Abstract
Naltrexone/bupropion ER (Contrave): newly approved treatment option for chronic weight management in obese adults
INTRODUCTION
Obesity is becoming an increasingly serious and costly health problem in the United States, with more than one-third of adults (34.9%) and 17% of children suffering from this condition.1 Obesity is defined as having a body mass index (BMI) greater than 30, while being overweight is defined as having a BMI between 25 and 29.9.2 Besides being a psychological burden on the patient, obesity is associated with increased risk of stroke, cardiovascular disease, type-2 diabetes, and the development of certain types of cancers, including esophageal, pancreatic, colon, and breast cancers.3,4 Obesity represents a huge burden to the U.S. health care system—$147 billion was spent on obesity in 2008 alone. Health care costs for obese patients were on average $1,429 higher than for patients of normal weight.5 The physiological, psychological, and financial effects associated with obesity have pushed health care professionals to come up with appropriate solutions to manage obesity and achieve better health outcomes.
The Food and Drug Administration (FDA) has recently added to the effort to curb obesity with the approval of medications for chronic weight management, including phentermine/topiramate extended release (Qsymia, VIVUS, Inc.), lorcaserin (Belviq, Eisai, Inc.), and naltrexone/bupropion extended release (Contrave, Orexigen Therapeutics, Inc.). Contrave’s two active components, naltrexone and bupropion, are both approved by the FDA (naltrexone to treat alcohol and opioid dependence, and bupropion to treat depression and seasonal affective disorder and as an aid to smoking cessation treatment). Initially, Contrave was rejected by the FDA in February 2011 because of the need for long-term clinical trials evaluating the cardiovascular effects of the drug.6 Contrave was finally approved in September 2014 after subsequent clinical trials demonstrated its safety and efficacy.7
CHEMICAL PROPERTIES8
Contrave is an extended-release (ER), fixed-dose combination product containing naltrexone, an opioid antagonist, and bupropion, an aminoketone antidepressant, with chemical formulas of morphinan-6-one, 17-(cyclopropylmethyl)4,5-epoxy-3,14-dihydroxy-, hydrochloride, (5α)- and (±)-1-(3chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propranone hydrochloride, respectively. It is available as a trilayer, film-coated, blue, round, biconvex, ER tablet debossed with NB-890 on one side and smooth on the other. Each tablet has a trilayer core composed of two drug layers containing 8 mg of naltrexone, 90 mg of bupropion, and excipients, which are separated by a rapid-dissolving inert layer. Inactive ingredients include edetate disodium, hydroxypropyl cellulose, microcrystal-line cellulose, lactose anhydrous, magnesium stearate, L-cysteine hydrochloride, hypromellose, lactose monohydrate, crospovidone, Opadry II Blue, FD&C Blue #2 Aluminum Lake, and colloidal silicon dioxide. The structures of both drugs are illustrated in Figure 1.
Figure 1.
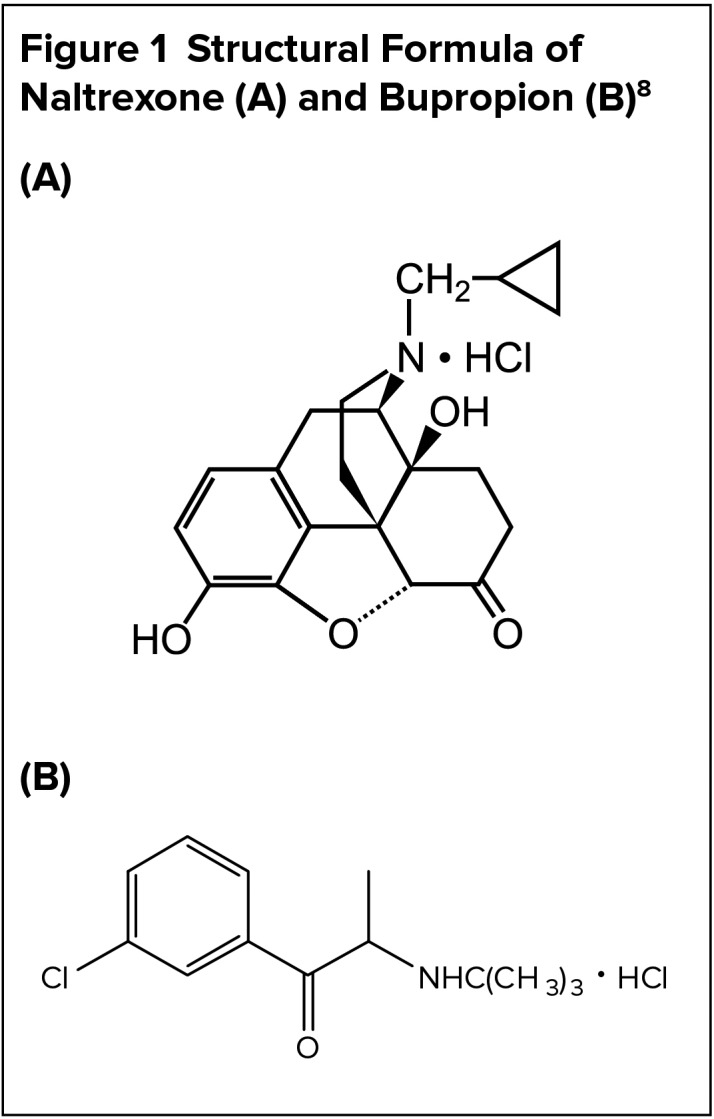
Structural Formula of Naltrexone (A) and Bupropion (B)8
MECHANISM OF ACTION
The exact neurochemical mechanism of the naltrexone/bupropion combination leading to weight loss is not fully understood.8 However, from preclinical study data, the combination is theorized to work synergistically in the hypothalamus and the mesolimbic dopamine circuit to promote satiety, reduce food intake, and enhance energy expenditure.9,10
Pro-opiomelanocortin (POMC) cells found in the arcuate nucleus of the hypothalamus produce melanocyte-stimulating hormone (alpha-MSH) and beta-endorphin, an endogenous opioid.11,12 The alpha-MSH activates the melanocortin-4 receptor (MC4R), leading to decreased food intake, increased energy expenditure, and weight loss.13,14 Beta-endorphin reduces activity of POMC cells by binding to the inhibitory mu-opioid receptor (MOP-R).15 Bupropion, a weak dopamine and norepinephrine reuptake inhibitor, enhances POMC cell production and release of alpha-MSH and beta-endorphin in vitro.10 Naltrexone, an opioid antagonist, blocks the MOP-R, therefore disrupting beta-endorphin inhibitory feedback on POMC cells.10 The naltrexone/bupropion combination enhances the effect of POMC signaling more than either drug alone.16
In addition, a preclinical study in hungry mice showed that direct injection of naltrexone and bupropion into the ventral tegmental area of the mesolimbic circuit produced a reduction in food intake.17 The reduction in food intake was notably larger than the reduction seen with either agent alone, suggesting that these drugs have independent yet synergistic actions in the mesolimbic dopamine circuit.17
INDICATION AND DOSAGE8
Naltrexone/bupropion is indicated as an adjunct to increased physical activity and a reduced-calorie diet for chronic weight management in obese adults (BMI of 30 kg/m2 or greater) or in overweight adults (BMI of 27 kg/m2 or greater) with at least one weight-related comorbid condition, such as hypertension, type-2 diabetes, or dyslipidemia.
Dosing recommendations are based on the number of tablets, which contain 8 mg of naltrexone and 90 mg of bupropion. Contrave should initially be prescribed as one tablet taken by mouth in the morning for one week. At the start of week 2, another tablet should be added to the regimen in the evening. This titration should continue weekly until the optimal dosing of two tablets twice daily is reached at week 4 for a total daily dose of 32 mg of naltrexone and 360 mg of bupropion.
After 12 weeks of treatment with naltrexone/bupropion, a patient should have achieved at least a 5% weight loss since initiation of therapy. If this result is not attained within 12 weeks, then naltrexone/bupropion should be discontinued because it is unlikely that the patient will derive benefit from it.
The drug has not been evaluated in patients with renal or hepatic impairment. The manufacturer’s dosing recommendations are based on data for naltrexone and bupropion individually (Table 1).
Table 1.
Renal and Hepatic Impairment Dosing Recommendations8
Renal Impairment
Hepatic Impairment
|
Too few trial participants were 65 years of age or older to determine if this age group responds differently than younger patients. Patients older than 65 years of age may be more sensitive to naltrexone/bupropion’s central nervous system adverse effects. No specific dose adjustments are recommended based solely on age, but using caution is advised. Safety and efficacy studies for patients younger than 18 years of age have not been performed, and the drug is not recommended for this age group.
DRUG INTERACTIONS8
The naltrexone component of Contrave may prevent patients from achieving full benefit from opioid-containing medications such as cough suppressants, antidiarrheal drugs, or opioid analgesics. If a patient requires intermittent treatment with opioids, Contrave should be discontinued and standard doses of the opioid should not be exceeded. If a patient uses opioids chronically, the opioid should be discontinued for seven to 10 days before Contrave is initiated to prevent precipitation of opioid withdrawal.8
The concomitant use of bupropion with a monoamine oxidase inhibitor (MAOI) is contraindicated due to risk of hypertensive reactions. A 14-day washout period should elapse before the initiation of Contrave or an MAOI after the discontinuation of the other medication.18
Bupropion is a strong inhibitor of cytochrome P450 (CYP 450) 2D6 isozyme and is primarily metabolized by CYP2B6 isozyme to the active metabolite hydroxybupropion.19 When initiating medications metabolized by CYP2D6 in a patient already receiving Contrave, start the concurrent medication at the lower end of its dosage range and titrate cautiously. Conversely, when initiating Contrave in a patient already receiving a drug metabolized by CYP2D6, a dose reduction of the original medication should be considered. Contrave should not exceed one tablet in the morning and evening when used concomitantly with CYP2B6 inhibitors.20 Avoiding the use of Contrave with CYP2B6 inducers is recommended as well.19
Additionally, bupropion has a dose-related propensity to cause seizures. Extra caution should be exercised when administering Contrave with anti-psychotics, antidepressants, theophylline, or systemic corticosteroids because of an enhanced risk of seizure. The use of bupropion with levodopa or amantadine has been reported to cause restlessness, agitation, tremor, ataxia, gait disturbance, vertigo, and dizziness due to combined dopamine agonistic effects.8 Use caution and monitor the patient for similar adverse events if Contrave is concurrently administered with these drugs.
PHARMACOKINETICS8
Following oral administration in healthy subjects, the peak concentrations (Cmax) and time to Cmax (Tmax) were 1.4 ng/mL in two hours and 168 ng/mL in three hours for naltrexone and bupropion, respectively. Naltrexone/bupropion administration is not recommended following a high-fat meal due to a significant increase in systemic exposure. Naltrexone demonstrated a 1.7-fold increase in area under the curve (AUC) and a 1.9-fold increase in Cmax. Bupropion exhibited a less significant 1.1-fold increase in AUC and 1.3-fold increase in Cmax.8 The increase in AUC and Cmax for both drugs is even more pronounced prior to reaching steady state. For more information on bioavailability, distribution, metabolism, and excretion, see Table 2.
Table 2.
Pharmacokinetic Profile of Contrave8
| Naltrexone | Bupropion | |
|---|---|---|
| Mean apparent volume of distribution at steady state (Vss/F) | 5,697 L | 880 L |
| Protein binding | 21% | 84% |
| Metabolism | Noncytochrome-mediated dehydrogenase | Extensively hepatic via CYP2B6 |
| Inhibition or induction of cytochrome P450 isozymes | None | Strong CYP2D6 inhibition |
| Metabolic first pass | Yes | No |
| Major metabolites | 6-beta-naltrexol (active) | Hydroxybupropion, erythrohydrobupropion, threohydrobupropion |
| Half-life |
|
|
| Excretion | Urine | Urine, feces |
EFFICACY IN CLINICAL TRIALS21–24
The Contrave Obesity Research-I (COR-I) study, the Contrave Obesity Research-II (COR-II) study, the Contrave Obesity Research-Intensive Behavior Modification study (COR-BMOD), and the Contrave Obesity Research-Diabetes study (COR-Diabetes) were four 56-week, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials enrolling 4,536 patients to evaluate the efficacy and safety of naltrexone/bupropion. The first three trials focused mainly on weight reduction in uncomplicated obesity or obesity with controlled hypertension and/or dyslipidemia. The COR-Diabetes study evaluated overweight and obese patients with type-2 diabetes.24 COR-I was the only study of the four that compared naltrexone/bupropion 16 mg/360 mg to naltrexone/bupropion 32 mg/360 mg and to placebo.21 All the studies combined low-intensity lifestyle modification counseling with naltrexone/bupropion or placebo except COR-BMOD. COR-BMOD utilized more intensive lifestyle modification counseling by exercise specialists, dietitians, and psychologists over 28 sessions.23 Interventions included strategically planned hypocaloric diets, calorie counting, maintaining food diaries, and gradual titration of exercise requirements. A full list of inclusion and exclusion criteria for the studies can be found in Table 3.
Table 3.
Inclusion and Exclusion Criteria for the Contrave Obesity Research (COR) Trial Program
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| COR-I Trial21 | Men and women ages 18–65 years, a BMI 30–45 kg/m2 with uncomplicated obesity, or a BMI of 27–45 kg/m2 with controlled hypertension and/or dyslipidemia | Pregnancy or lactation, type-1 or type-2 diabetes, obesity of known endocrine origin, cerebrovascular disease, cardiovascular disease, hepatic disease, renal disease, history of seizures, history of psychiatric illness, drug or alcohol misuse, previous surgical or device intervention for obesity, treatment with bupropion or naltrexone in the previous 12 months, loss or gain of more than 4 kg within 3 months prior to randomization |
| COR-II Trial22 | Same as COR-I plus a lack of any opioid medication for at least 7 days prior to randomization | Same as COR-I |
| COR-BMOD Trial23 | Same as COR-I | Same as COR-I except that patient cannot have used tobacco or nicotine within 6 months of study |
| COR-Diabetes Trial24 | Men and women ages 18–70 years with type-2 diabetes, a BMI of 27–45 kg/m2, an HbA1C of 7%–10%, a fasting blood glucose < 270 mg/dL, systolic and diastolic blood pressures < 145 and < 95 mm Hg, respectively; either no diabetes medications or stable doses of oral antidiabetic medications for ≥ 3 months prior to randomization | Type-1 diabetes, poorly controlled diabetes, diabetes deemed secondary to pancreatitis or pancreatectomy, severe microvascular or macrovascular complications of diabetes, serious medical conditions, loss or gain of > 5 kg weight within the previous 3 months, obesity of known endocrine origin, history of surgical or device intervention for obesity, history of seizures, history of serious psychiatric illness, history of drug or alcohol misuse, clinically significant cardiac abnormalities, receipt of prohibited concomitant medications |
BMI = body mass index; BMOD = behavioral modification; COR = Contrave Obesity Research; HbA1C = hemoglobin A1c.
In all four studies, naltrexone/bupropion was started at one-fourth of the full maintenance dose and titrated weekly until reaching the full maintenance dose at week 4. Efficacy analyses included an intention-to-treat (ITT) and modified intention-to-treat (mITT) approach with the last observation carried forward.
Baseline characteristics were similar among treatment arms in each of the four studies. In three of the four studies, the patient populations’ mean age was 44 to 46 years, the BMI was 36 kg/m2, more than 85% were women, and more than 68% were Caucasian. The COR-Diabetes trial, however, had a mean age of 54 years and 54% of the participants were female. These baseline characteristics could limit the external validity of these phase 3 studies.
Patients treated with naltrexone/bupropion 32 mg/360 mg achieved statistically and clinically significant weight reduction when compared with placebo in all four phase 3 studies. Additionally, the weight loss was maintained throughout the 56 weeks of all the trials. See Table 4 for overall weight loss percentage ranges. Patients in both arms of the COR-BMOD study lost on average more weight than patients in the other trials, most likely due to the more intensive lifestyle modification training.23
Table 4.
Body Weight Changes at 56 Weeks in Contrave Obesity Research (COR) Clinical Trials
| COR-I21 | COR-II22,a | COR-BMOD23 | COR-Diabetes24 | |||||
|---|---|---|---|---|---|---|---|---|
| Naltrexone/Bupropion 32/360 mg | Placebo | Naltrexone/Bupropion 32/360 mg | Placebo | Naltrexone/Bupropion 32/360 mg | Placebo | Naltrexone/Bupropion 32/360 mg | Placebo | |
| Intent-to-Treatb | n = 538 | n = 536 | n = 820 | n = 474 | n = 565 | n = 196 | n = 321 | n = 166 |
| Percent change in body weight from baseline, LS mean | −5.4%d | −1.3% | −5.6%d | −1.2% | −8.1%d | −4.9% | −3.7%d | −1.7% |
| Patients with ≥ 5% weight loss | 42%d | 17% | 47.9%d | 16.9% | 57%d | 43% | 36%d | 18% |
| Patients with ≥ 10% weight loss | 21%d | 7% | 28.1%d | 6.1% | 35%d | 21% | 15%e | 5% |
| Completersc | n = 296 | n = 290 | n = 434 | n = 267 | n = 301 | n = 106 | n = 175 | n = 100 |
| Percent change in body weight from baseline, LS mean | −8.1%f | −1.8% | −8.2%d | −1.4% | −11.5%d | −7.3% | −5.9%d | −2.2% |
| Patients with ≥ 5% weight loss | 62%f | 23% | 64.9%d | 21.7% | 80.4%d | 60.4% | 53.1%d | 24% |
| Patients with ≥ 10% weight loss | 34%f | 11% | 39.4%d | 7.9% | 55.2%d | 30.2% | 26.3%d | 8.0% |
BMOD = behavioral modification; COR = Contrave Obesity Research; LS = least squares.
56-week data was a secondary endpoint in the COR-II trial.
Includes all randomized patients with one baseline weight measurement and at least one post-baseline weight measurement during the defined treatment phase with the last observation carried forward.
Includes all randomized patients who completed 56 weeks of treatment.
Difference from placebo, P < 0.001.
Difference from placebo, P < 0.01.
Difference from placebo, P < 0.0001.
In the ITT population, the only cardiometabolic and glycemic parameters to show statistically significant improvements at 56 weeks after treatment with naltrexone/bupropion were waist circumference in COR-I and COR-BMOD and hemoglobin A1c (HbA1c) in the COR-Diabetes trial. When the analysis was changed to mITT, treatment with naltrexone/bupropion was associated with statistically significant improvements in waist circumference, triglycerides, and high-density lipoprotein-cholesterol (HDL-C). Moreover, in the COR-Diabetes trial, there was a clinically significant improvement in HbA1c and a lower portion of patients required rescue medications for glycemic control.24
Active treatment in the COR-I and COR-II trials was associated with significant improvements in eating control. Additionally, self-reported weight-related quality of life significantly improved in the COR-I, COR-II, and COR-Diabetes trials in the naltrexone/bupropion treatment arms.
The COR trial series provides adequate support for the efficacy of naltrexone/bupropion in chronic weight management, combined with lifestyle modifications.
SAFETY AND TOLERABILITY
Naltrexone/bupropion has been administered to 3,475 patients with more than 2,300 patient-years of exposure.25 The most common side effects experienced during the placebo-controlled trials are presented in Table 5. Contrave carries the antidepressant-class boxed warnings for suicidality and neuropsychiatric reactions in patients taking bupropion for smoking cessation, even though Contrave is not approved for depression or smoking cessation.8
Table 5.
Common Adverse Events Reported8
| Adverse Reactions | Naltrexone/Bupropion 32/360 mg n = 2,545 | Placebo n = 1,515 |
| Nausea | 32.5% | 6.7% |
| Constipation | 19.2% | 7.2% |
| Headache | 17.6% | 10.4% |
| Vomiting | 10.7% | 2.9% |
| Dizziness | 9.9% | 3.4% |
| Insomnia | 9.2% | 5.9% |
| Dry mouth | 8.1% | 2.3% |
| Diarrhea | 7.1% | 5.2% |
Naltrexone/bupropion has been associated with increases in resting heart rate and average blood pressure.8,25,26 In clinical trials, naltrexone/bupropion was not associated with a clinically significant change in blood pressure or electrocardiogram parameters.25 The number of cardiovascular events reported in clinical trials was considered to be too low to exclude a potential risk.25,26
P&T COMMITTEE CONSIDERATIONS
In February 2011, the FDA declined to approve Contrave due to concerns about the long-term cardiovascular safety profile; this was a substantial setback for Orexigen.6 Since that time, Orexigen has worked closely with the FDA and completed two additional studies. The FDA determined the additional studies were sufficient to grant approval of Contrave if Orexigen agreed to conduct post-marketing trials, including a long-term cardiovascular study called the LIGHT trial.27 However, Orexigen recently announced the termination of the LIGHT trial upon the recommendation of the lead researcher.28 Orexigen stated that the study is not being terminated due to the finding of superiority or harm but because protocol was breached.28 Interim study data was prematurely released in March 2015 indicating that naltrexone/bupropion may reduce cardiovascular risks, but these claims cannot be substantiated.28 Study protocol for a second post-marketing requirement, a cardiovascular outcomes trial, received FDA acceptance in April 2015, but Takeda and Orexigen have since entered into a legal dispute affecting the start date of the new trial.
Contrave’s approval came in the wake of the 2012 approvals of Qsymia and Belviq, and it is the fourth medication approved as an adjunct for chronic weight management. In the absence of head-to-head comparative studies, only data from the approved labeling can be used to determine general similarities and differences among products.
All currently approved antiobesity medications for chronic weight management have met the minimum FDA requirement of a 5% placebo-corrected weight loss and seem to have similar efficacy rates. While 5% to 10% weight loss in obese patients may appear to be only marginally beneficial, if at all, clinical benefits have been shown to exist even with modest reductions in weight. For example, weight loss of 5% to 10% is associated with reduced sleep apnea symptoms, improved mobility, and improvement in urinary stress incontinence.29 In patients with type-2 diabetes, weight loss of 5% to 10% is associated with reduced need for diabetes medications and a 0.6% to 1.0% reduction in HbA1c.30
Contrave, Qsymia, and Belviq have similar adverse effect profiles, which are relatively mild. Contrave has not been shown to have abuse potential and therefore is not a Schedule IV controlled substance like Qsymia and Belviq. Although Contrave has not been approved for any indication other than chronic weight management, it may have an advantage over weight-loss medications in patients who are overweight and concomitantly suffer from major depressive disorder, seasonal affective disorder, or attention-deficit disorder or who want to quit smoking. Conversely, it would be the last option, if used at all, in patients with uncontrolled hypertension, history of seizures, bulimia or anorexia nervosa, and chronic opioid use.
CONCLUSION
In recent years, obesity has evolved far beyond a simple cosmetic issue. It is now recognized at all levels of government as a major national health threat contributing significantly to many health conditions and mortality.30 In 2013, the American Medical Association passed a resolution that resulted in reclassification of obesity as a disease with physiological impairments requiring a range of medical interventions for treatment and prevention.31 Identification and treatment of underlying conditions contributing to obesity as well as intensive counseling on lifestyle modifications should remain first-line treatment. When first-line options provide unsatisfactory results, Contrave may serve as a valuable treatment adjunct to lifestyle modifications by promoting satiety, reducing feeding, enhancing energy expenditure, and ultimately helping patients achieve weight loss goals.
Footnotes
Disclosure: The authors report no commercial or financial interests in regard to this article.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Defining overweight and obesity. 2012. Available at: www.cdc.gov/obesity/adult/defining.html. Accessed January 1, 2015.
- 3.Centers for Disease Control and Prevention Adult obesity facts. 2014. Available at: www.cdc.gov/obesity/data/adult.html. Accessed January 1, 2015.
- 4.National Cancer Institute Obesity and cancer risk. 2012. Available at: www.cancer.gov/cancertopics/factsheet/Risk/obesity. Accessed January 1, 2015.
- 5.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff. 2009;28(5):822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 6.Pollack A. F.D.A. fails to approve diet drug. New York Times. 2011 Feb 2;:B1. Available at: www.nytimes.com/2011/02/02/business/02drug.html?_r=1&ref=orexigentherapeuticsinc. Accessed July 15, 2015. [Google Scholar]
- 7.Food and Drug Administration FDA approves weight-management drug Contrave. 2014. Available at: www.fda.gov/newsevents/newsroom/pressannouncements/ucm413896.htm. Accessed January 1, 2015.
- 8.Contrave (naltrexone HCl/bupropion HCl) prescribing information. Deerfield, Illinois: Takeda Pharmaceuticals America Inc; 2014. [Google Scholar]
- 9.Billes SK, Greenway FL. Combination therapy with naltrexone and bupropion for obesity. Expert Opin Pharmacother. 2011;12(11):1813–1826. doi: 10.1517/14656566.2011.591382. [DOI] [PubMed] [Google Scholar]
- 10.Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009;17(1):30–39. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 11.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 12.Liotta AS, Advis JP, Krause JE, et al. Demonstration of in vivo synthesis of pro-opiomelanocortin-, beta-endorphin-, and alpha-melanotropin-like species in the adult rat brain. J Neurosci. 1984;4(4):956–965. doi: 10.1523/JNEUROSCI.04-04-00956.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52(5):441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- 14.Fan W, Voss-Andreae A, Cao W-H, Morrison SF. Regulation of thermogenesis by the central melanocortin system. Peptides. 2005;26(10):1800–1813. doi: 10.1016/j.peptides.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Pennock RL, Hentges ST. Differential expression and sensitivity of presynaptic and postsynaptic opioid receptors regulating hypothalamic proopiomelanocortin neurons. J Neurosci. 2011;31(1):281–288. doi: 10.1523/JNEUROSCI.4654-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billes SK, Sinnayah P, Cowley MA. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res. 2014;84:1–11. doi: 10.1016/j.phrs.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Sinnayah P, Wallingford N, Evans A, Cowley MA. Bupropion and naltrexone interact synergistically to decrease food intake in mice [Abstract] Obesity (Silver Spring) 2007;15(9):A179. [Google Scholar]
- 18.Marcucci C, Sandson NB, Dunlap JA. Linezolid-bupropion interaction as possible etiology of severe intermittent intra-operative hypertension? Anesthesiology. 2004;101(6):1487–1488. doi: 10.1097/00000542-200412000-00051. [DOI] [PubMed] [Google Scholar]
- 19.Bjornsson TD, Callaghan JT, Einolf HJ, et al. The conduct of in vitro and in vivo drug–drug interaction studies: a PhRMA perspective. J Clin Pharmacol. 2003;43(5):443–469. [PubMed] [Google Scholar]
- 20.Turpeinen M, Tolonen A, Uusitalo J, et al. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2005;77(6):553–559. doi: 10.1016/j.clpt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 22.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19(1):110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orexigen Therapeutics Inc. Contrave (Naltrexone SR/Bupropion SR Combination) Advisory Committee Briefing Document. 2010. Available at: www.fda.gov/downloads/AdvisoryCommittees/Committees-MeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisory-Committee/UCM235672.pdf. Accessed July 15, 2015.
- 26.Mercer S. ACS chemical neuroscience molecule spotlight on Contrave. ACS Chem Neurosci. 2011;2(9):484–486. doi: 10.1021/cn200076y. Accessed May 30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ClinicalTrials.gov Cardiovascular outcomes study of naltrexone SR/bupropion SR in overweight and obese subjects with cardiovascular risk factors (the Light Study). NCT01601704. 2012. Available at: https://clinicaltrials.gov/ct2/show/NCT01601704?term=Naltrexone+SR+2FBupropion+SR&rank=3. Accessed May 12, 2015.
- 28. Dow Jones Business News. Orexigen terminates required study for diet drug Contrave. Nasdaq. Available at: www.nasdaq.com/article/orexigen-terminates-required-study-for-diet-drug-contrave-20150512-00828. Accessed May 12, 2015.
- 29.Bray GA. Why do we need drugs to treat the patient with obesity? Obesity. 2013;21(5):893–899. doi: 10.1002/oby.20394. Accessed July 15, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25):2985–3023. doi: 10.1016/j.jacc.2013.11.004. Accessed July 15, 2015. [DOI] [PubMed] [Google Scholar]
- 31.American Medical Association House of Delegates. Resolution 420, A-13; Recognition of Obesity as a Disease; 2013.; 2013. Available at: www.ama-assn.org/assets/meeting/2013a/a13-addendum-refcommd.pdf. Accessed July 15, 2015.


