 | 31 ± 4.1* |
aCells complete the first cell cycle and progress within the second cell cycle.
P < 0.05 vs. control.
Nevertheless, with PSMα3, the percentage of host cells in the G2/M phase was higher than that of untreated cells (31 ± 4.1 vs. 18 ± 4.1%).
PSMα1-induced G2/M transition delay is not related to the cytotoxic effect
To verify whether the observed G2/M phase transition delay was related to the cytotoxic effect of PSM treatment, the release of LDH was determined. LDH release from the HeLa cells treated with PSMα1 at concentrations of 0.1, 1, and 10 µg/ml or PSMα3 at concentrations of 0.1 and 1 µg/ml did not reveal a significant increase of LDH release. We observed a small increase in LDH release by cells exposed to 10 µg/ml of PSMα3 (Supplemental Fig. S1).
Isogenic USA300 mutant lacking the PSMα operon does not induce G2/M phase transition delay
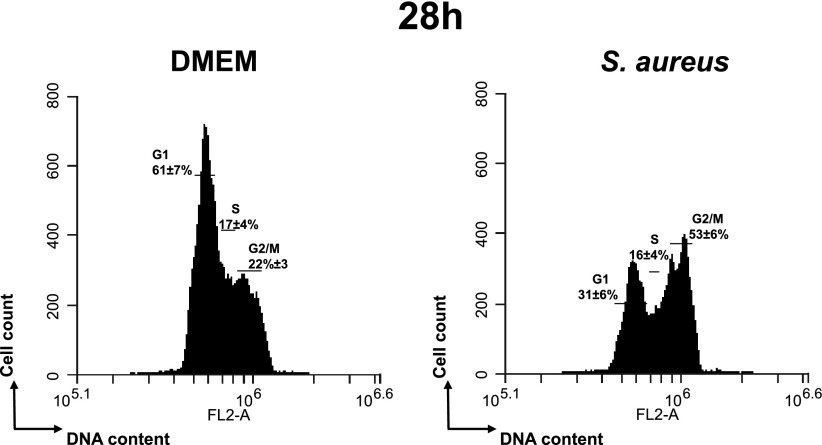
To further confirm the implication of PSMα in G2/M phase transition delay, the LAC WT strain and its isogenic mutant LAC∆psmα were compared for their capacity to alter the host cell cycle. The exposure of synchronized HeLa cells to increasing numbers of LAC WT cells (MOI ranging from 10:1 to 50:1) resulted in an increase in the percentage of the G2/M phase, whereas there were no effects on cell cycle phases between the isogenic mutant LAC∆psmα and the control cells (Table 4).
TABLE 4.
LAC∆psmα mutant does not induce the G2/M phase transition delay
| Experimental conditions | G1% | S% | G2/M% |
|---|---|---|---|
| Controla | 65 ± 10.2a | 19 ± 5.1a | 16 ± 4.3a |
| Cells+ LAC WT MOI 10:1 | 49 ± 9.4 | 20 ± 4.9 | 31 ± 5.1* |
| Cells+ LAC WT MOI 25:1 | 39 ± 7.1 | 25 ± 5.4 | 36 ± 5.3* |
| Cells+ LAC WT MOI 50:1 | 36 ± 6.4 | 24 ± 4.3 | 40 ± 6.1* |
| Cells+ LACΔpsm MOI 10:1 | 66 ± 8.3 | 15 ± 3.9 | 19 ± 3.9 |
| Cells+ LACΔpsm MOI 25:1 | 66 ± 7.9 | 14 ± 4.6 | 20 ± 4.3 |
| Cells+ LACΔpsm MOI 50:1 | 62 ± 8.2 | 15 ± 3.7 | 23 ± 4.2 |
aCells complete the first cell cycle and progress within the second cell cycle.
P < 0.05 vs. control.
Internalized LAC WT replicated inside HeLa cells in contrast to its isogenic LAC∆psmα mutant
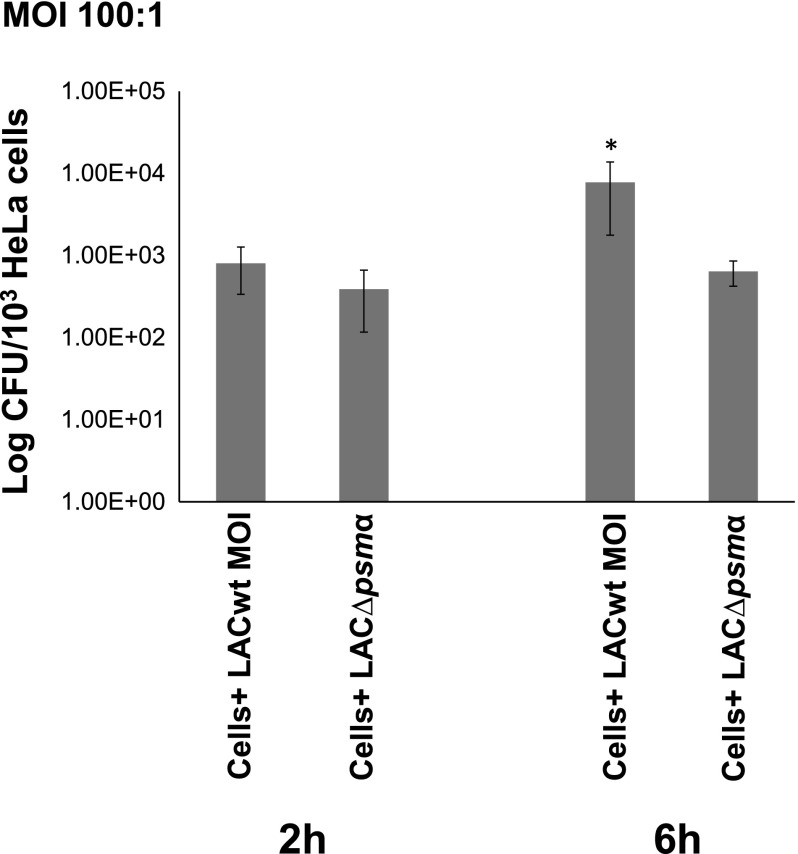
We previously showed (11) that the G2 phase is a prerequisite for the intracellular proliferation of internalized S. aureus. There was no difference between the CFU number of LAC WT and LAC∆psmα strains 2 hours after infection, indicating the equal number of internalized bacteria of both strains (Fig. 3). Given that PSMα peptides induce G2/M phase transition delay, we investigated whether they are responsible for the proliferation of internalized S. aureus inside of HeLa cells. Indeed, the CFUs of internalized LAC WT cells 6 hours after infection was 10-fold higher compared with LAC∆psmα. The CFU number 6 hours after infection was 6-fold higher than the value 2 hours after infection for LAC WT (P < 0.05), which is caused by its replication over time. In contrast, there was no statistically significant difference between the CFU number 2 and 6 hours after infection with LAC∆psmα, indicating that PSMα peptides not only induced cell cycle phase alteration, but also are responsible for bacterial intracellular proliferation.
Figure 3.
Replication of LAC WT strain internalized into HeLa cells. HeLa cells were infected with either LAC WT or its isogenic mutant LAC∆psmα at a multiplicity of infection of 100:1 for 1 hour in DMEM medium. HeLa cells were then incubated for 1 hour in DMEM supplemented with gentamicin. At T2 and T6 after infection, cells were lysed, and CFUs were determined. LAC WT strain replicates intracellularly because the CFU number of the LAC WT strain was increased 6 hours after infection. *P < 0.05. The data are presented as the logarithm of CFU/103 cells ± sd.
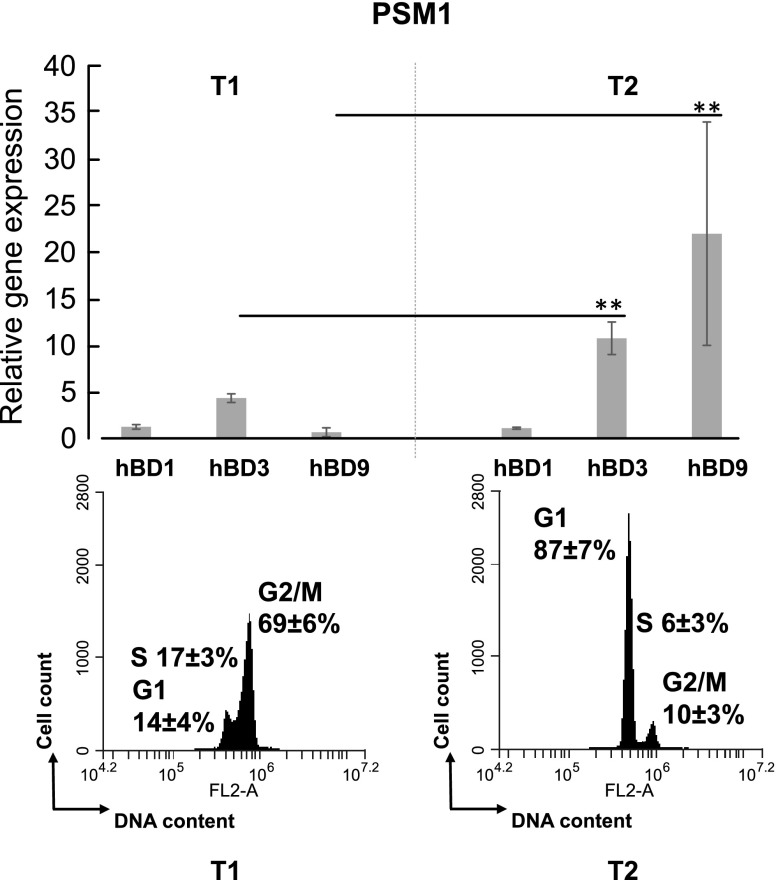
Level of PSMα-induced expression of hBDs depends on the cell cycle phase
Quantification of hBD-1, -3, and -9 expression was analyzed at the time when the majority of the synchronized cells were either in the G1 or in the G2/M phases. The expression of hBD-1 was constant at all times of the exposure of the cells either to PSMα1 or PSMα3 (Figs. 4 and 5). The expression of hBD-3 and -9 was 3.2 ± 0.5- and 10 ± 0.7-fold higher, respectively, by the cells in G1 phase (87 ± 7%) compared with the cells in the G2/M phase (69 ± 6%; Fig. 4).
Figure 4.
Analysis of mRNA levels of HBD-1, -3, and -9 in PSMα1-treated cells. Synchronized HeLa cells were treated with 1 µg/ml of PSMα1 and analyzed when the cells were mainly either in the G1 or G2/M phases. Defensin mRNA level was measured by RT-qPCR. Expression of all genes was normalized to the expression of housekeeping genes GAPDH and PPIA. *P < 0.01.
Figure 5.
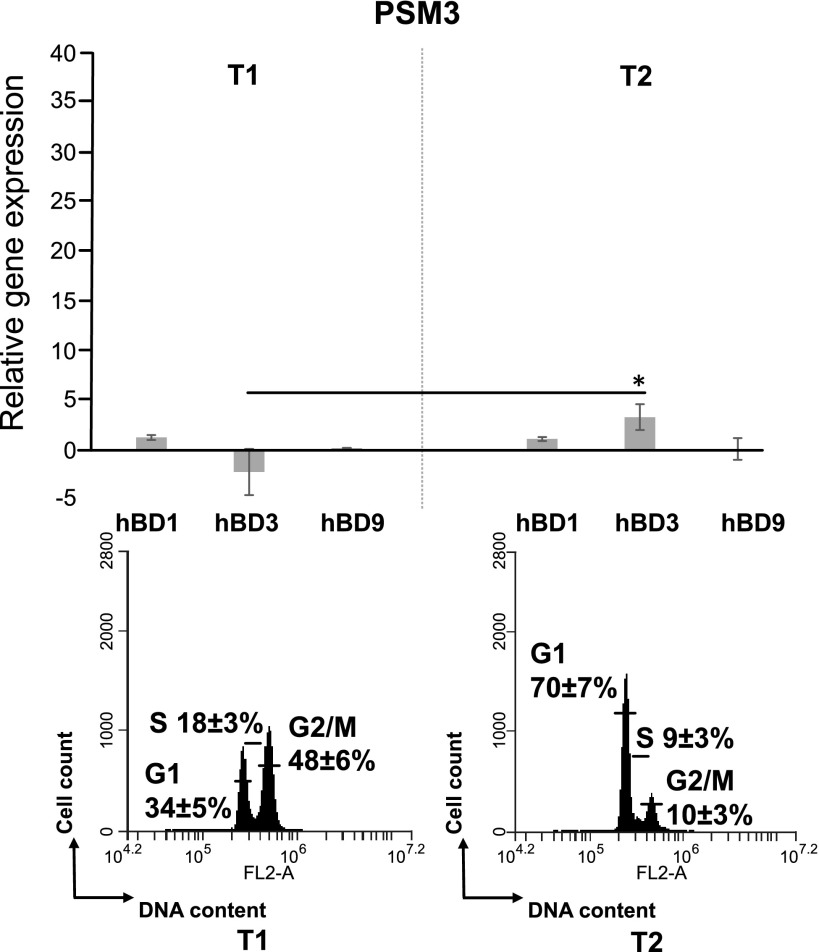
Analysis of mRNA levels of HBD-1, -3, and -9 in synchronized PSMα3-treated cells. Synchronized HeLa cells were treated with 1 µg/ml of PSMα3 and analyzed when the majority of the cells were either in the G1 or G2/M phases. Defensin mRNA level was measured by RT-qPCR. Gene expression was normalized to the expression of housekeeping genes GAPDH and PPIA. **P < 0.05.
We observed a faint decrease (1.7 ± 0.5-fold) of hBD-3 expression during PSMα3 treatment in the culture with 48 ± 6% of cells in the G2/M phase compared with those with the cells mainly in the G1 phase (70 ± 7%). There was no difference in hBD-9 expression, when the majority of the cells was either in the G1 or in the G2/M phase (Fig. 5).
Staphylococcus aureus clinical isolates are heterogeneous with respect to cell cycle delay activity
Thus far, we investigated the effect of S. aureus on cell cycles with clinical isolates that have been passaged extensively in laboratories. To understand whether the induction of cell cycle alteration is a common feature of S. aureus or is limited to certain strains, we tested the effect produced by freshly isolated clinical isolates.
The clinical isolate A980866 strongly delayed G2/M cell cycle transition, similar to MW2. Among HeLa cells that were exposed to concentrated A980866 supernatant for 20 hours, 86 ± 10.2% of cells were in the G2/M phase compared with 54 ± 8.3% in untreated cells and 10.4 ± 4.1% in cells exposed to concentrated DMEM (data not shown), whereas after 25 hours, 61 ± 8.1% of cells were in the G2/M phase compared with 29 ± 6.3% in untreated cells and 16 ± 5.4% in the culture exposed to concentrated DMEM (Table 5).
TABLE 5.
Cell cycle distribution of HeLa cells exposed to S. aureus clinical isolates
| Experimental conditions | G1% | S% | G2/M% |
|---|---|---|---|
| Controla | 52 ± 7.5a | 19 ± 3.8a | 29 ± 6.3a |
| DMEM | 68 ± 8.1 | 16 ± 3.6 | 16 ± 5.4 |
| Cells + A980866 strain | 32 ± 6.2 | 7 ± 3.5 | 61 ± 8.1* |
| Cells + DLY19990610 straina | 29 ± 5.2a | 16 ± 4.5a | 55 ± 7.8a,* |
| Cells + A970271 strain | 75 ± 7.4 | 12 ± 4.3 | 13 ± 4.6 |
| Cells + LY20000049 strain | 63 ± 6.6 | 19 ± 4.0 | 18 ± 3.9 |
aCells complete the first cell cycle and progress within the second cell cycle.
P < 0.05 vs. DMEM.
HeLa cell cycle phases were ∼10, 8, 3, and 1 hour, for G1, S, G2, and M phases, respectively. Because synchronized HeLa cells were exposed to A980866 supernatant 3 hours after the second DTB release, synchronizing cells in the G1/S border, the results suggest that HeLa cells did not complete the cell cycle but were delayed during the G2/M phase transition (Table 5).
In HeLa cells exposed to concentrated supernatant of another clinical strain, strain DLY19990610, for 20 hours, 63 ± 6.4% of cells were in the G1 phase and only 17 ± 3.1% were in the G2/M phase (data not shown), whereas 25 hours after infection, 55 ± 7.8% of HeLa cells were in G2/M compared with 29 ± 6.3% in untreated cells vs. 16 ± 5.4% in the culture exposed to concentrated DMEM . This suggests that HeLa cells exposed to DLY19990610 supernatant for 25 hours achieved a complete cycle and progressed to the G2/M phase of the second cell cycle.
The exposure of cells to the A970 271 or LY 200 000 49 supernatants did not induce the difference in cell cycle phase distribution (Table 5).
Supernatants of 4 clinical isolates were analyzed by reversed-phase HPLC coupled to tandem mass spectroscopy. The results of analysis showed that a small amount of PSMa1 was only detectable in A980866 supernatant that delay G2/M transition, whereas neither PSMa1 nor PSMa3 were detected in 3 other supernatants.
DISCUSSION
Pathogens have developed a large arsenal of strategies to subvert the host cell, such as the modulation of host cell apoptosis (23), the promotion of cell proliferation, and the inhibition of cell growth (24). Bacteria also have many virulence mechanisms that target the host cell cycle (9). Very few studies have described the capacity of S. aureus to modulate host cell cycle progression. It was shown that epidermal cell differentiation inhibitor affects keratinocyte differentiation (25) and that α-toxin increases the duration of S+G2/M phases (26).
We previously showed that S. aureus bacteria induce G2/M transition delay (11).
We demonstrated here that the capacity of S. aureus to alter the host cell cycle is caused by secreted products. SEC of S. aureus MW2 culture supernatant followed by mass spectrometry analysis of the active fractions revealed that the majority of the sequences of identified compounds in the biologically active fractions matched the sequence of peptides derived from PSMα1 and PSMα3. Consequently, we examined PSMα peptides as potential candidates to alter the host cell cycle.
PSMs have recently emerged as a novel toxin family that contributes to increased virulence and the spread and severity of S. aureus infection (27). PSMs stimulate inflammatory responses, are involved in biofilm development, and cause lysis of red and white blood cells (13). PSMs are divided into 2 groups, depending on their size: the short (20–25 amino acids) α-type peptides (PSMα1–PSMα4 and δ-toxin) and the long (44 amino acids) β-type peptides (PSMβ1 and PSMβ2). The PSMα peptide genes are grouped in 1 operon and apparently arose from gene duplication events (27). The production of PSMs is controlled by the accessory gene regulator (agr), a quorum sensing system that tightly controls virulence expression in S. aureus (6).
N-formylated synthetic PSMα1 and PSMα3 were tested for their capacity to alter the host cell cycle. With respect to cell cycle delay, PSMα1 was more active than PSMα3. This result was unexpected because PSMα3 has been reported to exert the strongest lytic activity against eukaryotic cells (6, 28) by far.
The decreased effect on G2/M transition delay of the internalized USA300Δpsmα mutant lacking the psmα operon further confirmed the involvement of PSMα on host cell cycle alteration. Our results suggest that PSMα act as a bacterial cyclomodulin, which would be a new property of PSMs. Cyclomodulins are a growing family of toxins that hijack the eukaryotic cell cycle (29). The role of cyclomodulins in bacterial pathogenicity is not well established thus far. However, the high prevalence of mucosa-associated E. coli that produce cyclomodulin and genotoxin in colon cancer has been described (30).
To verify the capacity to alter the host cell cycle by freshly isolated S. aureus strains and to determine whether G2/M transition delay is a common feature of S. aureus strains, we tested 4 human clinical isolates. A delayed G2/M phase transition was shown in only 1 clinical isolate and was correlated with the detection of PSMα1 in its supernatant. This result corroborated our findings with LAC WT and LAC∆psmα mutant strains.
It is worth mentioning that synthetic PSMα1 induced G2/M transition delay in HeLa cells at a concentration of 0.1 µg/ml, but a stronger effect was observed at a concentration of 10 µg/ml, suggesting a dose-dependent effect. PSMα1 concentration in the supernatant of the S. aureus clinical isolate was <0.1 µg/ml, although it induced a clear G2/M delay. At this level, we cannot exclude the presence of other molecules in bacterial supernatants that influence the host cell cycle, as has been shown with the multiplicity of E. coli cyclomodulins (29).
Some pathogens arrest the cells in a cell cycle phase, which is advantageous for their own life cycle. For example, it has been reported that the respiratory syncytial virus induces G1/G0 phase arrest of the airway cells and that this phase was favorable for virus production (31). Analysis of the proliferation of LAC WT and its isogenic mutant LAC∆psmα showed higher proliferation rates of internalized LAC WT. These results are in accordance with findings that demonstrated intracellular replication of USA300 LAC WT within human embryonic kidney 293 cells, in contrast to its LAC∆psmα mutant (20). On the one hand, PSMα causes a delay of the host cell cycle, which appears to be crucial for intracellular proliferation, and, on the other, PSMα plays a key role in intracellular replication. Consequently, the role of PSMα as a virulence factor is strengthened.
Upregulation of hBD-3 (32, 33) and downregulation of hBD-9 (32) were found in the S. aureus-infected skin (33) and in the ocular surface of the patients with bacterial keratitis (32). The hBD-3 expression was increased during the first day of the infection and then was constant within the following 2 days, as was observed in the human S. aureus-infected epidermal keratinocytes (12).
We show here that PSMα1 strongly induces hBD-3 and -9 expression in the G1 phase cells compared to the G2/M phase cells. In contrast, only faint difference in hBD-3 expression were observed between cells in the G1 phase and the G2/M phase in PSMα3-treated cells. This discrepancy between PSMα1 and PSMα3 treatments may be explained by the lower capacity of PSMα3 to induce the G2/M transition delay.
Given that hBDs expression was lower, when the cells were in the G2/M phase, it is likely that PSMα induces G2/M phase delay deteriorates antibacterial state of the epithelial surface and impairs the innate immune response during S. aureus infection.
In addition to the antimicrobial effect of hBD-3 within the innate immune system, it was shown that hBD3 is chemotactic for a broad spectrum of leukocytes, such as immature dendritic cells and T cells, monocytes, macrophages, and neutrophils, which are indispensable at the site of the infection (34). Thus, our data suggest that PSMα-induced host cell cycle alteration, which results in decreased hBD-3 expression, may impair an adaptive immune response during S. aureus infection.
In conclusion, our study shows that particularly PSMα1 interferes in various ways with host cell cycle and host cell response. PSMα1 induces G2/M phase transition delay, which prolongs the period of low hBD3 and -9 expression. Moreover, PSMα1 promotes intracellular proliferation of S. aureus in HeLa cells. This is a novel mechanistic strategy of how S. aureus is able to subvert cell cycle progression accompanied by decreased immune response.
Supplementary Material
Acknowledgments
The authors thank Gail Wagman and Mary Bret for revising the English. This work was supported by French National Institute for Agricultural Research (INRA), Ruminflame P10552 (to N.B., M.D., J.J., G.H., and Y.L.L.), the U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases' Intramural Research Program (to M.O.), and the German Research Foundation, SFB766 (to F.G.). R.A.E.-A.F. is a recipient of a Ph.D. fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Brazil), and L.A. is a recipient of a postdoctoral fellowship (France-Russia Bilateral Collaboration). The authors declare no conflicts of interest.
Glossary
- BHI
brain heart infusion
- CFU
colony-forming unit
- DTB
double thymidine block
- FCS
fetal calf serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- hBD
human β-defensin
- LDH
lactate dehydrogenase
- MI
mitotic index
- MOI
multiplicity of infection
- NCBI
National Center for Biotechnology Information
- PI
propidium iodide
- PPIA
peptidylproyl isomerase A
- PSMα
phenol-soluble modulin α
- SEC
size exclusion chromatography
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Edwards A. M., Massey R. C. (2011) How does Staphylococcus aureus escape the bloodstream? Trends Microbiol. 19, 184–190 [DOI] [PubMed] [Google Scholar]
- 2.Miller L. S., Cho J. S. (2011) Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 11, 505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casewell, M. W., Hill, R. L. (1986) The carrier state: methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 18(Suppl A), 1–12 [DOI] [PubMed]
- 4.Christie C. D., Lynch-Ballard E., Andiman W. A. (1988) Staphylococcal enterocolitis revisited: cytotoxic properties of Staphylococcus aureus from a neonate with enterocolitis. Pediatr. Infect. Dis. J. 7, 791–795 [PubMed] [Google Scholar]
- 5.Foster T. J., Geoghegan J. A., Ganesh V. K., Höök M. (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 12, 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Queck S. Y., Jameson-Lee M., Villaruz A. E., Bach T. H., Khan B. A., Sturdevant D. E., Ricklefs S. M., Li M., Otto M. (2008) RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32, 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto M. (2012) How Staphylococcus aureus breaches our skin to cause infection. J. Infect. Dis. 205, 1483–1485 [DOI] [PubMed] [Google Scholar]
- 8.Nikitas G., Deschamps C., Disson O., Niault T., Cossart P., Lecuit M. (2011) Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J. Exp. Med. 208, 2263–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nougayrède J. P., Homburg S., Taieb F., Boury M., Brzuszkiewicz E., Gottschalk G., Buchrieser C., Hacker J., Dobrindt U., Oswald E. (2006) Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851 [DOI] [PubMed] [Google Scholar]
- 10.Varon C., Mocan I., Mihi B., Péré-Védrenne C., Aboubacar A., Moraté C., Oleastro M., Doignon F., Laharie D., Mégraud F., Ménard A. (2014) Helicobacter pullorum cytolethal distending toxin targets vinculin and cortactin and triggers formation of lamellipodia in intestinal epithelial cells. J. Infect. Dis. 209, 588–599 [DOI] [PubMed] [Google Scholar]
- 11.Alekseeva L., Rault L., Almeida S., Legembre P., Edmond V., Azevedo V., Miyoshi A., Even S., Taieb F., Arlot-Bonnemains Y., Le Loir Y., Berkova N. (2013) Staphylococcus aureus-induced G2/M phase transition delay in host epithelial cells increases bacterial infective efficiency. PLoS ONE 8, e63279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park K., Ommori R., Imoto K., Asada H. (2014) Epidermal growth factor receptor inhibitors selectively inhibit the expressions of human β-defensins induced by Staphylococcus epidermidis. J. Dermatol. Sci. 75, 94–99 [DOI] [PubMed] [Google Scholar]
- 13.Wang R., Braughton K. R., Kretschmer D., Bach T. H., Queck S. Y., Li M., Kennedy A. D., Dorward D. W., Klebanoff S. J., Peschel A., DeLeo F. R., Otto M. (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514 [DOI] [PubMed] [Google Scholar]
- 14.Le Maréchal C., Jan G., Even S., McCulloch J. A., Azevedo V., Thiéry R., Vautor E., Le Loir Y. (2009) Development of serological proteome analysis of mastitis by Staphylococcus aureus in ewes. J. Microbiol. Methods 79, 131–136 [DOI] [PubMed] [Google Scholar]
- 15.Owen T. A., Soprano D. R., Soprano K. J. (1989) Analysis of the growth factor requirements for stimulation of WI-38 cells after extended periods of density-dependent growth arrest. J. Cell. Physiol. 139, 424–431 [DOI] [PubMed] [Google Scholar]
- 16.Tuchscherr L., Medina E., Hussain M., Völker W., Heitmann V., Niemann S., Holzinger D., Roth J., Proctor R. A., Becker K., Peters G., Löffler B. (2011) Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 3, 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surewaard B. G., Nijland R., Spaan A. N., Kruijtzer J. A., de Haas C. J., van Strijp J. A. (2012) Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog. 8, e1002606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig R., Beavis R. C. (2004) TANDEM: matching proteins with tandem mass spectra. Bioinformatics 20, 1466–1467 [DOI] [PubMed] [Google Scholar]
- 19.Alkuwaity K., Taylor A., Heckels J. E., Doran K. S., Christodoulides M. (2012) Group B Streptococcus interactions with human meningeal cells and astrocytes in vitro. PLoS ONE 7, e42660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosz M., Kolter J., Paprotka K., Winkler A. C., Schäfer D., Chatterjee S. S., Geiger T., Wolz C., Ohlsen K., Otto M., Rudel T., Sinha B., Fraunholz M. (2014) Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α. Cell. Microbiol. 16, 451–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 22.Cude K., Wang Y., Choi H. J., Hsuan S. L., Zhang H., Wang C. Y., Xia Z. (2007) Regulation of the G2-M cell cycle progression by the ERK5-NFkappaB signaling pathway. J. Cell Biol. 177, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkova N., Lair-Fulleringer S., Féménia F., Huet D., Wagner M. C., Gorna K., Tournier F., Ibrahim-Granet O., Guillot J., Chermette R., Boireau P., Latgé J. P. (2006) Aspergillus fumigatus conidia inhibit tumour necrosis factor- or staurosporine-induced apoptosis in epithelial cells. Int. Immunol. 18, 139–150 [DOI] [PubMed] [Google Scholar]
- 24.Bhavsar A. P., Guttman J. A., Finlay B. B. (2007) Manipulation of host-cell pathways by bacterial pathogens. Nature 449, 827–834 [DOI] [PubMed] [Google Scholar]
- 25.Sugai M., Hashimoto K., Kikuchi A., Inoue S., Okumura H., Matsumoto K., Goto Y., Ohgai H., Moriishi K., Syuto B., Yoshikawa K., Suginaka H., Takai Y. (1992) Epidermal cell differentiation inhibitor ADP-ribosylates small GTP-binding proteins and induces hyperplasia of epidermis. J. Biol. Chem. 267, 2600–2604 [PubMed] [Google Scholar]
- 26.Haugwitz U., Bobkiewicz W., Han S. R., Beckmann E., Veerachato G., Shaid S., Biehl S., Dersch K., Bhakdi S., Husmann M. (2006) Pore-forming Staphylococcus aureus alpha-toxin triggers epidermal growth factor receptor-dependent proliferation. Cell. Microbiol. 8, 1591–1600 [DOI] [PubMed] [Google Scholar]
- 27.Peschel A., Otto M. (2013) Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 11, 667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung G. Y., Duong A. C., Otto M. (2012) Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microbes Infect. 14, 380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nougayrède J. P., Taieb F., De Rycke J., Oswald E. (2005) Cyclomodulins: bacterial effectors that modulate the eukaryotic cell cycle. Trends Microbiol. 13, 103–110 [DOI] [PubMed] [Google Scholar]
- 30.Buc E., Dubois D., Sauvanet P., Raisch J., Delmas J., Darfeuille-Michaud A., Pezet D., Bonnet R. (2013) High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 8, e56964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W., Munday D. C., Howell G., Platt G., Barr J. N., Hiscox J. A. (2011) Characterization of the interaction between human respiratory syncytial virus and the cell cycle in continuous cell culture and primary human airway epithelial cells. J. Virol. 85, 10300–10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otri A. M., Mohammed I., Al-Aqaba M. A., Fares U., Peng C., Hopkinson A., Dua H. S. (2012) Variable expression of human Beta defensins 3 and 9 at the human ocular surface in infectious keratitis. Invest. Ophthalmol. Vis. Sci. 53, 757–761 [DOI] [PubMed] [Google Scholar]
- 33.Zanger P., Holzer J., Schleucher R., Scherbaum H., Schittek B., Gabrysch S. (2010) Severity of Staphylococcus aureus infection of the skin is associated with inducibility of human β-defensin 3 but not human β-defensin 2. Infect. Immun. 78, 3112–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Röhrl J., Yang D., Oppenheim J. J., Hehlgans T. (2010) Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J. Immunol. 184, 6688–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.