Abstract
A multimodality system combining a clinical prototype digital breast tomosynthesis with its imaging geometry modified to facilitate near-infrared spectroscopic imaging has been developed. The accuracy of parameters recovered from near-infrared spectroscopy is dependent on fibroglandular tissue content. Hence, in this study, volumetric estimates of fibroglandular tissue from tomosynthesis reconstructions were determined. A kernel-based fuzzy c-means algorithm was implemented to segment tomosynthesis reconstructed slices in order to estimate fibroglandular content and to provide anatomic priors for near-infrared spectroscopy. This algorithm was used to determine volumetric breast density (VBD), defined as the ratio of fibroglandular tissue volume to the total breast volume, expressed as percentage, from 62 tomosynthesis reconstructions of 34 study participants. For a subset of study participants who subsequently underwent mammography, VBD from mammography matched for subject, breast laterality and mammographic view was quantified using commercial software and statistically analyzed to determine if it differed from tomosynthesis. Summary statistics of the VBD from all study participants were compared with prior independent studies. The fibroglandular volume from tomosynthesis and mammography were not statistically different (p=0.211, paired t-test). After accounting for the compressed breast thickness, which were different between tomosynthesis and mammography, the VBD from tomosynthesis was correlated with (r =0.809, p<0.001), did not statistically differ from (p>0.99, paired t-test), and was linearly related to, the VBD from mammography. Summary statistics of the VBD from tomosynthesis were not statistically different from prior studies using high-resolution dedicated breast computed tomography. The observation of correlation and linear association in VBD between mammography and tomosynthesis suggests that breast density associated risk measures determined for mammography are translatable to tomosynthesis. Accounting for compressed breast thickness is important when it differs between the two modalities. The fibroglandular volume from tomosynthesis reconstructions is similar to mammography indicating suitability for use during near-infrared spectroscopy.
Keywords: Breast [A01.236], Spectroscopy, Near-Infrared [E01.370.350.750], Mammography [E01.370.350.700.500], Digital Breast Tomosynthesis, Breast Density
I. INTRODUCTION
Digital breast tomosynthesis (DBT) is a limited-angle x-ray tomographic technique that can reduce tissue superposition (Niklason et al., 1997, Suryanarayanan et al., 2000, Suryanarayanan et al., 2001). In DBT, multiple projection views of the compressed breast are acquired and reconstructed to provide quasi-three-dimensional (3D) image data. The reconstructed breast volumes are viewed along planes parallel to the detector and are often referred to as “slices” during interpretation. For clarity, the term “slice” in a DBT reconstructed volume is used to represent a plane parallel to the detector. Prospective clinical trials (Skaane et al., 2013, Ciatto et al., 2013) in screening populations have shown that DBT in combination with full-field digital mammography (FFDM) can significantly reduce false-positive rates and increase cancer detection, particularly for invasive disease. Retrospective observational studies (Rose et al., 2013, Friedewald et al., 2014) have also shown a significant decrease in false-positive rate with the combined DBT and FFDM screen. Several multi-reader multi-case retrospective studies (Rafferty et al., 2013, Wallis et al., 2012, Gennaro et al., 2013, Svahn et al., 2012) have shown that DBT improves performance measures, such as the area under the receiver operating characteristic curve (AUC). However, DBT provides only anatomical images.
Near Infrared Spectroscopy (NIRS) can measure several physiological parameters noninvasively such as hemoglobin concentration, oxygen saturation, water, and lipid content without the use of exogenous contrast agents. Malignant tumors are expected to exhibit higher hemoglobin concentration due to angiogenesis associated neovascularity, and lower oxygen saturation due to higher metabolism in cancers. NIRS alone exhibits poor spatial resolution. In the absence of any prior knowledge on tissue distribution, i.e., when NIRS is used as a stand-alone modality, its limited spatial resolution (>1 cm) resulted in an AUC for discriminating breast cancer from normal tissue of 0.67 (Poplack et al., 2007). For a subgroup of patients with lesions larger than 6 mm, the AUC improved to 0.88 (Poplack et al., 2007). When anatomical priors are provided from another imaging modality, substantial improvements in NIRS spatial resolution have been reported enabling visualization of abnormalities in the millimeter range (Dehghani et al., 2003, Pogue et al., 2006, Fang et al., 2009) and increasing the accuracy of recovered optical properties (Brooksby et al., 2005, Brooksby et al., 2006, Carpenter et al., 2007).
The combination of DBT and NIRS in a single imaging platform would retain the benefits of DBT in terms of reduced false-positive rates and increased cancer detection, while improving discrimination of carcinoma from benign lesions through the addition of NIRS. The concept of integrating NIRS with DBT was first described by researchers from Massachusetts General Hospital (Li et al., 2003, Boverman et al., 2007, Fang et al., 2011). We are investigating the potential of DBT-guided NIRS using a simpler NIRS configuration (Vedantham et al., 2011, Krishnaswamy et al., 2012, Michaelsen et al., 2012, Michaelsen et al., 2014). In the study by Fang et al, hemoglobin concentration determined from NIRS was correlated with fibroglandular tissue content in the breast (Fang et al., 2011). Hence, in addition to providing spatial priors for NIRS image reconstruction, determining fibroglandular volume is important for estimating hemoglobin concentration, accurately. Thus, segmenting DBT reconstructions not only provides spatial priors for use during NIRS image reconstruction, but also determines fibroglandular content which improves the accuracy of hemoglobin concentration estimates from NIRS.
To achieve DBT segmentation, an algorithm previously described (Vedantham et al., 2011) has been refined to eliminate intermediate steps that did not alter the segmentation results. Relative to extracting spatial priors, DBT segmentations were discretized into 3-D meshes with tetrahedral elements for finite element method analyses (Mimics®, Materialise NV, Leuven, Belgium). Simulation studies showed that meshes with tetrahedral elements containing 30,000 nodes were sufficient for accurate NIRS parameter recovery without substantially increasing computation time (Michaelsen, 2014). Hence, the DBT segmentations were down-sampled to 20% prior to mesh generation, and yielded suitably resolved discretizations of approximately 35,000 nodes. The spacing between the nodes of the tetrahedral elements was approximately 0.25 cm, resulting in a volume of approximately 1.84 mm3. In this work, we focused on the other important task of determining fibroglandular volume from DBT using full-resolution data (i.e., prior to down-sampling), and used these results as a framework for validating the DBT segmentation method.
Results from DBT segmentation offer other potential benefits as well, and could be used to estimate breast density quantitatively. Several studies (Saftlas et al., 1991, Boyd et al., 1995, Vachon et al., 2007, Greenwood et al., 2011) have suggested an association between breast density and breast cancer risk. A meta-analysis (McCormack and dos Santos Silva, 2006) observed that the relative risk was ~4.7 for women with >75% fibroglandular content compared to those with <5% fibroglandular content. Along with female sex, age and genetic or syndromic predisposition, breast density is amongst the most important cancer risk factors (Veronesi et al., 2005). Recognizing the significance of breast density as a risk factor and the fact that the sensitivity of mammography is lower for women with dense breasts (Kolb et al., 2002), some states in the USA now require that a woman undergoing screening mammography must be informed of her breast density.
Methods involving commercial and non-commercial software are available for determining breast density from FFDM. Given that at least one vendor has received regulatory approval from the United States Food and Drug Administration for the clinical use of a two-dimensional (2-D) synthetic mammogram (C-View™, Hologic Inc., Bedford, MA) generated from data acquired during DBT, one approach would be to estimate breast density from such a synthesized mammogram. However, the algorithm used for generating the synthesized mammogram is vendor-specific and may have an effect on the quantitative estimate. A few studies have reported on breast density associated measures from DBT. Bakic et al found that an area-based breast density (ABD) from the central projection of the DBT acquisition was correlated with the ABD from FFDM (Bakic et al., 2009). In a subsequent study, Kontos et al (Kontos et al., 2011) extracted six parenchymal texture features from the FFDM and from the central plane parallel to the detector (central slice) of the reconstructed DBT breast volumes. With respect to the ABD, texture features from the DBT central slice were better correlated than the texture features from FFDM. Tagliafico et al determined the average ABD from all DBT projections using two algorithms and observed that the ABDs from the DBT projections differed from FFDM (Tagliafico et al., 2012). A subsequent study compared the average ABD from all DBT projections, the average ABD from MRI on a per slice basis, and the ABD from FFDM, and noted that the estimate from FFDM significantly differed from DBT and MRI, while the estimates from DBT and MRI were similar (Tagliafico et al., 2013a). When ABD was computed on a per slice basis from DBT reconstructions and averaged, it also differed from the ABD from FFDM (Tagliafico et al., 2013b). A prospective case-control study with 1,100 subjects showed that breast cancer risk is more accurately predicted when volumetric estimates of breast density rather ABDs are used in conjunction with other known risk factors (Shepherd et al., 2011). Additionally, a recent study found that volumetric breast density (VBD) was more reliable than ABD (Alonzo-Proulx et al., 2015).
Hence, quantitatively determining the volumetric breast density (VBD) appears to be important, is readily accomplished with our DBT segmentation algorithm, and is another important aspect of this work. VBD is defined as the ratio of the fibroglandular volume to the total breast volume excluding the skin and was determined from the reconstructed breast volume. DBT reconstructions, rather than projections, were chosen for segmentation to facilitate widespread adaptation of the algorithm as the projection images may have manufacturer-specific formats. Currently available commercial software for determining VBD from DBT requires projection images. Also, an algorithm utilizing DBT reconstructions would facilitate retrospective clinical studies, as the projection images are often not archived. Hence, in this study we report on the quantitative estimates of fibroglandular volume and VBD determined using the developed algorithm (Vedantham et al., 2011) from DBT reconstructed breast volumes of women who participated in a clinical study of DBT-guided NIRS. A subset of study participants underwent routine annual (12-month) follow-up FFDM, subsequent to the DBT-guided NIRS exam. For these study participants, fibroglandular volume and VBD were determined using a US FDA-approved tool (Quantra™, Hologic Inc., Bedford, MA) for comparative analysis.
Thus, the study seeks to determine if the fibroglandular volume determined from DBT reconstructions is similar to estimates obtained from FFDM for the cohort of women who underwent both DBT-guided NIRS and subsequent annual FFDM follow-up exams, and provides a form of validation of the DBT segmentation as a way of determining fibroglandular content. Since the DBT and FFDM data were acquired on two different imaging platforms with differing compressed breast thickness, the statistical association in VBD between DBT and FFDM was determined, and then VBD from all participants in the DBT-guided NIRS exams was compared with values from prior independent studies to investigate the reliability of the DBT segmentation method.
II. METHODS AND MATERIALS
II.a. Human Subjects
All study participants underwent the combined multimodality DBT-guided NIRS imaging in adherence to an Institutional Review Board (IRB)-approved, Hospital Insurance Portability and Accountability Act (HIPAA)-compliant protocol at the Breast Imaging Center, Dartmouth-Hitchcock Medical Center, Lebanon, NH. Written informed consent was obtained from all study participants.
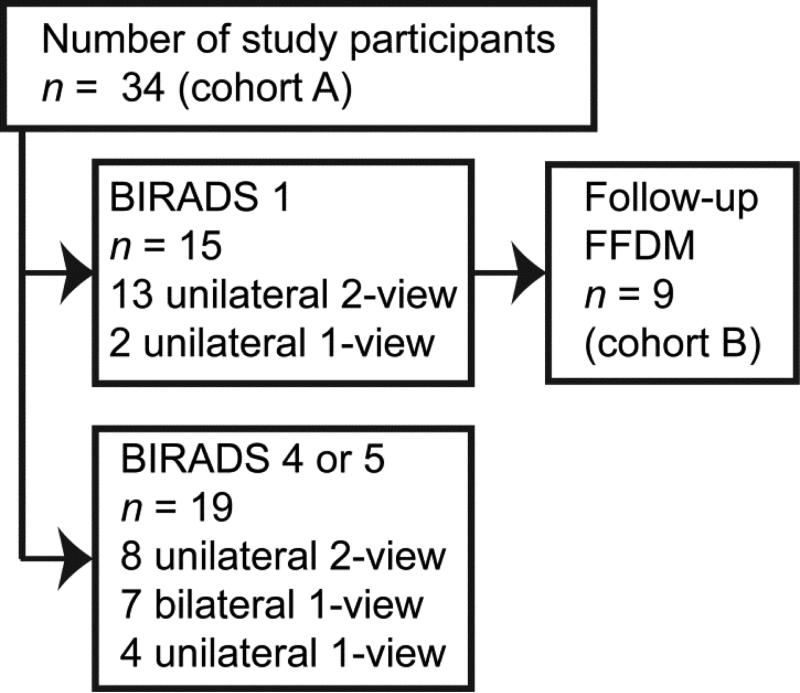
Cohort A corresponds to all study participants (n = 34) who underwent DBT-NIRS imaging (Figure 1). Fifteen of the study participants were assigned American College of Radiology (ACR) Breast-Imaging Reporting and Data System (BIRADS®) diagnostic assessment category 1 (“negative” or normal), and the remainder (n = 19) were assigned BIRADS® diagnostic assessment categories 4 or 5 that correspond to “suspicious finding” or “highly suggestive of malignancy”, respectively, and require tissue sampling (biopsy). In order to minimize the radiation dose to study participants, the number of views and orientations (craniocaudal, CC or mediolateral oblique, MLO) for each subject was limited to meet research objectives, such as evaluating the consistency in NIRS derived parameters for 2 views of the same breast, and determining differences between ipsilateral and contralateral breasts in the same view. A total of 41 CC-views and 21-MLO views of 41 breasts from these 34 study participants was available for analysis. None of the study participants underwent a MLO-view alone.
Figure 1.
Distribution of imaging views among study participants
Quantra™ (Hologic Inc., Bedford, MA) requires DICOM “for processing” images from FFDM to compute VBD. As is customary at most institutions, the DICOM “for processing” images from FFDM acquired at the time of DBT-guided NIRS exam were not archived; only the DICOM “for presentation” images were saved. Hence, for a subset of study participants (cohort B: n = 9 subjects) that returned at 12 months following their DBT-guided NIRS exam for their annual mammogram (Selenia®, Hologic Inc., Bedford, MA), the DICOM “for processing” mammograms were used to determine VBD using Quantra™. For these 9 study participants, a total of 17 DBT reconstructions was acquired at the time of DBT-guided NIRS imaging: 6 unilateral 2-view (CC and MLO), 2 bilateral 1-view (CC alone), and 1 unilateral 1-view (CC alone) DBT-NIRS imaging. VBD estimates from mammograms acquired at matched laterality and orientation to the DBT reconstructions were used for comparative analysis. A schematic of the study groups is shown in Figure 1.
II.b. System Description and Imaging Protocol
The study utilized a prototype DBT system (Genesis, Hologic Inc., Bedford, MA) that was modified for NIRS imaging. Detailed description of the NIRS imaging component and its integration with the DBT system has been reported (Krishnaswamy et al., 2012). The prototype DBT unit uses concentric motion of x-ray tube and detector assembly with source-to-detector distance of 66 cm. The axis of rotation (AOR) is located 58.4 cm from the focal spot and 11 projections are acquired spanning an angular range of ±7.5° referenced to the AOR. In order to accommodate the NIRS detector panel, a 3.1 cm thick NIRS detector housing is positioned on top of the breast support. This addition results in a slight magnification for DBT imaging. Study participants underwent DBT-guided NIRS imaging with the breast supported on the NIRS detector housing. DBT and NIRS imaging was performed sequentially without altering patient positioning and compression. During DBT imaging, the NIRS detector panel was removed and the technique factors for acquisition were manually selected using manufacturer provided charts. After completion of the DBT acquisition, the NIRS detector panel was inserted within its housing and the NIR data was acquired using a raster scan of the breast. The acquired DBT projections were reconstructed using filtered back-projection (FBP) provided with the system to a nominal slice thickness of 1 mm and in-plane pixel size of approximately 100 μm. A photograph of the system along with the coordinates is shown in Figure 2. Thus, the reconstructed slices viewed by the radiologists correspond to (x, y) planes. The anonymized DBT reconstructions were transferred to the researchers at the University of Massachusetts Medical Center for segmentation and classification to generate anatomic priors for NIRS reconstruction. The segmented DBT reconstructions were used to compute the VBD from DBT.
Figure 2.
Photographs of the developed DBT-guided NIRS system with the imaging coordinates labeled. The slices viewed by the radiologist correspond to x-y planes.
II.c. Image preparation
Prior to segmenting the reconstructed DBT volume, several image preparation steps were necessary due to the use of an earlier-generation clinical prototype system. For a compressed breast thickness of CBTT in mm, the DBT system reconstructed ⌈CBTT⌉+ 31 slices, each 1 mm thick, where ⌈CBTT⌉ represents the compressed breast thickness rounded-up (ceiling function) to the nearest integer and the 31 slices correspond to the 31 mm thick NIRS detector housing positioned on top of the breast support. These 31 slices were removed prior to segmentation. The sequence of image processing steps is shown in Figure 3. On occasion, detector artifacts were observed in the reconstructed image volume (arrow 1 in Figure 3a, x-y plane). Also, the plastic screws of the NIRS detector housing were present within the DBT reconstructed field-of-view (FOV) located at the posterior and lateral edges of the image (arrow 2 in Figure 3a). These artifacts were removed from each slice by thresholding and area filtering (Figure 3b). If the plastic screw was within the reconstructed breast volume, which occurred often in MLO views, then the location corresponding to the screw was assigned the surrounding tissue type (often pectoralis muscle) post segmentation. The posterior edge of the NIRS detector housing also fell within the DBT FOV (arrow 3 in Figure 3b) and was manually identified and removed (Figure 3c). Since the posterior aspect of the breast seldom contains fibroglandular tissue, the breast region corresponding to Figure 3c was assigned as non-fibroglandular tissue (Figure 3d). The remainder of the breast region (Figure 3e) was used for segmentation. All of the aforementioned steps could be averted with redesign of the NIRS housing panel to be outside the DBT imaging FOV, and with a more robust detector.
Figure 3.
Illustration of the steps used for segmenting DBT reconstructions. Prior to segmentation, each slice (A; x-y plane) was corrected for detector artifacts (arrow 1), if present, and plastic screws from the NIRS detector housing (arrow 2). These artifacts were removed from each slice by thresholding and area filtering (B). The posterior edge of the NIRS detector housing fell within the DBT field-of-view (arrow 3 in B) and was manually identified and removed (C). Post-segmentation, the removed region was added back as adipose tissue (D). After repeating this process for all slices, a bilateral filter was applied in x-z plane (not shown) to provide the slice (E). The skin layer (F) was determined from the central slice and removed from each slice to provide the breast region excluding the skin (G). A kernel-based fuzzy c-means method was used to segment each slice into adipose (I) and non-adipose (H) regions.
II.d. DBT segmentation
In reconstructed DBT breast volume, the skin along the breast periphery had higher intensity than adipose tissue due to its higher linear attenuation coefficient. To avoid classification of skin as fibroglandular tissue, the skin thickness (ts, in mm) was determined as the average full-width at half-maximum of the gradient image computed along the medial-lateral direction from the central slice. The estimated ts from the central slice was used to identify the skin layer (Figure 3f) followed by its removal through isotropic erosion in each slice (Figure 3g). The estimated skin layer thickness was rounded-up to the nearest integer (⌈ts⌉), and ⌈ts⌉ slices in contact with the NIRS detector housing and compression paddle were removed from the breast volume. Thus, the breast volume used for segmentation excluded the skin. Upon completing these steps for all slices, the breast volume was preprocessed using an edge-preserving bilateral filter (Chen et al., 2007, Paris et al., 2009) with angular constraints (Vedantham et al., 2011) applied in the x-z planes. This result is not shown in Figure 3 as the slice used for interpretation corresponded to the x-y plane that is parallel to the detector, whereas the filter was applied in x-z planes.
After bilateral filtering the breast volume, an automated kernel-based fuzzy C-means (KFCM) clustering algorithm was used to segment the breast volume on a per-slice basis. Compared to the conventional fuzzy C-means (FCM) method, KFCM is more robust to noise and incomplete data (Wu and Yang, 2002, Zhang and Chen, 2003, Hathaway et al., 2005). The KFCM algorithm segmented the breast into two clusters: non-adipose (Figure 3h) and adipose (Figure 3i) tissues. If the pectoralis muscle was absent in the FOV (occurs only in CC views) then the segmented non-adipose tissue region was classified as fibroglandular tissue. Figure 3 is an example of such a case.
If the pectoralis muscle was present in the FOV and was separated from the fibroglandular region by adipose tissue, then a geographical area filter was applied to segment and classify the pectoralis muscle for expediency. In CC views, this geographical area filter was centered laterally and at the posterior aspect of the breast. In MLO views, the geographical area filter was positioned at the region corresponding to the axilla (Figure 4). The remainder of the non-adipose tissue region was classified as fibroglandular tissue. If the pectoralis muscle was connected to fibroglandular tissue (Figure 5), then a second iteration of KFCM was applied to the segmented non-adipose region to separate fibroglandular tissue from pectoralis muscle.
Figure 4.
Illustration of the method used for segmenting pectoralis muscle when it is separated from fibroglandular region. After preprocessing with a bilateral filter, the skin layer from a slice (A) from MLO-view DBT reconstruction is removed to provide (B) for segmenting into adipose (C) and non-adipose (D) regions. A geographical area filter is applied to the non-adipose region to provide fibroglandular region (E) and pectoralis muscle (F). VBD computation excludes the skin and the pectoralis muscle.
Figure 5.
Illustration of the method used for segmenting pectoralis muscle when it is connected to the fibroglandular region. After preprocessing and skin removal, the slice (A) is segmented into adipose (not shown) and non-adipose (B) regions. A second iteration of KFCM is applied to the non-adipose region to segment fibroglandular tissue (not shown) and pectoralis muscle (C). VBD computation excludes the skin and the pectoralis muscle.
II.e. VBD computation
The voxel size in each reconstructed slice was dependent on its distance from the detector and this information was provided by the manufacturer. For each slice, the fibroglandular volume within that slice was estimated from the voxel size and the segmented fibroglandular region (example, Figure 3h). The fibroglandular volume for the entire breast was computed by summing the fibroglandular volume from each slice. For each slice, the breast volume within that slice was determined from the voxel size and by summing the segmented regions corresponding to adipose and fibroglandular tissue (for example, regions corresponding to Figures 3d, 3h and 3i). When pectoralis muscle is present (for example, in Figures 4 and 5) then the segmented region corresponding to the pectoralis muscle was excluded from the breast volume estimate. The total breast volume was obtained by summing the breast volume from each slice. The VBD was determined as the ratio of fibroglandular volume for the entire breast to the total breast volume and was expressed in percentage. Thus, the VBD determined in this study excludes the skin and the pectoralis muscle, when present.
II.f. Statistical Methods
For cohort A, the VBD estimated from DBT reconstructions was compared with VBD from independent prior studies. For cohort B, the compressed breast thickness (CBTM) recorded in the FFDM DICOM image headers as well as estimates of the total breast volume (TBVM), the fibroglandular volume (GVM), and the volumetric breast density (VBDM) provided by Quantra were compared with the corresponding estimates from DBT, represented by the subscript “T”. Depending upon whether the data satisfied normality assumptions, appropriate parametric and nonparametric tests were used for statistical analysis. All data were analyzed either using SAS® (version 9.3, SAS Institute Inc., Cary, NC) or GraphPad Prism® (version 6.05, GraphPad Software, Inc., La Jolla, CA). All tests were two-tailed and effects associated with p < 0.05 were considered statistically significant.
III. RESULTS
III.a. Comparison between FFDM and DBT
For clarity, results of the paired comparison between FFDM and DBT that corresponds to cohort B are presented first, as this analysis permits validation of the method used to estimate VBDT. Summary statistics of compressed breast thickness, fibroglandular volume, total breast volume and VBD from FFDM and DBT reconstructions are provided in Table 1. The difference between FFDM and DBT for each of these metrics satisfied the normality assumption (p >0.109, Shapiro-Wilk's test).
Table 1.
Summary statistics and comparison of compressed breast thickness (CBT), fibroglandular volume (GV), total breast volume (TBV) and volumetric breast density (VBD) from FFDM (subscript ‘M’) and DBT (subscript ‘T’) reconstructions. The data were matched for study participant, breast laterality and view (n =17 DBT reconstructions). The paired data were statistically analyzed in terms of Pearson correlation coefficient (r) and paired t-tests.
| Metric (units) | Mean | Standard Deviation | Minimum | 1st Quartile | Median | 3rd Quartile | Maximum |
|---|---|---|---|---|---|---|---|
| CBTM (cm) | 5.6 | 0.8 | 4.4 | 5.1 | 5.7 | 6.1 | 7.1 |
| CBTT (cm) | 5.0 | 0.9 | 2.9 | 4.4 | 5.2 | 5.4 | 6.7 |
| CBTM – CBTT (cm) | 0.7 ± 1.05, p =0.014; r =0.27, p =0.302 | ||||||
| TBVM (cm3) | 690.5 | 303.3 | 227.7 | 497.9 | 624.0 | 869.9 | 1241.1 |
| TBVT (cm3) | 507.6 | 185.2 | 241.8 | 357.5 | 516.9 | 669.2 | 841.4 |
| TBVM – TBVT (cm3) | 196.3 ± 163, p <0.001; r =0.89, p <0.001 | ||||||
| GVM (cm3) | 85.5 | 32.9 | 28.0 | 71.2 | 80.0 | 107.9 | 144.2 |
| GVT (cm3) | 75.9 | 27.2 | 28.8 | 61.6 | 79.1 | 95.9 | 118.3 |
| GVM – GVT (cm3) | 9.65 ± 30.5, p =0.211; r =0.498, p =0.042 | ||||||
| VBDM (%) | 13.2 | 4.0 | 6.8 | 10.0 | 12.5 | 16.1 | 21.1 |
| VBDT (%) | 15.7 | 4.2 | 9.6 | 13.0 | 14.9 | 16.1 | 27.6 |
| VBDM – VBDT (%) | −2.46 ± 4.19, p =0.028; r =0.471, p =0.056 | ||||||
After matching for study participant, breast laterality and view, the fibroglandular volume from FFDM (GVM) provided by Quantra was statistically correlated (Pearson's r = 0.498, p = 0.042) with that from DBT (GVT) determined using the algorithm. Paired t-test indicated that the GVM and the GVT were not statistically different (p =0.211), with the GVM being slightly larger than the GVT (mean difference: 9.6 mm3).
After matching for study participant, breast laterality and view, the compressed breast thickness recorded by FFDM (CBTM) was not statistically correlated (Pearson's r = 0.266, p =0.302) with that recorded by DBT after correcting for the 31 mm thick NIRS detector housing (CBTT). Paired t-test indicated that the CBTM statistically differed (p =0.014) from the CBTT, with the CBTM being larger than the CBTT (mean difference: 0.7 mm). Consequently, the total breast volume from FFDM (TBVM) provided by Quantra also statistically differed (p <0.001, paired t-test) from the total breast volume from DBT (TBVT). The TBVM was larger than the TBVT (mean difference: 196.3 mm3).
The volumetric breast density from FFDM (VBDM) provided by Quantra was marginally correlated (Pearson's r = 0.471, p =0.056) with that from DBT (VBDT) determined using the algorithm. The VBDM expressed in percent, was significantly different (p =0.028, paired t-test) and lower than VBDT (mean difference: −2.46%), as the TBVM was different than TBVT.
In order to account for the differing compressed breast thickness between the modalities (DBT and FFDM) and as a consequence the estimated total breast volume, multivariable linear regression was used to model the association between VBDM and VBDT, with step-wise selection of parameters that statistically differed between FFDM and DBT as possible covariates. Thus, the compressed breast thickness and the total breast volume from both FFDM and DBT were considered. Since linear regression is sensitive to outliers, studentized residuals and Cook's D were assessed, which identified 2 (out of 17) outliers and were excluded. These 2 outliers corresponded to DBT reconstructions with substantial detector artifacts that could not be completely corrected. With VBDT as the outcome, step-wise selection resulted in a statistically significant model (p =0.008, ANOVA) with VBDM (p =0.004), CBTT (p =0.007) and CBTM (p =0.048) as predictors. VBDT was related to VBDM as per equation (1):
| (1) |
where, a = −13.9649514, b = 0.7320357, c = 1.97264191, and d = 1.7686961. However, the additive model given in equation (1) does not impose non-negativity constraint for VBDT. Hence, linear regression using log-transformed variables was used to provide a multiplicative model that was statistically significant (p =0.0008) and is shown in equation (2).
| (2) |
where, the fit parameters k0 = 5.8818068×10−2, k1 = 1.1651628 and k2 = 0.6454340. All predictors were statistically significant (p <0.0132). After adjusting for the compressed breast thickness (using equation 2), the model estimated VBD, represented as VBDT, was determined. Figure 6 shows the plot of VBDM provided by Quantra vs. VBDT. VBDT was statistically correlated with VBDM (Pearson's r =0.843, p <0.001) and was not statistically different (p =0.9473, paired t-test).
Figure 6.
Scatter plot of the VBD from mammography and the VBD from DBT reconstructions after accounting for compressed breast thickness.
III.b. Comparison of VBDT with independent prior studies
The VBDT from all study participants (cohort A) for each breast were used for comparison with prior studies. A total of 41 CC-views and 21-MLO views of 41 breasts was available from these 34 study participants. For the 21 study participants who underwent CC and MLO views of the same breast during DBT-guided NIRS imaging, the VBDT was analyzed to determine if a statistical difference occurred between the 2-views. The difference in VBDT between CC and MLO views of the same breast satisfied the normality assumption (p =0.193, Shapiro-Wilks test). Paired t-test indicated that the VBDT from CC-view was not statistically different (p =0.243) from MLO-view, and hence averaged. Summary statistics of the VBDT from CC-views (n =41 breasts), MLO-views (n =21 breasts), all DBT reconstructions (n =62), and the estimate for each breast (n =41 breasts) from all study participants (cohort A) are provided in Table 2. Summary statistics derived from VBDT for each breast were compared with prior independent studies of VBD using fully 3-D, high-resolution dedicated breast CT (Nelson et al., 2008, Yaffe et al., 2009, Vedantham et al., 2012). The results from the analysis (unpaired t-test with Welch's correction) are summarized in Table 3. To account for the multiple comparisons, Sidak correction was applied, and hence, effects associated with p <0.017 were considered statistically significant. The analysis indicated that the VBDT from this study was not statistically different from prior independent studies reporting VBD from dedicated breast CT.
Table 2.
Summary statistics of VBDT (in %) from all CC-views (n =41 breasts), all MLO-views (n =21 breasts), all views (n =62), and from all breasts (n =41).
| Mean | Standard Deviation | Minimum | 1st Quartile | Median | 3rd Quartile | Maximum | |
|---|---|---|---|---|---|---|---|
| CC-views | 17.04 | 4.40 | 9.75 | 14.31 | 16.11 | 19.38 | 27.8 |
| MLO-views | 15.30 | 5.21 | 7.59 | 11.94 | 13 | 18.7 | 24.75 |
| All views | 16.45 | 4.72 | 7.59 | 13 | 15.235 | 19.38 | 27.8 |
| All breasts | 16.62 | 4.29 | 9.75 | 13.72 | 15.34 | 19.33 | 27.8 |
Table 3.
Comparison of VBD (in %) from this study using DBT reconstructions with prior studies using dedicated breast CT (Yaffe et al., 2009, Nelson et al., 2008, Vedantham et al., 2012). Summary data were compared using unpaired t-test with Welch's correction (2-tailed). To account for the multiple comparisons, Sidak correction was applied. Hence, effects associated with p <0.017 were considered statistically significant. (SD – standard deviation).
| Mean ± SD | p -value | Difference between means | |
|---|---|---|---|
| Comparison of VBD (in %) | |||
| This study | 16.6 ± 4.30 | ||
| Nelson et al., 2008 | 17.1 ± 15.2 | 0.769 | 0.48 ± 1.6 |
| Yaffe et al. 2009 (Group A) | 14.3 ± 10.3 | 0.022 | −2.32 ± 1.0 |
| Vedantham et al. 2012 | 15.8 ± 13.0 | 0.528 | −0.82 ± 1.3 |
IV. DISCUSSION
The study did not observe a significant difference in fibroglandular volume between DBT and FFDM as most of the fibroglandular tissue was concentrated at the retro-areolar and central breast regions, which were included in the imaged FOV with both DBT and FFDM. This indicates suitability of the method for providing the fibroglandular volume during NIRS reconstruction. The study observed statistically significant differences in compressed breast thickness and total breast volume between DBT and FFDM, with the estimates being higher with FFDM. The thick detector assembly of the prototype DBT/NIRS system (~19 cm inclusive of NIRS detector housing) impeded proper positioning of the study participants. This restriction reduced inclusion of the posterior aspect of the breast in the imaged FOV and contributed to a reduction in compressed breast thickness and total breast volume. Consequently, the VBDM was lower than the VBDT. However, after accounting for the compressed breast thicknesses in DBT and FFDM, the VBDT was correlated with, and linearly related to, VBDM. Hence, breast density related risk estimates developed for mammography can be applied readily for VBDT determined from DBT reconstructions using the method investigated. At present, most of the clinical knowledge is based on the combined DBT+FFDM exam, where projection data for DBT and the digital mammogram are acquired under the same compression. However, this result in approximate doubling of the radiation dose compared to DBT imaging alone (Sechopoulos et al., 2007, Feng and Sechopoulos, 2012, Vedantham et al., 2015). One approach to reduce the radiation dose is to replace the FFDM with the synthesized mammogram generated from data acquired for DBT. To our knowledge, no prior studies have demonstrated agreement in quantitative breast density between a synthesized mammogram and FFDM. Also, systems capable of DBT imaging alone have been developed (Wallis et al., 2012). While the VBD can be estimated from DBT projections, these images can be in vendor-specific format posing a challenge for widespread use. The method investigated here relies on DBT reconstructions and not projections, which would allow for retrospective studies investigating the association between quantitative breast density and risk factors with imaging performance. Hence, it is of clinical importance in addressing the current needs as well as facilitating retrospective studies.
Equation (2) can be written as: . If the breast compression is maintained the same during DBT and FFDM acquisition, i.e., CBTT = CBTM = CBT then equation (2) simplifies to: VBDT = k0 VBDM (CBT)k1+k2. Clinical systems capable of DBT and mammographic acquisitions under the same compression are currently available and could provide a platform for future validation.
With regard to comparison with prior studies, the choice of dedicated breast CT needs clarification. Since we validated the VBDT with FFDM, and dedicated breast CT provides higher resolution than breast MRI (Vedantham et al., 2014, O'Connell et al., 2014), VBD determined using breast CT was chosen for comparison. Also, both DBT and breast CT attempt to reconstruct the linear attenuation coefficients thus providing a direct comparison of a physical measure based breast density estimate. One study using breast MRI reported that the quantitative estimate of density was dependent on the image acquisition sequence (Tagliafico et al., 2014).
Additionally, the skin thickness (ts) determined from DBT reconstructions was compared with prior independent studies using dedicated breast CT (Huang et al., 2008, Shi et al., 2013). The mean ± standard deviation of ts determined from DBT was 1.78 ± 0.09 mm (n =41 breasts). Compared to the study by Huang et al. (Huang et al., 2008), the mean skin thickness statistically differed (p <0.001) and on average the ts from DBT was higher by 0.33 ± 0.04 mm. Compared to the study by Shi et al. (Shi et al., 2013), the ts from DBT statistically differed (p <0.001) and on average the ts from DBT was higher by 0.34 ± 0.03 mm. Importantly, ts estimated by x-ray imaging modalities includes the combined thickness of epidermis and dermis and excludes subcutaneous fat, as it is often indistinguishable from adipose tissue within the breast. A slight overestimation of ts was expected with DBT, partly due to the curvature of the breast at its periphery and due to the nominal slice thickness of 1 mm.
In terms of developing an algorithm for segmenting and generating anatomic (structural) priors from DBT for use during NIRS reconstruction, these observations suggest that the fibroglandular volume determined from DBT reconstructions is similar to FFDM. Evaluation of concordance in radiologists’ marking of fibroglandular regions with algorithm-segmented regions in the central DBT reconstructed slice using metrics such as dice ratio and overlap ratio are currently ongoing. We did not statistically analyze the association between radiologists’ assignment of BI-RADS® breast density categories with the VBD, as data existed from only 34 study participants. Importantly, the BI-RADS density assignment is based on bilateral assessment and corresponds to the denser of the two breasts, if they differ. Since only 7 of the 34 study participants had undergone bilateral DBT-guided NIRS exams, we did not perform this analysis. Future work will investigate the association between BI-RADS density assignment and VBD from DBT.
In this study, we focused on the analysis of fibroglandular volume and VBD from DBT reconstructions obtained during DBT-guided NIRS imaging. Results from radiologists’ interpretation of the combined DBT-guided NIRS exam and its association with pathological results and/or follow-up imaging exams are ongoing and will be reported in future.
CONCLUSIONS
The primary conclusion from this study is that the fibroglandular volume determined from DBT reconstructions was similar to that from FFDM, indicating the suitability of the segmentation algorithm for DBT-guided NIRS. Additional, key observations from this study were the linear association in VBD between DBT and FFDM, and the significant correlation between VBDs from DBT and FFDM, after accounting for compressed breast thickness. Hence, mammographic breast density based risk estimates are applicable to DBT. This finding is of clinical importance as DBT continues its transition to breast cancer screening. The observation that the summary statistics of VBD from DBT reconstructions was similar to prior studies using high-resolution dedicated breast CT indicates that the method described here could be suitable for population studies investigating the association between breast density, breast cancer risk, and imaging performance. The analysis also underscored the importance of accounting for compressed breast thickness when it differs between the two modalities.
ACKNOWLEDGMENTS
The authors thank Chris Ruth, PhD and Yiheng Zhang, PhD from Hologic Inc., for providing information pertaining to the geometry of the prototype DBT system and for computing the breast density and associated parameters using Quantra™. This work was supported in part by National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R01 CA139449 and R21 CA176470. The contents are solely the responsibility of the authors and do not reflect the official views of the NIH or the NCI. The NIRS/DBT imaging system described in this paper is not US FDA approved, and was used experimentally under IRB-approved protocols at Dartmouth. Preliminary and partial contents of this work were presented at the 56th Annual Meeting of the American Association of Physicists in Medicine (AAPM), Austin, TX, 2014.
Footnotes
Publisher's Disclaimer: “This is an author-created, un-copyedited version of an article accepted for publication in Biomedical Physics & Engineering Express. The publisher is not responsible for any errors or omissions in this version of the manuscript or any version derived from it. The Version of Record is available online at http://dx.doi.org/10.1088/2057-1976/1/4/045202”
Disclosures/Conflicts: All other authors have no conflicts related to this study. The NIRS/DBT imaging system described in this paper is not US FDA approved, and was used experimentally under IRB-approved protocols at Dartmouth.
REFERENCES
- Alonzo-Proulx O, Mawdsley GE, Patrie JT, Yaffe MJ, Harvey JA. Reliability of automated breast density measurements. Radiology. 2015;275:366–76. doi: 10.1148/radiol.15141686. [DOI] [PubMed] [Google Scholar]
- Bakic PR, Carton AK, Kontos D, Zhang C, Troxel AB, Maidment AD. Breast percent density: estimation on digital mammograms and central tomosynthesis projections. Radiology. 2009;252:40–9. doi: 10.1148/radiol.2521081621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boverman G, Fang Q, Carp SA, Miller EL, Brooks DH, Selb J, Moore RH, Kopans DB, Boas DA. Spatio-temporal imaging of the hemoglobin in the compressed breast with diffuse optical tomography. Phys Med Biol. 2007;52:3619–41. doi: 10.1088/0031-9155/52/12/018. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–5. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- Brooksby B, Jiang S, Dehghani H, Pogue BW, Paulsen KD, Weaver JB, Kogel C, Poplack SP. Combining near-infrared tomography and magnetic resonance imaging to study in vivo breast tissue: implementation of a Laplacian-type regularization to incorporate magnetic resonance structure. J. Biomed. Opt. 2005;10:051504. doi: 10.1117/1.2098627. [DOI] [PubMed] [Google Scholar]
- Brooksby B, et al. Imaging breast adipose and fibroglandular tissue molecular signatures by using hybrid MRI-guided near-infrared spectral tomography. Proc Natl Acad Sci U S A. 2006;103:8828–33. doi: 10.1073/pnas.0509636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CM, et al. Image-guided optical spectroscopy provides molecular-specific information in vivo: MRI-guided spectroscopy of breast cancer hemoglobin, water, and scatterer size. Opt Lett. 2007;32:933–5. doi: 10.1364/ol.32.000933. [DOI] [PubMed] [Google Scholar]
- Chen J, Paris S, Durand F. Real-time edge-aware image processing with the bilateral grid. ACM Transactions on Graphics - Proceedings of ACM SIGGRAPH. 2007;2007:26. [Google Scholar]
- Ciatto S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14:583–9. doi: 10.1016/S1470-2045(13)70134-7. [DOI] [PubMed] [Google Scholar]
- Dehghani H, Pogue BW, Shudong J, Brooksby B, Paulsen KD. Three-dimensional optical tomography: resolution in small-object imaging. Applied optics. 2003;42:3117–28. doi: 10.1364/ao.42.003117. [DOI] [PubMed] [Google Scholar]
- Fang Q, Selb J, Carp SA, Boverman G, Miller EL, Brooks DH, Moore RH, Kopans DB, Boas DA. Combined optical and X-ray tomosynthesis breast imaging. Radiology. 2011;258:89–97. doi: 10.1148/radiol.10082176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang QQ, et al. Combined Optical Imaging and Mammography of the Healthy Breast: Optical Contrast Derived From Breast Structure and Compression. IEEE Transactions on Medical Imaging. 2009;28:30–42. doi: 10.1109/TMI.2008.925082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng SS, Sechopoulos I. Clinical digital breast tomosynthesis system: dosimetric characterization. Radiology. 2012;263:35–42. doi: 10.1148/radiol.11111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald SM, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311:2499–507. doi: 10.1001/jama.2014.6095. [DOI] [PubMed] [Google Scholar]
- Gennaro G, et al. Combination of one-view digital breast tomosynthesis with one-view digital mammography versus standard two-view digital mammography: per lesion analysis. Eur Radiol. 2013;23:2087–94. doi: 10.1007/s00330-013-2831-0. [DOI] [PubMed] [Google Scholar]
- Greenwood CM, et al. A genome-wide linkage study of mammographic density, a risk factor for breast cancer. Breast Cancer Res. 2011;13:R132. doi: 10.1186/bcr3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway RJ, Huband JM, Bezdek JC. Kernelized Non-Euclidean Relational Fuzzy c-Means Algorithm.. Proc. 14th International Conference on Fuzzy Systems; Reno, NV.. 2005.pp. 414–419. [Google Scholar]
- Huang SY, Boone JM, Yang K, Kwan AL, Packard NJ. The effect of skin thickness determined using breast CT on mammographic dosimetry. Med Phys. 2008;35:1199–206. doi: 10.1118/1.2841938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–75. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- Kontos D, Ikejimba LC, Bakic PR, Troxel AB, Conant EF, Maidment AD. Analysis of parenchymal texture with digital breast tomosynthesis: comparison with digital mammography and implications for cancer risk assessment. Radiology. 2011;261:80–91. doi: 10.1148/radiol.11100966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy V, Michaelsen KE, Pogue BW, Poplack SP, Shaw I, Defrietas K, Brooks K, Paulsen KD. A digital x-ray tomosynthesis coupled near infrared spectral tomography system for dual-modality breast imaging. Opt Express. 2012;20:19125–36. doi: 10.1364/OE.20.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, et al. Tomographic optical breast imaging guided by three-dimensional mammography. Appl Opt. 2003;42:5181–90. doi: 10.1364/ao.42.005181. [DOI] [PubMed] [Google Scholar]
- Mccormack VA, Dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- Michaelsen K, Krishnaswamy V, Pogue BW, Poplack SP, Paulsen KD. Near-infrared spectral tomography integrated with digital breast tomosynthesis: effects of tissue scattering on optical data acquisition design. Med Phys. 2012;39:4579–87. doi: 10.1118/1.4728228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen KE. PhD. Dartmouth College; 2014. Combined Digital Breast Tomosynthesis and Near-Infrared Spectral Tomography for Breast Lesion Characterization. [Google Scholar]
- Michaelsen KE, Krishnaswamy V, Poplack SP, Shi L, Vedantham S, Pogue BW, Paulsen KD. Digital Breast Tomosynthesis Guided Near-Infrared Spectral Tomography: Early Clinical Results. Biomedical Optics 2014, 2014. Optical Society of America,Vol. OSA Technical Digest (online) BM3A. 78 [Google Scholar]
- Nelson TR, Cervino LI, Boone JM, Lindfors KK. Classification of breast computed tomography data. Med Phys. 2008;35:1078–86. doi: 10.1118/1.2839439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklason LT, et al. Digital tomosynthesis in breast imaging. Radiology. 1997;205:399–406. doi: 10.1148/radiology.205.2.9356620. [DOI] [PubMed] [Google Scholar]
- O'connell AM, Karellas A, Vedantham S. The potential role of dedicated 3D breast CT as a diagnostic tool: review and early clinical examples. Breast J. 2014;20:592–605. doi: 10.1111/tbj.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S, Kornprobst P, Tumblin J, Durand F. Bilateral Filtering: Theory and Applications. Foundations and Trends in Computer Graphics and Vision. 2009;4:1–73. [Google Scholar]
- Pogue BW, Davis SC, Song X, Brooksby BA, Dehghani H, Paulsen KD. Image analysis methods for diffuse optical tomography. J. Biomed. Opt. 2006;11:033001. doi: 10.1117/1.2209908. [DOI] [PubMed] [Google Scholar]
- Poplack SP, et al. Electromagnetic breast imaging: results of a pilot study in women with abnormal mammograms. Radiology. 2007;243:350–9. doi: 10.1148/radiol.2432060286. [DOI] [PubMed] [Google Scholar]
- Rafferty EA, Park JM, Philpotts LE, Poplack SP, Sumkin JH, Halpern EF, Niklason LT. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology. 2013;266:104–13. doi: 10.1148/radiol.12120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R., Jr. Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am J Roentgenol. 2013;200:1401–8. doi: 10.2214/AJR.12.9672. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Hoover RN, Brinton LA, Szklo M, Olson DR, Salane M, Wolfe JN. Mammographic densities and risk of breast cancer. Cancer. 1991;67:2833–8. doi: 10.1002/1097-0142(19910601)67:11<2833::aid-cncr2820671121>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sechopoulos I, Suryanarayanan S, Vedantham S, D'orsi CJ, Karellas A. Computation of the glandular radiation dose in digital tomosynthesis of the breast. Med Phys. 2007;34:221–32. doi: 10.1118/1.2400836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, Malkov S, Vittinghoff E, Cummings SR. Volume of mammographic density and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1473–82. doi: 10.1158/1055-9965.EPI-10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Vedantham S, Karellas A, O'connell AM. Technical Note: Skin thickness measurements using high-resolution flat-panel cone-beam dedicated breast CT. Med Phys. 2013;40:031913. doi: 10.1118/1.4793257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaane P, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267:47–56. doi: 10.1148/radiol.12121373. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S, Karellas A, Vedantham S, Baker SP, Glick SJ, D'orsi CJ, Webber RL. Evaluation of linear and nonlinear tomosynthetic reconstruction methods in digital mammography. Acad Radiol. 2001;8:219–24. doi: 10.1016/S1076-6332(03)80530-5. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S, Karellas A, Vedantham S, Glick SJ, D'orsi CJ, Baker SP, Webber RL. Comparison of tomosynthesis methods used with digital mammography. Acad Radiol. 2000;7:1085–97. doi: 10.1016/s1076-6332(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Svahn TM, Chakraborty DP, Ikeda D, Zackrisson S, Do Y, Mattsson S, Andersson I. Breast tomosynthesis and digital mammography: a comparison of diagnostic accuracy. Br J Radiol. 2012;85:e1074–82. doi: 10.1259/bjr/53282892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliafico A, et al. Breast density assessment using a 3T MRI system: comparison among different sequences. PLoS One. 2014;9:e99027. doi: 10.1371/journal.pone.0099027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliafico A, Tagliafico G, Astengo D, Airaldi S, Calabrese M, Houssami N. Comparative estimation of percentage breast tissue density for digital mammography, digital breast tomosynthesis, and magnetic resonance imaging. Breast Cancer Res Treat. 2013a;138:311–7. doi: 10.1007/s10549-013-2419-z. [DOI] [PubMed] [Google Scholar]
- Tagliafico A, Tagliafico G, Astengo D, Cavagnetto F, Rosasco R, Rescinito G, Monetti F, Calabrese M. Mammographic density estimation: one-to-one comparison of digital mammography and digital breast tomosynthesis using fully automated software. Eur Radiol. 2012;22:1265–70. doi: 10.1007/s00330-012-2380-y. [DOI] [PubMed] [Google Scholar]
- Tagliafico AS, Tagliafico G, Cavagnetto F, Calabrese M, Houssami N. Estimation of percentage breast tissue density: comparison between digital mammography (2D full field digital mammography) and digital breast tomosynthesis according to different BI-RADS categories. Br J Radiol. 2013b;86:20130255. doi: 10.1259/bjr.20130255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Sellers TA. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:43–9. doi: 10.1158/1055-9965.EPI-06-0738. [DOI] [PubMed] [Google Scholar]
- Vedantham S, Karellas A, Vijayaraghavan GR, Kopans DB. Digital Breast Tomosynthesis: State-of-the-art. Radiology. 2015 doi: 10.1148/radiol.2015141303. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham S, O'connell AM, Shi L, Karellas A, Huston AJ, Skinner KA. Dedicated Breast CT: Feasibility for Monitoring Neoadjuvant Chemotherapy Treatment. J Clin Imaging Sci. 2014;4:64. doi: 10.4103/2156-7514.145867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham S, Shi L, Karellas A, Michaelsen KE, Krishnaswamy V, Pogue BW, Paulsen KD. Semi-automated segmentation and classification of digital breast tomosynthesis reconstructed images. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6188–91. doi: 10.1109/IEMBS.2011.6091528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham S, Shi L, Karellas A, O'connell AM. Dedicated breast CT: fibroglandular volume measurements in a diagnostic population. Med Phys. 2012;39:7317–28. doi: 10.1118/1.4765050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast cancer. Lancet. 2005;365:1727–41. doi: 10.1016/S0140-6736(05)66546-4. [DOI] [PubMed] [Google Scholar]
- Wallis MG, Moa E, Zanca F, Leifland K, Danielsson M. Two-view and single-view tomosynthesis versus full-field digital mammography: high-resolution X-ray imaging observer study. Radiology. 2012;262:788–96. doi: 10.1148/radiol.11103514. [DOI] [PubMed] [Google Scholar]
- Wu KL, Yang MS. Alternative c-means clustering algorithms. Pattern Recognition. 2002;35:2267–2278. [Google Scholar]
- Yaffe MJ, Boone JM, Packard N, Alonzo-Proulx O, Huang SY, Peressotti CL, Al-Mayah A, Brock K. The myth of the 50-50 breast. Med Phys. 2009;36:5437–43. doi: 10.1118/1.3250863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Chen SC. Clustering incomplete data using kernel-based fuzzy C-means algorithm. Neural Processing Letters. 2003;18:155–162. [Google Scholar]